Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Water SA
On-line version ISSN 1816-7950
Print version ISSN 0378-4738
Water SA vol.49 n.1 Pretoria Jan. 2023
http://dx.doi.org/10.17159/wsa/2023.v49.i1.3947
RESEARCH PAPER
Prevalence of free-living acanthamoeba and its associated bacteria in energy-efficient hot water systems in South Africa
SJ MoodleyI, II; P MuchesaIII, IV; C BartieV; TG BarnardIII; R ClarkeI; A MasengeVI; SN VenterII
IEskom Research, Testing & Development, Private Bag 40175, Cleveland, Johannesburg, South Africa
IIDepartment of Biochemistry, Genetics and Microbiology, University of Pretoria, South Africa
IIIWater and Health Research Centre, University of Johannesburg, South Africa
IVBiotechnology and Biochemistry Department, University of Zimbabwe, Zimbabwe
VCB Scientific, Roodepoort, Gauteng, South Africa
VIInternal Statistical Consultation Services, Department of Statistics, University of Pretoria, South Africa
ABSTRACT
As part of the Eskom rebate programme, energy-efficient hot water systems such as solar water heaters (low pressure), heat pumps and energy-efficient showerheads were rolled out to the public as a measure to conserve and save energy. There has been a concern that these systems may not reach the required high temperatures, especially during winter, and, as a result of this, Acanthamoeba and its associated bacteria such as Legionella pneumophila, Pseudomonas aeruginosa and nontuberculous mycobacteria could flourish within these systems causing a potential health risk to consumers. This study examined the relationship between Acanthamoeba and its associated bacteria at different temperature ranges. A total of 156 water (69) and biofilm samples (87) were collected from a solar water heater, heat pump, geyser and showerheads and examined for these organisms using amoebal enrichment and molecular techniques. Amoeba could be cultivated from 45 (65.2%) of the water samples and 56 (64.4%) of the biofilm samples. The study confirmed the presence of Legionella pneumophila, Pseudomonas aeruginosa and nontuberculous mycobacteria in the hot water systems at both of the simulated winter (20°C to 30°C) and higher summer (40°C to 55°C) temperatures as well as the control system. There was a significant positive correlation between the presence of Acanthamoeba and the presence of Pseudomonas. Based on this association it is suggested that Pseudomonas aeruginosa could be investigated as an indicator organism for the presence of Acanthamoeba and opportunistic pathogens.
Keywords: free-living amoeba, hot water systems, Legionella pneumophila, Acanthamoeba, Pseudomonas aeruginosa, nontuberculous mycobacteria
INTRODUCTION
The provision of safe and clean water to communities is an important step for improving the general health of people. Proper treatment steps, and management of water distribution systems which supply buildings, are important steps to provide and maintain microbial drinking water quality. Household water systems must protect consumers from any potential health risks that may be associated with water supply (WHO, 2011). This is of paramount importance in a country like South Africa where water is a scarce resource. These water systems have a distinct microbial ecology, which consists of bacteria, viruses, fungi, algae and protozoa (Douterelo et al., 2019). Among these organisms, the growth of opportunistic pathogens in household water systems is an increasing public health threat which needs to be addressed to prevent outbreaks.
These opportunistic pathogens include free-living amoebae (FLA) and their associated bacteria. Free-living amoeba, such as Acanthamoeba, Naegleria, Balamuthia and Sappinia, have been identified as potential risks for causing opportunistic eye, skin and central nervous system infections (Scheid, 2018). In addition to causing infections, these organisms can also interact and act as reservoirs for other bacterial opportunistic pathogens, such as nontuberculous mycobacteria (NTM), Legionella pneumophila and Pseudomonas aeruginosa, in water systems. These bacteria can coexist and are often able to infect, survive and multiply in FLA as they have the ability to digest FLA (Greub and Raoult, 2004; Shaheen et al., 2019). Bacteria in this category are referred to as amoeba-resistant bacteria. A review by Thomas et al. (2010) reported that, of the 539 bacterial species listed as pathogenic to humans and/or animals (Taylor et al., 2001), 102 species were known as amoeba-resistant bacteria, and 27 species were suspected to resist digestion and survive or grow within FLA. Despite the worldwide occurrence of FLA and their associated bacteria, to date few studies have been done in Africa, particularly for the warm, stagnant conditions in building plumbing which usually create ideal conditions for regrowth for a number of opportunistic pathogens and their associated FLA. These hot water systems are characterized by different conditions, such as increased organic matter, temperature stratifications, flow rates and residence times in the hot water tank and the distribution system (Falkinham et al., 2015). These conditions may also influence the distribution of opportunistic pathogens in the HWS and favour a great diversity of microorganisms.
The South African National Standard drinking water specifications, SANS 241:2015 (SABS 2015), state the numerical limits for physical, chemical and microbial quality parameters for potable water, with the main focus on indicator organisms such as E. coli, total coliforms, coliphages and heterotrophic plate counts, as well as pathogens such as protozoan parasites and enteric viruses. However, FLA and their associated opportunistic pathogens, such as nontuberculous mycobacteria, Legionella pneumophila and Pseudomonas aeruginosa are not included in the drinking water specifications. These organisms can grow and thrive as part of complex microbial communities inhabiting building plumbing supplied by water considered safe for drinking, and therefore do not necessarily respond to traditional approaches for pathogen control geared towards indicator organisms (Berry et al., 2006). These organisms can become the leading source of waterborne disease in countries like South Africa.
The current study focused on domestic energy-efficient hot water systems (HWS) such as solar water heaters, heat pumps and energy-efficient showerheads which were rolled out by Eskom with the objective to save and conserve energy. This Residential Mass Roll-Out rebate programme formed part of Eskom's Integrated Demand Management initiative (Begemann and Lipchin, 2012). As these water heating systems are dependent on the temperature of the environment, water temperatures may fluctuate and create conditions that could enable the growth, proliferation and spread of FLA and its associated opportunistic pathogens. This study investigated the presence of Acanthamoeba and its associated bacteria within a laboratory setting. The aim of this study was to test the impact of temperature (20°C to 30°C and 40°C to 55°C) on the presence Acanthamoeba and its associated bacteria, to establish whether there will be greater exposure risk to selected opportunistic pathogens associated with these energy-efficient systems.
METHODS AND MATERIALS
Sampling protocol
The sampling focused on the presence of Acanthamoeba and its associated bacteria within 4 separate laboratory-based systems: a low-pressure solar water heater, a heat pump, a geyser (control) and energy-efficient showerheads. The pilot hot water systems were not connected to each other and operated independently from each other. All systems obtained their water supply from the same municipal connection. Figure 1 shows the hot water systems as well as the layout and sampling points for each individual system.
The solar water heater (SWH) is a low-pressure system which does not have a back-up element and mimics systems used in low-income communities. Three probes were installed inside the unit to measure the temperature of the water. The first temperature probe was installed at the bottom of the tank, the second temperature probe was installed at the top of the tank and the third temperature probe was installed where the water exits the system. The temperature of the unit was carefully controlled to obtain the desired temperatures. The water samples were taken from the tap which was located close to the SWH (Fig. 1A). When the SWH temperature increased above the set temperature, cold water was pumped into the system and hot water was pumped out into the dump tank, thus enabling the SWH temperatures to remain at the set temperatures. The temperatures were set at 20°C to 30°C and 40°C to 55°C during the experimental period.
This was done to test the impact of specific temperature ranges representing summer and winter conditions on the presence of Acanthamoeba and its associated bacteria.
The temperature of the heat pump (HP) was controlled by the control box. Two probes were installed to measure the temperature of the water inside the HP as wells as the water exiting the HP. The temperature range was set for a period of 3 months between 40°C and 55°C and for the next 3 months to operate between 20°C and 30°C. A tap was used to sample the water from the HP (Fig. 1B).
As a control, a system simulating the operation of a normal electrical geyser was also included. A 3 kW heating element was inserted in a 100 L plastic tank so that the water could reach temperatures of 55°C during the experiment. Geysers are typically operated at 55°C to 60°C. Biofilm coupons in a casing were placed inside the tank under the water level. The water samples were taken from the tap and the biofilm coupons were swabbed (Fig. 1C).
The showerhead test unit had two perspex boxes; an energy-efficient showerhead was housed in Box B and a conventional showerhead was housed in Box A (control). The plastic container was filled with approximately 20 L of water and the pressure was regulated to ensure that both the efficient and conventional showerhead were run at the same pressures while the water was run constantly through the system. The water samples were taken from each showerhead and, in addition to this, the showerheads were swabbed (Fig. 1D).
Sample collection
Water and biofilm samples were collected for microbiological analysis every 2 weeks for a period of 6 months from March 2017 to August 2017. The samples were stored in a portable ice chest and processed on the same day. Water samples were collected after running each system for approximately 1 to 2 min before collecting the sample in a 1 L sampling bottle. All bottles contained 5 mg/L of sodium thiosulphate to neutralize the chlorine or any oxidizing biocides which may be present at the time of sampling. Taps connected to these systems were also swabbed on the inside to collect biofilms that may have formed. The swaps were placed into 20 mL of Page's modified Neff's amoebae saline (PAS) solution. At the same time water samples were also collected for the municipal connection feeding into all of the experimental systems.
Isolation of FLA
Five hundred milliliters (500 mL) of water sample were concentrated by filtration using a nitrocellulose membrane (Millipore, SA) with a pore size of 0.45 μm. Swabs were vortexed at maximum speed for 30 s in 10 mL Page's amoebal saline buffer (PAS) in individual sterile tubes and the suspension was concentrated by membrane filtration similarly to the water samples. Each filter membrane was placed upside-down onto a non-nutrient agar (NNA) plate overlaid with a suspension of heat-killed Escherichia coli ATCC 25922 (100 μL for each plate) (Muchesa et al., 2014). The plates were incubated aerobically at 32°C and examined daily for 3 weeks under a light microscope (Olympus, Japan) with 10 x objective for the appearance of amoebal trophozoites and/or cysts. Plates were recorded as negative if no amoebae were observed after 3 weeks. Plates containing amoebae were sub-cultured by cutting small agar plugs, which were placed upside-down onto fresh NNA-E. coli plates and incubated as before. Sub-culturing was done 3 times to purify amoebae cells. Once purified, amoebae cells were harvested by gently scraping the agar surface andre-suspending the cells in 1 mL PAS. To further remove extracellular bacteria and debris, the suspension was centrifuged 3 times at 1 000 g for 20 min. The washed pellet was re-suspended in 1 mL PAS. The suspension was inoculated into 24-well microtitre plate wells (Nunc, USA) and incubated at 32°C. The plates were checked daily under an inverted microscope (Leica, Germany), with a 40 x objective, for the morphological appearance of free-living amoeba. Isolated Acanthamoeba and potentially associated Legionella pneumophila, Pseudomonas aeruginosa and nontuberculous mycobacteria were identified by PCR assays using specific primers for Acanthamoeba and the different bacteria.
DNA extraction
DNA was extracted from amoeba-positive samples. Briefly, the amoebae suspension was centrifuged for 2 min at 13 000 r/min to concentrate cells. The supernatant was discarded and the pellet kept for DNA extraction. To the pellet, 700 μL of lysis buffer was added and incubated at 70°C for 10 min. A volume of 250 μL 100% ethanol was added to the mixture and further incubated at 56°C for 10 min. To this mixture 50 μL of Celite was added and incubated at room temperature for 10 min. The mixture was then loaded onto a spin column containing a DNA binding membrane and centrifuged. The column was washed in 2 steps with 700 μL wash buffer and centrifuged. Final washing was done with 70% ethanol in 2 steps of 700 μL each. DNA was eluted using 100 μL of a TRIS EDTA buffer (10mM Tris-Cl and 0.5 M EDTA; pH 9) after incubation at room temperature for 2 min and stored at -20°C. The extracted DNA was used as a template in all PCR reactions.
PCR analysis
Acanthamoeba was detected by amplification of the extracted DNA using the primers targeting the 18S rDNA of free-living amoeba and specific regions of the 16S rDNA for Legionella, mycobacteria or Pseudomonas aeruginosa (Table 1). The samples were amplified in a 20 μL reaction mixture containing 10 μL of the 2 x Qiagen m-PCR master mix (Hotstart Taq DNA polymerase, 10 x buffer, 2 mM MgCl2 and dNTP mix), 1 μL 5 x Q-solution, 1 μL of PCR grade water, 2 μL 25 mM MgCl2, 4 μL of template DNA and 0.5 μL of primers. The PCRs were carried out with the listed forward and reverse primers. The cycling conditions were: an initial enzyme activation of 95°C for 15 min, followed by 30 cycles of denaturation at 94°C for 1 min, annealing at 62°C for 90 s and elongation at 72°C for 1 min with final extension of 72°C for 10 min. The primers yielded 750-1 000 bp fragments. All amplicons were separated on a 2% (w/v) agarose gel.
Statistical analysis
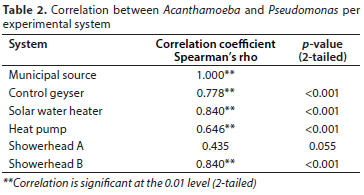
The presence of Acanthamoeba was used to compare the different experimental and control systems. The Bonferroni method was performed to adjust the p-values for multiple comparisons of proportions. The Spearman, non-parametric correlation test was conducted to test for associations between the Acanthamoeba detection data and the presence of Legionella pneumophila, Pseudomonas aeruginosa or nontuberculous mycobacteria. The Spearman correlations (r) range between -1 and 1, with 1 being a perfect positive correlation or relationship, i.e., the occurrence of Acanthamoeba is associated with the occurrence of the bacterial group it was compared with. Correlations were considered to highly significant at the 0.01 level (2-tailed).
RESULTS
This study investigated the presence of Acanthamoeba and its associated bacteria in prototype energy-efficient HWS systems and associated appliances within a laboratory setting. These results were compared with the municipal water supply that served as source water as well as a protype of a normal geyser operated at 55°C. A total of 156 water and bio film samples were collected during the experimental process from March until August, of which 69 were water samples and 87 were biofilm samples. Of the 69 water samples, growth of amoeba was observed for 45 samples (65.26%), and of the 87 biofilm samples, 56 showed positive growth (64.4%). Nontuberculous mycobacteria had a rather low (11.4%) association with the Acanthamoeba cultures, compared to Pseudomonas aeruginosa at 93,2% and even Legionella (33.0%). Based on the PCR analysis, 88 of the 101 free-living amoeba cultures (87.1%) could be identified as Acanthamoeba. When the different systems were compared with each other based on the presence of Acanthamoeba, no significant differences at the 0.05 level were found.
Municipal source and geyser
Based on the PCR analysis, the municipal water supply (Fig. 2A) showed Acanthamoeba, Legionella pneumophilia, Pseudomonas aeruginosa and nontuberculous mycobacteria to be present within the distribution system during the period of 6 months within which samples were collected. Acanthamoeba was present in 31.6%, Legionella pneumophilia in 10.5%, nontuberculous mycobacteria in 5.3% and Pseudomonas aeruginosa in 31.6% of municipal water and biofilm samples (31.6%). Although the geyser (Fig. 2B) was set at 55°C, Acanthamoeba and Pseudomonas could regularly be detected in both the water and biofilms in 68.8% and 62.5% of all the samples, respectively. The geyser system also had the highest percentage of samples positive for the presence of Acanthamoeba for all the systems tested.
Legionella pneumophila was only detected in 10 (20.8%) of the samples in spite of the fact that it was run at an elevated temperature. Mycobacterium was absent from the geyser, apart from one of the coupons (2.1%).
Solar water heater and heat pump
Acanthamoeba was present in around half of all the SWH and HP samples during both of the experiments, with 43.5% of the samples collected from these two systems positive for Pseudomonas (Fig. 3). Legionella pneumophilia was observed in the SWH at 20°C to 30°C (41.7%) but at a lower percentage of 27.3% at 40°C to 55°C. For the HP the values were even lower, ranging between 16.7% (20°C to 30°C) and 9% (40°C to 55°C). Nontuberculous mycobacteria were only present in 3 (13%) of the SWH samples and 1 (4.3%) of the HP samples (Fig. 3).
Showerhead results
Figure 4 shows the Showerhead A (conventional) and Showerhead B (energy-efficient) data. Acanthamoeba was present in both the water and biofilm samples and the percentage of positive samples was 65% and 56.5% for the two showerheads, respectively. Although Showerhead B showed a slightly lower presence of Acanthamoeba, higher levels of Legionella pneumophilia (21.7%) were detected in this system compared to the absence of Legionella in Showerhead A samples. No major differences were observed for the nontuberculous mycobacteria with only one sample was positive for each of the systems.
Bacterial associations with Acanthamoeba
In this study there was a strong positive correlation between the presence of Acanthamoeba and Pseudomonas (Table 2). For all systems apart from Showerhead A there was a high correlation based on Spearman's rho at the level of 0.01 (2-tailed). The only other highly significant correlation was between Acanthamoeba and Legionella. A Spearman's rho of r = 0.699 (p < 0.001) was observed for the solar water heater. None of the other associations between Acanthamoeba and Legionella or the mycobacteria were significant at the 0.01 level.
DISCUSSION
From our investigations, it was observed that Acanthamoeba and Pseudomonas aeruginosa were regularly detected in all heating systems tested as well as the municipal supply. Legionella and nontuberculous mycobacteria, known to be associated with Acanthamoeba, were also detected, but at lower levels compared to Pseudomonas. These observations were in line with the findings of other investigations which showed that these opportunistic pathogens were present in electric water heaters (Stone et al., 2019), premise plumbing (Falkinham et al., 2015) and different hospital water systems (Muchesa et al., 2016), and if not well managed could pose a risk to consumers.
One of the concerns raised during the rollout of the energy-efficient hot water systems was that these systems could pose a health risk when operated at sub-optimal temperatures, especially during winter. Acanthamoeba is known to grow optimally at 30°C (Lakhundi et al., 2014). Muchesa et al. (2016) stated that the high prevalence of Acanthamoeba in their study could be a result of the relatively lower temperatures of cold-water samples compared with the relatively high temperatures of HWS analysed in other studies. In this study Acanthamoeba was present in high numbers at both the high and lower temperatures. The results showed that this organism has a high temperature tolerance, as it was regularly detected in the control geyser operated at 55°C as well as the experiments conducted at around 40°C to 55°C. The results therefore did not indicate an increased risk associated with the operation of the energy-efficient systems at the lower temperature range of20-30°C.
Legionella pneumophilia was intermittently detected in Acanthamoeba cultures obtained from both water and biofilm samples of all the systems, apart from Showerhead A. Valcina et al. (2019) reported that for the samples collected during their study, Legionella was always isolated along with FLA. Apart from the solar water heater there was no significant correlation between the presence of Acanthamoeba and Legionella. The association of Legionella pneumophilia with Acanthamoeba in the water systems may still indicate an increased health risk as Acanthamoeba are able to provide long-term persistence and transmission of Legionella pneumophilia.
The low levels of nontuberculous mycobacteria (11.4%) associated with Acanthamoeba isolated from water and biofilm samples were in line with other studies. Thomas et al. (2006) detected them in 20.5% of the amoebal cultures obtained from water and biofilm samples in the water network of a hospital. A South African based study reported that nontuberculous mycobacteria could be cultured from 17.9% of the drinking water samples tested (September et al., 2004). In contrast to these studies, Delafont et al. (2014) reported that nontuberculous mycobacteria could be recovered from nearly 88% of the amoebal cultures obtained from drinking water networks. The reasons for these differences would need to be investigated further.
The interactions between FLA and Pseudomonas aeruginosa were previously studied and showed a frequent recovery of this bacterial genus. In this study Pseudomonas aeruginosa was present in most of the Acanthamoeba cultures isolated from the systems as well as the municipal source. This was supported by Delafont et al. (2016) and reported that Pseudomonas and Burkholderi a significantly co-occurred with Acanthamoeba in water samples. It is possible that intracellular multiplication of Pseudomonas aeruginosa within Acanthamoeba polyphaga could take place, as was reported in synthetic drinking water (Bedard et al., 2016).
This study showed that there was a clear correlation between Acanthamoeba and Pseudomonas aeruginosa, irrespective of the environmental temperature they were exposed to, as similar levels of Acanthamoeba and Pseudomonas aeruginosa were found at both the high and low temperatures. The presence of Pseudomonas aeruginosa and Acanthamoeba in both the water and biofilm samples at similar levels was also noted. Highly significant correlations based on Spearman's rho supported this association which was independent of temperature or sample type. It can therefore be proposed that Pseudomonas aeruginosa could be investigated as an indicator organism in order to establish the presence of FLA in water systems.
CONCLUSIONS
Several studies have indicated the presence of Acanthamoeba and its associated bacteria in hot water systems. This study confirmed the presence of Acanthamoeba and its associated bacteria in both normal and energy-efficient hot water systems. Acanthamoeba, Pseudomonas aeruginosa and Legionella pneumophilia were found to be temperature tolerant with the potential to survive at different temperatures. As these organisms are associated with both conventional electric geysers and efficient hot water systems, their potential health risk should be addressed. It is recommended that detailed studies should be conducted to understand the behaviour of these organisms within hot water distribution systems and how physical and biological parameters impact their dynamics. One of the important factors that still needs attention is the effect of temperature gradients on the survival of these heat-tolerant organisms. This will help us to balance our need for saving energy and at the same time reducing the risk to users.
This study also confirmed that Acanthamoeba, Legionella pneumophilia, nontuberculous mycobacteria and Pseudomonas aeruginosa were present in the municipal water. As such this water could act as a source of these organisms in domestic water supply and heating systems. Currently, there are guidelines and risk assessments for the presence of Legionella pneumophilia in water systems. However, there is a lack of risk management and intervention plans for hot water systems in South Africa. We propose that Pseudomonas aeruginosa, Acanthamoeba and associated bacterial pathogens should also be considered as they could pose a potential risk in hot water systems as was shown by the experimental systems as well as the conventional geyser. It is therefore highly recommended that a risk management plan be developed for water heating systems in South Africa in order to control the risk to water users.
CONFLICT OF INTEREST
There was no conflict of interest for any party involved with this study.
ACKNOWLEDGEMENTS
The author would like to acknowledge the Eskom Research, Testing and Development Department for the research funding for this study and the University of Johannesburg for providing technical assistance.
AUTHOR CONTRIBUTIONS
SJM - conceptualisation of study, data collection and analysis, writing of manuscript; PM - data collection and analysis, writing of manuscript; CB - conceptualisation of study, data collection and analysis, writing of manuscript; TGB - conceptualisation of study, data collection and analysis, writing of manuscript; RC -conceptualisation of study, writing of manuscript; AM - statistical analysis, writing of manuscript; SNV - data analysis, writing of manuscript
ORCIDS
SJ Moodley: https://orcid.org/0000-0003-1091-0051
P Muchesa: https://orcid.org/0000-0003-1670-4777
C Bartie: https://orcid.org/0000-0002-7505-8122
TG Barnard: https://orcid.org/0000-0002-6831-1361
A Masenge: https://orcid.org/0000-0001-8372-2356
SN Venter: https://orcid.org/0000-0002-5726-4918
REFERENCES
BÉDARD E, PRÉVOST M and DÉZIE E (2016) Pseudomonas aeruginosa in premise plumbing of large buildings. Microbiol. Open 5 (6) 937-956. https://doi.org/10.1002/mbo3.391 [ Links ]
BEGEMANN M and LIPCHIN B (2012) The Eskom IDM suite of solutions. Mining and Industrial Energy Optimisation (MIEO), Cape Town. 28-30. URL: https://www.ee.co.za/wpcontent/uploads/legacy/Vector%202012/eskom_the%20eskom%20idm%20suite%20of%20solutions.pdf (Accessed 17 July 2022). [ Links ]
BERRY D, XI CW and RASKIN L (2006) Microbial ecology of drinking water distribution systems. Curr. Opin. Biotechnol. 17 (3) 297-302. https://doi.org/10.1016/jxopbio.2006.05.007 [ Links ]
COSKUN KA, SEMRA OZÇELIK S, TUTAR L, ELALD N and TUTAR Y (2013) Isolation and identification of free-living amoebae from tap water in Sivas, Turkey. Biomed Res. Int. 2013 675145. https://doi.org/10.1155/2013/675145 [ Links ]
DELAFONT V, BOUCHON D, HECHARD Y and YANN M (2016) Environmental factors shaping cultured free-living amoebae and their associated bacterial community within drinking water network. Water Res. 100 382-392. https://doi.org/10.1016/j.watres.2016.05.044 [ Links ]
DELAFONT V, MOUGARI F, CAMBAU E, JOYEUX M, BOUCHON D, HECHARD Y and MOULIN L (2014) First evidence of amoeba-mycobacteria association in drinking water network. Environ. Sci. Technol. 48 11872-11882. https://doi.org/10.1021/es5036255 [ Links ]
DOUTERELO I, SHARPE RL, HUSBAND S, FISH KE and BOXALL JB (2019) Understanding microbial ecology to improve management of drinking water distribution systems. Wiley Interdisc. Rev. Water 6 e01325. https://doi.org/10.1002/wat2.1325 [ Links ]
FALKINHAM JO, ILBORN ED, ARDUINO MJ, PRUDEN A and EDWARDS MA (2015) Epidemiology and ecology of opportunistic premise plumbing pathogens: Legionella pneumophila, Mycobacterium avium, and Pseudomonas aeruginosa. Environ. Health Perspect. 123 749-758. https://doi.org/10.1289/ehp.1408692 [ Links ]
FALKINHAM JO, PRUDEN A and EDWARDS MA (2015) Opportunistic premise plumbing pathogens: Increasingly important pathogens in drinking water. Pathogens 4 373-386. https://doi.org/10.3390/pathogens4020373 [ Links ]
GREUB G and RAOULT D (2004) Microorganisms resistant to free-living amoebae. Clin. Microbiol. Rev. 17 413-433. https://doi.org/10.1128/CMR.17.2.413-433.2004 [ Links ]
LAKUNDI S, KHAN NA and SIDDIQUI R (2014) The effect of environmental and physiological conditions on excystation of Acanthamoeba castellanii belonging to the T4 genotype. Parasitol. Res. 113 2809-2816. https://doi.org/10.1007/s00436-014-3941-6 [ Links ]
MUCHESA P, LEIFELS M, JURZIK L, BARNARD TG and BARTIE C (2016) Free-living amoebae isolated from a hospital water system in South Africa: a potential source of nosocomial and occupational infection. Water Suppl. 16 70-78. https://doi.org/10.2166/ws.2015.106 [ Links ]
MUCHESA P, MWAMBA O, BARNARD TG and BARTIE C (2014) Detection of free-living amoebae using amoebal enrichment in a wastewater treatment plant of Gauteng province, South Africa. BioMed Res. Int. 2014 575297. https://doi.org/10.1155/2014/575297 [ Links ]
RAFIEE M, JAHANGIRI-RAD M, HAJJARAN H, MESDAGHINIA A and HAJAGHAZADEH M (2014) Detection and identification of Legionella species in hospital water supplies through polymerase chain reaction (16S rRNA). J. Environ. Health Sci. Eng. 12 83. https://doi.org/10.1186/2052-336X-12-83 [ Links ]
SABS (2015) South African National Standard 241-1: Drinking water, Part 1: Microbiological, physical aesthetic and chemical determinants. South African Bureau of Standards, Pretoria. [ Links ]
SCHEID P (2018) Free-living amoebae as human parasites and hosts for pathogenic microorganisms. Multidisc. Dig. Publ. Inst. Proc. 2 692. https://doi.org/10.3390/proceedings2110692 [ Links ]
SEPTEMBER SM, BROZEL VS and VENTER SN (2004) Diversity of nontuberculoid Mycobacterium species in biofilms of urban and semiurban drinking water distribution systems. Applied Environ. Microbiol. 70 7571-7573. https://doi.org/10.1128/AEM.70.12.7571-7573.2004 [ Links ]
SHAHEEN M, SCOTT C and ASHBOLT NJ (2019) Long-term persistence of infectious Legionella with free-living amoebae in drinking water biofilms. Int. J. Hyg. Environ. Health 222 678-686. https://doi.org/10.1016/j.ijheh.2019.04.007 [ Links ]
SPILKER T, COENYE, T, VANDAMME P and LIPUMA JJ (2004) PCR-based assay for differentiation of Pseudomonas aeruginosa from other Pseudomonas species recovered from cystic fibrosis patients. J. Clin. Microbiol. 42 2074-2079. https://doi.org/10.1128/JCM.42.5.2074-2079.2004 [ Links ]
STONE W, LOUW TM, GAKINGO GK, NIEUWOUDT MJ and BOOYSEN MJ (2019) A potential source of undiagnosed Legionellosis: Legionella growth in domestic water heating systems in South Africa. Energ. Sustain. Dev. 48 130-138. https://doi.org/10.31224/osf.io/23fzc [ Links ]
TAYLOR LH, LATHAM SM and WOOLHOUSE ME (2001) Risk factors for human disease emergence. Philos. Trans. R. Soc. B 356 983-989. https://doi.org/10.1098/rstb.2001.0888 [ Links ]
THOMAS V, HERRERA-RIMANN K, BLANCS DS and GREUB G (2006) Biodiversity of amoebae and amoeba-resisting bacteria in a hospital water network. Appl. Environ. Microbiol. 72 2428-2438. https://doi.org/10.1128/AEM.72.4.2428-2438.2006 [ Links ]
THOMAS V, MCDONELL G, DENYER S and MAILLARD JY (2010) Free-living amoebae and their intra-cellular pathogenic microorganisms: risks for water quality. FEMS Microbiol. Rev. 34 231-259. https://doi.org/10.1111/j.1574-6976.2009.00190.x [ Links ]
VALCINA O, PULE D, MALISEVS A, TROFIMOVA J, MAKAROVA S, KONVISERS G, BERZIN A and KRUMINA A (2019) Cooccurrence off free-living amoeba and Legionella in drinking water supply systems. Medicina 55 492. https://doi.org/10.3390/medicina55080492 [ Links ]
WORLD HEALTH ORGANIZATION (2011) Guidelines for drinking-water quality, 4th edition. WHO Press, Geneva. 631 pp. URL: www.who.int/publications/i/item/9789241549950 (Accessed 14 September 2021). [ Links ]
 Correspondence:
Correspondence:
SJ Moodley
Email: makardsj@eskom.co.za
Received: 8 October 2021
Accepted: 14 November 2022














