Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Water SA
On-line version ISSN 1816-7950
Print version ISSN 0378-4738
Water SA vol.49 n.1 Pretoria Jan. 2023
http://dx.doi.org/10.17159/wsa/2023.v49.i1.3970
RESEARCH PAPER
Adsorptive removal of BTEX compounds from wastewater using activated carbon derived from macadamia nut shells
Kedibone MelaphiI; Olawumi O SadareI; Geoffrey S SimateII; Stephan WagenaarIII; Kapil MoothiI
IDepartment of Chemical Engineering, Faculty of Engineering and the Built Environment, Doornfontein Campus, University of Johannesburg, PO Box 17011, Doornfontein 2028, Johannesburg, South Africa
IISchool of Chemical and Metallurgical Engineering, Faculty of Engineering and the Built Environment, University of the Witwatersrand, Johannesburg, Wits 2050, South Africa
IIIDepartment of Chemical Sciences, Faculty of Science, University of Johannesburg, PO Box 17011, Doornfontein 2028, Johannesburg, South Africa
ABSTRACT
In this study, adsorptive removal of benzene, toluene, ethylbenzene and xylenes (BTEX) from synthetic water using activated carbon adsorbent derived from macadamia nut shells was investigated. The surface functional groups of the synthesized adsorbents were assessed by Fourier transform infrared spectra. The specific surface area, pore size and pore volume at 77 K nitrogen adsorption, surface morphology, and the crystalline structure of the adsorbents were determined using Brunauer-Emmett-Teller, scanning electron microscopy and x-ray diffraction, respectively. Batch adsorption mode was used to evaluate the performance of the activated carbon. The stock solutions of synthetic wastewater were prepared by dissolving 100 mg/L of each of the BTEX compound into distilled water in a 250 mL volumetric flask. Effect of initial concentration of BTEX compounds, contact time, and mass of adsorbent on the removal of BTEX compounds from the synthetic wastewater was investigated. The macadamia nut shell-derived activated carbon (MAC) proved to be an effective adsorbent for BTEX compounds, with a large surface area of 405.56 m2/g. The exposure time to reach equilibrium for maximum removal of BTEX was observed to be 20 min. The adsorption capacity of the BTEX compounds by MAC followed the following adsorption order: benzene > toluene > ethylbenzene > xylene.
Keywords: wastewater, activated carbon, adsorption, BTEX compounds, macadamia nut shell
INTRODUCTION
The presence of organic compounds such as benzene, toluene, ethylbenzene and xylenes (BTEX) in industrial and municipal wastewaters has been recognized as a serious environmental problem (Aghdam et al., 2015; Mohammadi et al., 2020). The BTEX compounds are common groundwater and potable water pollutants which are introduced to the environment through leakages from underground storage tanks, accidental spillages, and improper waste disposal practices, causing contamination of surface soils, seawater and the groundwater environment (Chriac et al., 2007; Slack et al., 2005; Mitra et al., 2011). The United States Environmental Protection Agency (USEPA) has classified BTEX as priority pollutants that pose significant threats to human health and the environment due to their carcinogenic properties (Mohammadi et al., 2017; Hackbarth et al., 2014; Mottaleb et al., 2003). Health concerns relating to BTEX compounds include irritation of mucous membranes, irritation of organs and respiratory problems (Nourmoradi et al., 2012). In spite of the negative health effects, BTEX pollutants remain untreated or undetected in municipal water treatment systems (Fayemiwo et al., 2018; Makhathini et al., 2017). This makes their removal from wastewater essential.
Many approaches, such as bioremediation, chemical oxidation and natural attenuation, have been employed to remove BTEX from wastewater. These techniques have proved to be successful; however, they are time-consuming and expensive (Fayemiwo et al., 2017; Agarwal et al., 2017). In the recent past, attention of researchers has been drawn towards the application of adsorption techniques, because these can be carried out at lower temperatures and pressures, making the process less energy intensive. In addition, the adsorption approach has low operational cost, is easy to operate, and adsorbents can be easily regenerated (Edet et al., 2020; Rodrigues et al., 2013). Several adsorbents, such as polysterin resin, organoclay, natural clays and activated carbon (AC) have been used to remove BTEX compounds from water through adsorption processes (Makhathini et al., 2017; Nasrollahpour et al., 2020; Carvallo et al., 2012). Although commercial AC is popular and widely utilized owing to its high surface area, microporous structure and chemical stability, its applications have been hampered due to high cost of starting material (e.g., wood or coal). Thus, it is imperative to use an efficient, cheap, and locally available adsorbent that is derived from renewable biomass materials (Dao et al., 2021).
Macadamia nuts are an agricultural product, which is grown in subtropical regions around the world. The macadamia tree (Macadamia spp.) is native to Australia, where it was first discovered in in 1857 (Dao et al., 2021). Macadamia nuts are grown commercially in Limpopo, the northernmost province of South Africa. The production of AC using different agricultural waste products has been extensively documented in literature, whereas macadamia nut shells have received little attention. Moreover, utilisation of macadamia nut shells as a starting material to produce AC for removal of BTEX compounds is scantily reported in literature.
Macadamia nut shells are an abundant resource. Australia produces 47 000 tons of macadamia nuts with about 10 800120 000 tons of macadamia nut shells discarded as solid waste per annum, posing a significant waste management issue (Dao et al., 2020). However, recycling of macadamia nut shells has played a vital role in producing biochar, which has been used to make activated carbon, because the shells have high carbon content (Dao et al., 2021). In fact, agricultural waste materials that are carbon based can be used as effective raw materials for making AC. Recent studies have investigated the use of agricultural waste such as, cocoa husk, corn cob, nut shell, macadamia nut shells and orange peels as raw materials for synthesis of adsorbents (Dejang et al., 2015; Jawad et al., 2017). Activated carbons derived from macadamia nut shells have shown to be promising adsorbents for the removal of various pollutants (Pakade et al., 2016; Wongcharee et al., 2017; Dao et al., 2020).
In view of the aforementioned, application of macadamia nut shell as a promising adsorbent precursor for production of powdered and granular activated carbons could solve the challenge associated with its disposal and reduce environmental pollution. Macadamia nut shell-derived activated carbons (MACs) are a common carbon-based group of nanomaterials derived from biomass. They have microporous and mesoporous structures with a large surface area of 700-1 100 m2/g (Rotorcarb Activated Carbon, 2020). Furthermore, MACs have many reactive sites on their surface, resulting in high adsorption capacity. The unique structure of MACs makes it suitable for bridging the gap between conventional coal and coconut carbons (Rotorcarb Activated Carbon, 2020). They have many applications in water purification, such as odour removal, colour correction, alcohol removal and toxin removal (Rodrigues et al., 2013). Therefore, they can be extensively used as effective and affordable adsorbent for removal of BTEX compounds from industrial wastewater. In a previous study, Dao et al. (2020) reported the synthesis of macadamia nut shell by H3PO4 activation for effective removal of Cu2+ and Zn2+ from wastewater. The adsorption efficiencies obtained were 84.02% and 53.42%, respectively. Wongcharee et al. (2018) also reported the use of MAC synthesized through carbon dioxide activation for adsorption of methylene blue. The results showed adsorption capacities in the range of 134-144 mg/g. In other studies, rice husk activated carbons (Yakout et al., 2014), paper mill sludge activated carbons (Aghdam et al., 2015) and biological activated carbons (Zhang et al., 2011) have been reported to be effective adsorbents for removal of BTEX compounds from wastewater. However, as far as it can be ascertained, no studies have been reported in literature on the use of activated carbon derived from macadamia nut shells as an adsorbent for removal of BTEX compounds from synthetic wastewater.
Against this background, this study investigated the performance of MACs in a batch adsorption mode for the removal of BTEX compounds from synthetic wastewater. In addition, the study provides information on the adsorption behaviour and kinetics of the adsorbent during the adsorption process.
MATERIALS AND METHODS
The powdered macadamia nut shell activated carbons (PMAC, 250 μm) used in this study were donated by Rotocarb Activated Carbon, Olifantsfontein, South Africa. The MAC was used as received from the manufacturer without any further purification. The BTEX chemicals, consisting of benzene (purity: 99.7%), toluene (purity: 99.7%), ethylbenzene (purity: 99.7%), and xylene (purity: 99.7%) were purchased from Sigma-Aldrich Johannesburg, South Africa. The chemicals used in this study were used without further purification.
Physico-chemical characterization of MAC
Specific surface area, pore size, and pore volume was obtained by Brunauer-Emmet-Teller (BET). The samples were analysed by physical adsorption of nitrogen at 77K using a Nova 3200e instrument. The samples were degassed at 150°C up to 4 h prior to each measurement. The SEM JOEL JSM-5600 scanning electron microscope (SEM) was used to examine the surface morphology of the adsorbent. The samples were coated with carbon to obtain better images. X-ray diffraction (XRD) analysis was used to examine the crystalline structure of the MACs. XRD patterns were obtained with Bruker XRD machine carried out in the two theta (2θ) on a D8 diffractometer. Functional groups of the adsorbent sample were assessed with Fourier-transform infrared (FTIR) spectroscopy Perkin Elmer Two ATR-FTIR equipment to detect surface functional groups. A small amount of KBr powder was mixed with MACs. The mixture was ground for 5 min in a mortar to fine powder. The pellets were placed on the FTIR sample holder for analysis. The spectra for MACs were obtained in a frequency band range from 500 to 4 000 cm-1.
Preparation of synthetic BTEX solution
The synthetic wastewater was prepared by dissolving 100 mg/L of each of the compounds (benzene, toluene, ethylbenzene and xylene), using a micropipette, in distilled water in a 250 mL volumetric flask to prepare the stock solution. The flask was closed with a lid and placed in the orbital shaker incubator running at 180 r/min at room temperature for 60 min to allow dissolution of BTEX compounds (Fadaei et al., 2017). New solutions were prepared daily with the initial concentration of the adsorbate calculated before the start of each experiment. Dilutions of the stock solution were prepared for calibration standard.
Performance evaluation of MAC for removal of BTEX compounds
For adsorption capacity studies, BTEX compounds were dissolved in distilled water and 0.055 g of the adsorbent was added to the flask containing different initial concentrations of BTEX compounds, ranging from 50 mg/L to 250 mg/L, in water. The experiments were conducted at 25°C for 60 min; the samples were then analysed with UV-vis (SHIMADZU UV MINI 1240 model) between 215 and 261 nm. The experiments were conducted in duplicate. The adsorption capacity of the adsorbent was calculated using Eq. 1.

where: Qt = adsorption capacity of adsorbent (mg/g); C0 = initial concentration of adsorbate (mg/L); Ct = concentration at time t (mg/L); V = solution volume (L); and m = mass of adsorbent used (g).
To determine the BTEX removal percentage, Eq. 2 was used:

where Ci is the initial dose of the pollutants and Ce is the effluent concentration in mg/L.
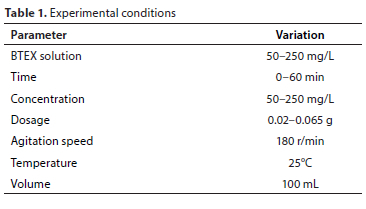
Table 1 provides detailed information on the conditions of the experiment: contact time, adsorbent dosage, and concentration.
Adsorption isotherms
The results obtained from Eq. 1 were used to determine the adsorption isotherms. The Langmuir and Freundlich isotherms were used to examine the adsorption mechanism of MAC for the removal of BTEX compounds from synthetic BTEX-containing wastewater.
Langmuir Isotherm
The Langmuir isotherm model assumes homogeneous monolayer adsorption; adsorption occurs via a fixed number of the same sorption sites (Makhathini et al., 2017). The model is expressed in Eq. 3:
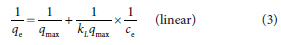
where qe (mg/g) is the amount of BTEX pollutants adsorbed at equilibrium, KL is the Langmuir constant, Ce is the equilibrium concentration of BTEX pollutants in solution after adsorption (mg/L) and qmax is the maximum amount of adsorption equivalent to complete monolayer coverage on the surface (mg/g) (Makhathini et al., 2017).
In this experiment, Ce was measured and qe is calculated for different sets of conditions. The Langmuir model produced a graph of1/qe vs. 1/Ce to establish the best-fit line and determine the R2 values for each pollutant adsorbed onto the MAC adsorbents. The feasibility of the processes was evaluated using the separation factor RL. A dimensionless constant or separation factor (RL) is represented by Eq. 4:

where RL is used to describe the favourable nature of the adsorption process whereby RL > 1 is unfavorable, RL = 0 is linear, 0 < RL < 1 is favourable, and RL = 0 is irreversible (Foo et al., 2010)
Freundlich isotherm
The Freundlich isotherm describes a heterogeneous process whereby the amount of solute adsorbed per unit adsorbent mass grows gradually (Edet et al., 2020). The Freundlich isotherm model was originally developed as an empirical model expressed as follows:

where Kf is the constant of the Freundlich isotherm (L/g) and n is an isotherm constant. The value of n is determined by the nature and strength of the adsorption process as well as the available active sites (Saeed et al., 1996).
The n value is an empirical constant related to heterogeneity of the adsorbent surface. The parameter n indicates the nature of the adsorption process whereby if n lies between 0 and 1, adsorption is favourable, if n > 1 adsorption is unfavourable, and n = 1 represents linear adsorption, and if n = 0 the process is irreversible. The values of n and KF are calculated from the slope and intercept of the plot of ln qe versus ln Ce (Aziam et al., 2017).
The best-fit kinetic model is selected by considering the regression coefficient of determination (R2) which is a measure of how well predicted values from a forecast model match the experimental data. The Freundlich kinetic model is applied to plot ln qe vs. ln Ce graph for the best-fit line that determines the R2 values for each pollutant adsorbed onto MAC.
Adsorption kinetics
The adsorption kinetics for adsorption of BTEX compounds onto MACs was investigated using the pseudo-first-order (PFO) and pseudo-second-order (PSO) kinetic models. The PFO kinetic model describes the relationship between the rate at which the sorption sites of the adsorbents are occupied and the number of unoccupied sites (Sadare and Daramola, 2019). It is defined using Eq. 6:

where qe and qt are the amounts of BTEX adsorbed at equilibrium and at time t (min), respectively, and k1 is the rate constant of adsorption (min-1). The linear plot of ln (qe - qt) against time is used to determine the rate constant k1.
The PSO kinetic model describes the dependency ofthe adsorption capacity of the adsorbent on time and can be determined based on Eq. 7:

where qt and qe are the amounts of BTEX adsorbed at equilibrium and at time t (min), respectively, and k2 is the pseudo-second-order rate constant (g/(mg-min). The linear plot of t/qi against time is used to determine qe and k2 from the slope and intercept, respectively,
RESULTS AND DISCUSSION
Physicochemical characterization of MAC
Figure 1 depicts the surface morphology of MAC, whereby the surface was observed to be glossy and smooth. The pores of the MAC were elongated, and the microstructure had high porosity (Fab et al., 2018). The alignment indicates that the macadamia kept the structure oflignocellulose-based plant materials, showing that during the activation process non-carbon materials were removed. There was also an increase in the presence of mesopores and macropores which confirms the high specific surface area of MACs from BET results. This is an indication that MACs could be a suitable adsorbent for effective adsorption of BTEX pollutants (Wongcharee et al., 2018)
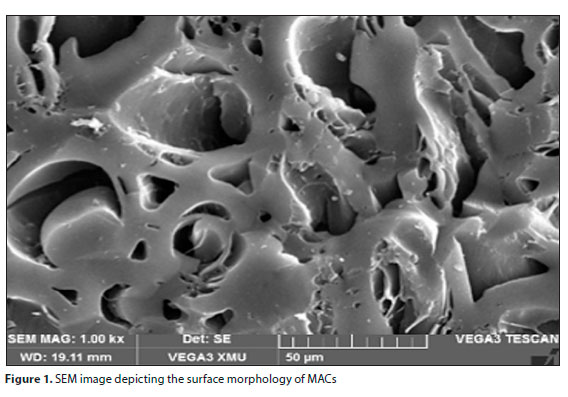
Figure 2 illustrates the surface functional groups on MACs as assessed by FTIR spectroscopy. Surface functional groups on the MACs play an essential role in the adsorption and provide selectivity and affinity towards BTEX. The infrared spectrum of activated carbon derived from macadamia nut shell showed a strong peak at 3 443 cm-1, representing the OH stretching vibration of hydroxyl functional groups, which is commonly seen when water is used in the preparation process (Makhathini et al., 2015). The aliphatic stretching vibration C-H is observed at a very weak band at 2 847 cm-1 while the peak at 2 909 cm-1 represents the presence of CH2 stretching (Fan et al., 2018). The peaks at 1 631 cm-1 to 1 080 cm-1 represent C-O and C=O which is attributed to the presence of carboxylic acids (Phele et al., 2019; Wongcharee et al., 2018). The peak at 1 351 cm-1 corresponds to C=C stretching of the aromatic rings, which indicates the presence of carbon in the macadamia nut shells (Mopoung et al., 2015). This could be formed by decomposition of C-H bonds at high activation temperature. The peak at 1 080 cm-1 represents C-OH stretching of phenol groups (Phele at al., 2019).
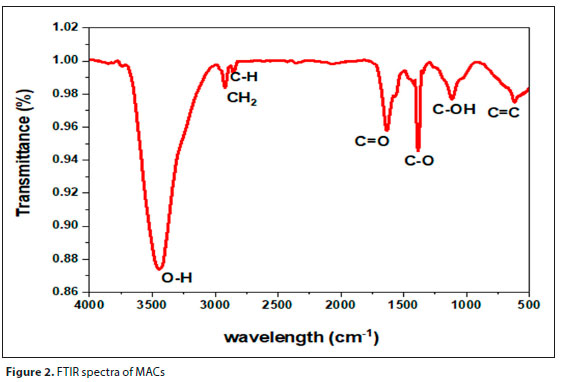
These are the main functional groups that can be attributed to the macadamia activated carbon component. The results observed in this study are comparable to literature (Mopoung et al., 2015; Phele et al., 2019; Wongcharee et al., 2018).
Figure 3 depicts the crystalline structure of the MAC composite. From the XRD graph (Fig. 4), a broad intensity at 22.6° which correlates to carbon and graphite at 38.8° is observed at the initial phase of carbon powder, which can be indexed as 002 and 222 plane reflection. The peaks obtained correspond to amorphous carbon and graphite at high broad peaks. At high temperature, applied during the activation process, the macadamia nut shell was transformed to a crystalline structure of carbon (Dejang et al., 2015). The results indicate the presence of graphite crystallite in the macadamia nut shell (Xie et al., 2014). Similar observations were reported by Wongcharee et al. (2017) and Dejang et al. (2015).
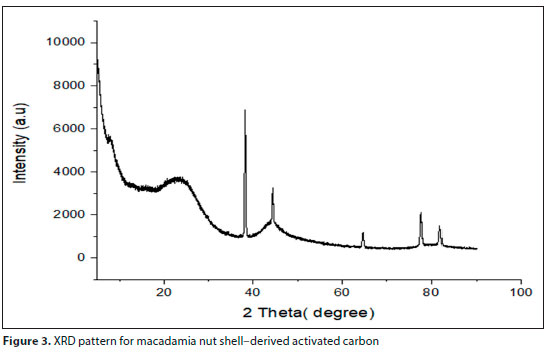
Table 2 presents the textural properties of the MAC adsorbent. The BET technique was used to characterize the textural properties of activated carbon. The adsorbent materials were characterized in terms of pore sizes and surface area. From Table 2, it can be seen that the activated carbon has a specific surface area of 405.56 m2/g. This could be due to its micro-pore size of 241.89 Â and the temperature employed during synthesis. Kemp et al. (2015) claimed that temperature employed during the synthesis of an adsorbent determines the development of surface areas. Makhathini et al. (2015) reported that the porous structure of activated carbons is positively or negatively affected by the surface oxidation. In a positive way, reaction of oxygen with a reactive carbon skeleton can form new micropores which increase the specific surface area. However, in a negative way, the increase in size of pores, from micropore to macropore, can result in an overall decrease in the specific surface area (Makhathini et al., 2015).
Performance evaluation of MAC for adsorptive removal of BTEX compounds
Effect of adsorbent contact time
The effect of contact time on the adsorptive removal of BTEX compounds from synthetic BTEX solution is depicted in Fig. 4. The contact time was varied from 2 to 60 min while all other parameters remained constant. Percentage BTEX removal increased with time. The rapid increase in the adsorption of BTEX compound from 2-20 min might be due to the numerous adsorption sites that are available at the beginning of the reaction, which subsequently decreased with increase in contact time as the adsorption sites become saturated (Sadare and Daramola, 2019; Sadare et al., 2020). Mekonnen et al. (2015) and Kaya et al. (2014) reported similar trends, using papaya peels and wheat bran-derived adsorbents for removal of Cr6+, respectively. Dao et al. (2020) also observed a similar trend during investigation of Cu2+ and Zn2+ removal using activated carbon derived from macadamia nut shell. The adsorption rate was said to be quicker before the reaction approached equilibrium and slower as the reaction attained equilibrium (Wongcharee et al., 2017; Sadare et al., 2020). The results also showed that xylene was the most adsorbed compound among the BTEX compounds, and the order of percentage removal was B < T < E < X. This can be attributed to their differing water solubility, at 1 780 mg/L for benzene, 500 mg/L toluene, 150 mg/L for ethylbenzene and 150 mg/L for o, m, p xylenes (Su et al., 2010). This observed trend could also be attributed to their hydrophobicity (based on log Kow) with benzene = 2.13, toluene = 2.69, ethylbenzene = 3.15 and xylenes = 3.15. Similar results were obtained by Nourmoradi et al. (2012) and Jodeh et al. (2015) using various adsorbents.
Effect of adsorbent dosage
Figure 5 presents the percentage removal of BTEX compounds from synthetic aqueous solution. The adsorbent dosage was varied from 0.02-0.0655 g, while all other parameters remained constant. Percentage removal of BTEX compounds increased as the adsorbent mass was increased. This was attributed to the additional active sorption sites and surface area of MAC, which was 405.56 m2/g as obtained from BET analysis (Edet et al., 2020). It could be observed that at 0.025 g of MAC, 85.56%, 78.49%, 70.40% and 58.90% removal were obtained for xylene, ethylbenzene, toluene and benzene, respectively. At 0.025 g adsorbent dosage, no further adsorption took place; this is because most of the BTEX compounds were adsorbed (Jodeh et al., 2015). The removal percentage for BTEX followed the order B < T < E < X. Less hydrophobic compounds such as benzene, which is more soluble compared to the other compounds, has less affinity toward the MAC adsorbent. Recent studies have concluded that BTEX adsorption onto various promising adsorbents favours the adsorption rate order of B < T < E < X (Nourmoradi et al., 2012, Kong et al., 2020). This is possibly because of the decrease in water solubility as well as the combination of molecular weight and hydrophobicity (log Kow).
Effect of initial concentration
The effect of initial adsorbate concentration on the removal of BTEX compounds by MAC is shown in Fig. 6. The initial concentration of BTEX was varied from 0-250 mg/L while all other adsorption parameters remained constant. At lower BTEX concentrations (0-100 mg/L) the percentage removal of BTEX increased. This could be due to the availability of more vacant adsorption sites at low concentrations of BTEX compounds. At higher concentrations from 100 mg/L to 250 mg/L, it was observed that the percentage removal decreased, and equilibrium was reached. The decrease in percentage removal at higher initial concentration could be attributed to the saturation of adsorption sites (Owalude and Tella, 2016; Sadare and Daramola., 2019).
Comparison of results with literature
Table 3 summarises and compares published studies where various adsorbents have been used to remove BTEX pollutants from aqueous solutions. Jodeh et al. (2015) reported the use of date palm pits for removing BTEX at 0.1 adsorbent dosage, 20°C and 100 mL BTEX solution. The percentage removals obtained were 68% for benzene, 72% for toluene and 77% for xylene. Similarly, in a study conducted by Yakout et al. (2014) in which the removal of BTEX was achieved by using activated carbon prepared from rise husk, the removal percentage was 22% benzene, 33% toluene, 58% ethylbenzene, and 18.8% of p-xylene.
In this study, removal of BTEX was investigated using MAC at 0.055 g dosage, 100 mL/g BTEX solution at 25°C. The removal percentages obtained were 85.56%, 78.49%, 70.40% and 58.90% for xylene, ethylbenzene, toluene and benzene, respectively. The results obtained from the current study showed higher adsorption performance compared to Jodeh et al. (2015) and Yakout et al., (2014). Removal of BTEX compounds using MAC proved to give high removal efficiencies. It is expected that percentage removal will increase with increase in surface area. However, this was not the case in this study, where surface area for MAC was 405.56 m2/g before modification, while surface area for date pit and rice husk activated carbon was 893.78 m2/g and 956.51 m2/g, respectively, after modification (Anjum et al., 2017). This indicates that specific surface area is not the main factor which determines high removal percentages. Bansode et al. (2013) established that activated carbons with high surface areas do not always result in good aromatic adsorption. In another example, in the removal of BTEX using paper mill sludge activated carbon the surface area was 613.38 m2/g with removal efficiencies above 92% for all for compounds at 10 mL/g and 1 g/L dosage (Aghdam et al., 2015).
Other factors such as selection of raw materials and activation play an essential role (Anjum et al., 2017). Raw materials that have abundant lignin content have a microporous structure while raw materials that have high cellulosic content have a macroporous structure. Surface functional groups such as carboxylic groups are also the main feature enabling adsorption of BTEX (Aghdam et al., 2015). Therefore, further studies should consider how the surface of the adsorbent can be modified in order to improve the efficiency of the MAC adsorbent.
Adsorption isotherm studies
Equilibrium data were fitted to the Langmuir and Freundlich isotherm models as described by Eqs 4 and 6. Figure 7a shows the Freundlich isotherm plot of BTEX sorption onto MAC adsorbent. The isotherm failed to adequately fit in the data, with R2 = 0.7109. The Freundlich constant 1/n was found to be 0.4264, which indicates that adsorption of BTEX by the MAC was favourable. Similar results were obtained in a study conducted by Sivakumar et al. (2019) for the removal of Azure A dye in wastewater. The Langmuir isotherm presented in Fig.7b fitted the data perfectly with R2 = 1.000, suggesting that the adsorption process occurs as a monolayer without a lot of interactions between molecules of the adsorbate. Edokpayi et al. (2020) and Wongcharee et al. (2018) reported similar results using macadamia nut shell for sorption of methylene blue.
The feasibility of the processes was evaluated using the separation factor RL. In this study, the value for RL is 0.019; this indicates that the adsorption process is favourable (Wongcharee et al., 2018). In other studies to investigate the adsorption of BTEX compounds, the results have differed from the findings of this study. Nourmoradi et al. (2012) reported that the Freundlich isotherm was a better fit for BTEX adsorption on montmorillonite adsorbent. On the other hand, Makhathini et al. (2017) reported that the Langmuir isotherm was a better fit for BTEX adsorption on PAD polystyrenic resins. This indicates that the adsorption of BTEX can be described by different isotherms, possibly based on the nature of the adsorbent used.
Adsorption kinetics
In this study, PFO and PSO were evaluated for BTEX compounds adsorbed on the MAC. It is evident from the plots (Fig. 8a and b) that the PSO fitted the data better than PFO. R2 for the PSO was above 0.9 and for the PFO was less than 0.9. This observation indicates that that the rate-limiting step for adsorption of BTEX on MACs may be chemisorption. In chemical adsorption, the BTEX molecules stick to the adsorbent surface by forming strong covalent bonds. The data show large difference between the experimental and calculated adsorption capacity (qe) for BTEX in the PFO model.
CONCLUSION
This study has successfully established a proof-of-concept that activated carbon derived from macadamia nut shell is a promising adsorbent for effective removal of BTEX compounds from synthetic aqueous solution. The adsorption capacity was affected by the initial BTEX concentration, adsorbent dose, and contact time. It reached a maximum concentration of 100 mg/L at adsorbent dose of 0.025 g and a sorption time of 20 min. The sorption capacity of BTEX increased in the order B < T < E < X, due to their differing molecular weights. The increase in percentage removal compared to other activated carbons was also attributed to the surface area of MAC which was 405.56 m2/g. This confirms that the adsorbent has a mesoporous structure. The equilibrium adsorption was better represented by the Langmuir isotherm which indicates a monolayer coverage of the adsorbate on the surface of the MAC. The kinetic study indicated that the PSO described the data better with R2 of 0.99.
Waste macadamia nut shells are readily available, hence making the adsorption process a cheaper alternative to other conventional techniques of treating BTEX-contaminated water. The results of this study could stimulate further research in this direction. Furthermore, the results reported in this study could be instrumental in addressing the challenge posed by the disposal of waste macadamia nut shell and thus alleviate environmental pollution.
AUTHOR CONTRIBUTIONS
Conceptualization by KM and KM. The manuscript was written by KeM, KaM, OOS. KaM, OOS and GSS contributed to the preparation and review of this manuscript. Experiments were performed by KeM and data analyses were carried out by KeM in discussion with OOS. The project was supervised by KaM and GSS with project administration by KaM. All authors have read and agreed to the published version of the manuscript.
ACKNOWLEDGEMENTS
The authors acknowledge the financial support provided by National Research Fund (NRF) and BP Education Foundation SA.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
AGARWAL M and SINGH K (2017) Heavy metal removal from wastewater using various adsorbents: a review. J. Water Reuse Desalin. 7 (4) 387-419. https://doi.org/10.2166/wrd.2016.104 [ Links ]
AGHDAM E, AMINZADEH B, BAGHDADI M and FARD MA (2015) Removal of BTEX from aqueous solutions by paper mill sludge-based activated carbon. J. Adv. Chem. 11 (1). https://doi.org/10.24297/jac.v11i1.6688 [ Links ]
ANJUM H, CHEMAT F, GNANASUNDARAM N, ARUNAGIRI A and THANABALAN M (2017) Impact of surface modification of activated carbon on BTEX removal from aqueous solutions: a review. In: Sen TK (ed.) Air, Gas, and Water Pollution Control Using Industrial and Agricultural Solid Wastes Adsorbents, CRC Press. 293-312. [ Links ]
AZIAM R, CHIBAN M, EDDAOUDI H, SOUDANI A, ZERBET M and SINAN F (2017) Kinetic modelling, equilibrium isotherm and thermodynamic studies on a batch adsorption of anionic dye onto eco-friendly dried Carpobrotus edulis plant. Eur. Phys. J. Spec. Topics 226 (5) 977-992. https://doi.org/10.1140/epjst/e2016-60256-x [ Links ]
CARRIERO G, NERI L, FAMULARI D, DI LONARDO S, PISCITELLI D, MANCO A, ESPOSITO A, CHIRICO A, FACINI O, FINARDI S and TINARELLI G (2018) Composition and emission of VOC from biogas produced by illegally managed waste landfills in Giugliano (Campania, Italy) and potential impact on the local population. Sci. Total Environ. 640 377-386. https://doi.org/10.1016/j.scitotenv.2018.05.318 [ Links ]
DAO MT, NGUYEN TT, NGUYEN XD, LA DD, NGUYEN DD, CHANG SW, CHUNG WJ, and NGUYEN VK (2020) Toxic metal adsorption from aqueous solution by activated biochars produced from macadamia nut shell waste. Sustainability 12 (19) 7909. https://doi.org/10.3390/su12197909 [ Links ]
DAO MT, TRAN TPL, VO DT, NGUYEN VK and HOANG LTTT (2021) Utilization of macadamia nut shell residue for the synthesis of magnetic activated carbon toward zinc (II) ion removal. Adv. Mater. Sci. Eng. 2021 Article ID 2543197, 10 pp. https://doi.org/10.1155/2021/2543197 [ Links ]
DEJANG N, SOMPRASIT O and CHINDARUKSA S (2015) A preparation of activated carbon from Macadamia shell by microwave irradiation activation. Energ. Proced. 79 727-732. https://doi.org/10.1016/j.egypro.2015.11.556 [ Links ]
EDET UA and IFELEBUEGU AO (2020) Kinetics, isotherms, and thermodynamic modeling of the adsorption of phosphates from model wastewater using recycled brick waste. Processes 8 (6) 665. https://doi.org/10.3390/pr8060665 [ Links ]
EDOKPAYI JN, ALAYANDE SO, ADETORO A and ODIYO JO (2020) The Equilibrium, kinetics, and thermodynamics studies of the sorption of methylene blue from aqueous solution using pulverized raw macadamia nut shells. J. Anal. Meth. Chem. 2020 Article ID 8840666, 10 pp. https://doi.org/10.1155/2020/8840666 [ Links ]
FAN F, YANG Z, LI H, SHI Z and KAN H (2018) Preparation and properties of hydro chars from macadamia nut shell via hydrothermal carbonization. R. Soc. Open Sci. 5 (10) 181126. https://doi.org/10.1098/rsos.181126 [ Links ]
FADAEI S, MOGHADAM FN, HASHEMI M and POURZAMANI H (2017) BTEX removal from aqueous solution by modified multi-walled carbon nanotubes with ozone. An. Inst. Geoci. 40 (1) 235-242. https://doi.org/10.11137/2017_1_235_242 [ Links ]
FAYEMIWO O, MOOTHI K and DARAMOLA M (2017) BTEX compounds in water-future trends and directions for water treatment. Water SA 43 (4) 602-613. https://doi.org/10.4314/wsa.v43i4.08 [ Links ]
FAYEMIWO OM, DARAMOLA MO and MOOTHI K (2018) Tannin-based adsorbents from green tea for removal of monoaromatic hydrocarbons in water: preliminary investigations. Chem. Eng. Comm. 205 (4) 549-556. https://doi.org/10.1080/00986445.2017.1409738 [ Links ]
JAWAD AH, MAMAT NFH, ABDULLAH MF and ISMAIL K (2017) Adsorption of methylene blue onto acid-treated mango peels: kinetic, equilibrium and thermodynamic study. Desalin. Water Treat. 59 210-219. [ Links ]
JODEH S, AHMAD R, SULEIMAN M, RADI S, EMRANK M, SALGHI R, WARAD I and HADDA TB (2015) Kinetics, thermodynamics and adsorption of BTX removal from aqueous solution via date-palm pits carbonization using SPME/GC-MS. J. Mater. Environ. Sci 6 (10) 2853-2870. [ Links ]
KONG H, MAT H and YUNUS MAC (2020) Batch adsorptive removal of BTEX from aqueous solution: a review. In: Third International Conference on Separation Technology 2020 (ICoST2020). Atlantis Press. 303-318. https://doi.org/10.2991/aer.k.201229.040 [ Links ]
LEITE AB, SAUCIER C, LIMA EC, DOS REIS GS, UMPIERRES CS, MELLO BL, SHIRMARDI M, DIAS SL and SAMPAIO CH (2018) Activated carbons from avocado seed: optimisation and application for removal of several emerging organic compounds. Environ. Sci. Pollut. Res. 25 (8) 7647-7661. https://doi.org/10.1007/s11356-017-1105-9 [ Links ]
LIMA LF, DE ANDRADE JR, DA SILVA MG and VIEIRA MG (2017) Fixed bed adsorption of benzene, toluene, and xylene (BTX) contaminants from monocomponent and multicomponent solutions using a commercial organoclay. Indust. Eng. Chem. Res. 56 (21) 6326-6336. https://doi.org/10.1021/acs.iecr.7b00173 [ Links ]
MAKHATHINI TP and SUDESH R (2017) Investigation of BTEX compounds adsorption onto polystyrenic resin. S. Afr. J. Chem. Eng. 23 (2017) 71-80. https://doi.org/10.1016/j.sajce.2017.03.001 [ Links ]
MAKHATHINI TP (2015) Investigation of the adsorption performance of polystyrenic resin and GAC for the removal of BTEX compounds from industrial wastewater. PhD dissertation, Durban University of Technology, Durban. https://hdl.handle.net/10321/1496 [ Links ]
MITRA S and ROY P (2011) BTEX: a serious ground water contaminant. Res. J. Environ. Sci. 5 (5) 394-398. https://doi.org/10.3923/rjes.2011.394.398 [ Links ]
MOHAMMADI L, RAHDAR A, BAZRAFSHAN E, DAHMARDEH H, SUSAN ABH and KYZAS GZ (2020) Petroleum hydrocarbon removal from wastewaters: a review. Processes 8 (4) 447. https://doi.org/10.3390/pr8040447 [ Links ]
MOHAMMADI L, BAZRAFSHAN E, NOROOZIFAR M, ANSARI-MOGHADDAM A, BARAHUIE F and BALARAK D (2017) Adsorptive removal of benzene and toluene from aqueous environments by cupric oxide nanoparticles: kinetics and isotherm studies. J. Chem. 2017 Article ID 2069519. https://doi.org/10.1155/2017/2069519 [ Links ]
MOPOUNG S, MOONSRI P, PALAS W and KHUMPAI S (2015) Characterization and properties of activated carbon prepared from tamarind seeds by KOH activation for Fe (III) adsorption from aqueous solution. Sci. World J. 2015 Article ID 415961. 9 pp. https://doi.org/10.1155/2015/415961 [ Links ]
MOTTALEB MA, ABEDIN MZ and ISLAM MS (2003) Determination of benzene, toluene, ethylbenzene and xylene in river water by solid-phase extraction and gas chromatography. Anal. Sci. 19 (10) 1365-1369. https://doi.org/10.2116/analsci.19.1365 [ Links ]
NOURMORADI H, NIKAEEN M and KHIADANI M (2012) Removal of benzene, toluene, ethylbenzene and xylene (BTEX) from aqueous solutions by montmorillonite modified with nonionic surfactant: Equilibrium, kinetic and thermodynamic study. Chem. Eng. J. 191 341-348. https://doi.org/10.1016/jxej.2012.03.029 [ Links ]
NOURMORADI H, KHIADANI M and NIKAEEN M (2013) Multi-component adsorption of benzene, toluene, ethylbenzene, and xylene from aqueous solutions by montmorillonite modified with tetradecyl trimethyl ammonium bromide. J. Chem. 2013 Article ID 589354. 10 pp. https://doi.org/10.1155/2013/589354 [ Links ]
NASROLLAHPOUR S, DARYOUSH YK, MOHAMMAD G, and OMID GF (2020) Application of organically modified clay in removing BTEX from produced water. In: Geo-Congress 2020: Geo-Systems, Sustainability, Geoenvironmental Engineering, and Unsaturated Soil Mechanics. American Society of Civil Engineers, Reston, VA. 275-283. https://doi.org/10.1061/9780784482827.031 [ Links ]
OWALUDE SO and TELLA AC (2016) Removal of hexavalent chromium from aqueous solutions by adsorption on modified groundnut hull. Beni-Suef Univ. J. Basic Appl. Sci. 5 (4) 377-388. https://doi.org/10.1016/j.bjbas.2016.11.005 [ Links ]
PAKADE VE, NTULI T and OFOMAJA AE (2016) Biosorption of hexavalent chromium 128 from aqueous solutions by Macadamia nut shell powder. Appl. Water Sci. 6 1-16. https://doi.org/10.1007/s13201-016-0412-5 [ Links ]
PHELE MJ, EJIDIKE IP and MTUNZI FM (2019) Adsorption efficiency of activated macadamia nut shell for the removal Organochlorine pesticides: Endrin and 4, 4-DDT from aqueous solution. J. Pharm. Sci. Res. 11 (1) 258-262. [ Links ]
ROTORCARB-ACTIVATED CARBON. Rotorcarbs products. URL: www.rotocarb.co.za (Accessed 3 August 2020). [ Links ]
RODRIGUES LA, DE SOUSA RIBEIRO LA, THIM GP, FERREIRA RR, ALVAREZ-MENDEZ MO and DOS REIS COUTINHO A (2013) Activated carbon derived from macadamia nut shells: an effective adsorbent for phenol removal. J. Por. Mater. 20 (4) 619-627. https://doi.org/10.1007/s10934-012-9635-5 [ Links ]
SADARE OO and DARAMOLA MO (2019) Adsorptive removal of dibenzothiophene from petroleum distillates using pomegranate leaf (Punica granatum) powder as a greener adsorbent. Chem. Eng. Comm. 206 (3) 333-345. https://doi.org/10.1080/00986445.2018.1488691 [ Links ]
SADARE OO, AYENI AO and DARAMOLA MO (2020) Performance evaluation of green adsorbent (neem leaf powder) for desulfurization of petroleum distillate. Chem. Eng. Trans. 80 361-366. https://doi.org/10.3303/CET2080061 [ Links ]
SIVAKUMAR S, MUTHIRULAN P and MEENAKSHI SUNDARAM M (2019) Adsorption kinetic and isotherm studies of Azure A on various activated carbons derived from agricultural wastes. Arab. J. Chem. 12 (7) 1507-1514. https://doi.org/10.1016/j.arabjc.2014.10.028 [ Links ]
SLACK RJ, GRONOW JR and VOULVOULIS N (2005) Household hazardous waste in municipal landfills: contaminants in leachate. Sci. Total Environ. 337 (1-3) 119-137. https://doi.org/10.1016/j.scitotenv.2004.07.002 [ Links ]
SU F, LU C, JOHNSTON KR and HU S (2010) Chapter 5 - Kinetics, thermodynamics, and regeneration of BTEX adsorption in aqueous solutions via NaOCl-oxidized carbon nanotubes. In: Fan M, Huang C-P, Bland AE, Wang Z, Slimane R & Wright I (eds) Environanotechnology. Elsevier. 71-97. https://doi.org/10.1016/B978-0-08-054820-3.00005-8 [ Links ]
STOFELA SKF, DE ANDRADE JR and VIEIRA MGA (2017) Adsorption of benzene, toluene, and xylene (BTX) from binary aqueous solutions using commercial organoclay. Can. J. Chem. Eng. 95 (6) 1034-1044. https://doi.org/10.1002/cjce.22748 [ Links ]
YAKOUT SM and DAIFULLAH AAM (2013) Adsorption/desorption of BTEX on activated carbon prepared from rice husk. Desalin. Water Treat. 52 (22-24) 4485-4491. https://doi.org/10.1080/19443994.2013.821629 [ Links ]
WONGCHAREE S, ARAVINTHAN V, ERDEI L and SANONGRAJ W (2017) Use of macadamia nut shell residues as magnetic nanosorbents. Int. Biodeterior. Biodegrad. 124 276-287. https://doi.org/10.1016/j.ibiod.2017.04.004 [ Links ]
WONGCHAREE S, ARAVINTHAN V, ERDEI L and SANONGRAJ W (2018) Mesoporous activated carbon prepared from macadamia nut shell waste by carbon dioxide activation: Comparative characterisation and study of methylene blue removal from aqueous solution. Asia-Pacific J. Chem. Eng. 13 (2) 2179. https://doi.org/10.1002/apj.2179 [ Links ]
ZHANG W, DING W and YING W (2011) Biological activated carbon treatment for removing BTEX from water. Chin. Environ. Sci. 31 (12) 1965-1971. https://doi.org/10.1061/(ASCE)EE.1943-7870.0000731 [ Links ]
 Correspondence:
Correspondence:
Kapil Moothi
Email: kmoothi@uj.ac.za
Received: 10 December 2021
Accepted: 21 November 2022














