Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Water SA
On-line version ISSN 1816-7950
Print version ISSN 0378-4738
Water SA vol.48 n.4 Pretoria Oct. 2022
http://dx.doi.org/10.17159/wsa/2022.v48.i4.3971
RESEARCH PAPER
Application of isotherm models to combined filter systems for the prediction of iron and lead removal from automobile workshop stormwater runoff
Clement Oguche Ataguba; Isobel Brink
Department of Civil Engineering, Stellenbosch University, Private Bag X1, Matieland 7602, Stellenbosch, South Africa
ABSTRACT
Langmuir and Freundlich isotherm adsorption models were used to predict iron and lead removal from automobile workshop stormwater runoff. Combined low-cost filter systems consisting of granular activated carbon-rice husk (GAC-RH) and river gravel-granular activated carbon (GR-GAC) were used in this study. The effects of adsorbent dosage and contact time on the adsorption capacity of the adsorbents, as well as the removal efficiencies of the adsorbent systems, were also investigated. The results for the Langmuir model generally showed favourable adsorption processes., with all RL values < 1 (in the range 0.358-0.518). The Langmuir model gave better predictions for iron and lead removal, with high R2 values (in the range 0.8420.969), while the root mean square error (RMSE) values ranged from 0.002 to 2.366. The Freundlich model parameters indicated chemisorption processes with all n values < 1 (in the range 0.1296-0.4675). R2 values were in the range of 0.634-0.916 while RMSE values ranged from 0.002 to 0.1765. Additionally, the removal efficiencies for iron and lead using GAC-RH filter system (54% and 48%, respectively) were found to be higher than those obtained using GR-GAC filter system (35% and 25%, respectively). The adsorption capacities of the adsorbents decreased with increased dosages of the adsorbent, with optimum adsorbent dosage of 0.5 g and equilibrium contact time of 80 min for the combined filter adsorbents. Further research towards modifying adsorbents for removal of oil and grease from polluted automobile workshop stormwater runoff are warranted.
Keywords: granular activated carbon, rice husk, river gravel, iron and lead pollutants, adsorption model
INTRODUCTION
Stormwater runoff from different land uses in urban areas is a major source of pollution to receiving streams and rivers, which affects the health of receiving water bodies (Laurenson et al., 2013). Therefore, a major goal for stormwater management, with respect to the water quality standards of a receiving water body, is to minimize pollutant loads discharged. This is necessary in order to maintain the natural hydrological patterns of a catchment area as far as possible (NAP, 2009; Argue and Pezzaniti, 2005). Generally, the presence of heavy metals in stormwater/wastewater effluents can be very toxic and harmful to aquatic and terrestrial life when discharged into the environment without any treatment (Kavand et al., 2011). Accumulation of pollutants in a water environment can result from discharging untreated stormwater runoff into an environment that has limited natural purification capacity, with the result that the ecosystem cannot reasonably absorb the pollutants (UNEP, 2004).
Stormwater management has evolved beyond the conventional practice of immediate collection, conveyance and disposal of runoff using grey infrastructures, towards the use of low-cost and low-impact development (LID) technologies. These are less expensive technologies that collect, detain and gradually release the runoff for groundwater recharge or final disposal into the receiving water body. According to Guyer (2017), LID is a land planning or engineering strategy aimed at maintaining or restoring the natural hydrology of any catchment for the protection of the natural resources of the site towards achieving the goal of environmental regulatory requirements.
The removal of heavy metals from stormwater or wastewater via adsorption have been achieved in past studies using un-combined filter systems of granular activated carbon (GAC) as well as other low-cost agricultural wastes (Khan et al., 2004; Desta 2013). These technologies, however, required further effluent treatment to meet discharge standards (Reddy et al., 2014; Larm and Wahlsten, 2018; Wang et al., 2017). Ataguba and Brink (2021) therefore investigated and developed combined filter systems that use low-cost locally available materials specifically for application to water quality improvement of polluted stormwater runoff from automobile workshops in Nigeria. This became necessary as the application of conventional iron and lead removal technologies such as ultra-filtration, electrodialysis, ion exchange, chemical precipitation, reverse osmosis, etc., in developing countries have been reported to be uneconomical and technically cumbersome, due to the high cost of operation as well as nonavailability of required labour (Brown et al., 2000; Fu and Wang, 2011; Bahgat et al., 1999).
Adsorption, in the treatment of polluted water, has been referred to as a surface process where ions or molecules of the pollutants are removed from the water and attached to the surface of a solid or adsorbent (Piccin et al., 2017; Agunwamba, 2001). Adsorption using activated carbon has been in use for treatment of drinking water for over 100 years and has proved to be efficient in the removal of adsorbates in water (Worch, 2021). According to Worch (2021), there are two types of adsorption, namely, physisorption (physical adsorption) and chemisorption (chemical adsorption). While physisorption results from Van der Waal's forces, chemisorption is caused by a chemical reaction between the adsorbate and the surrounding surface of the adsorbent.
Stormwater treatment models can be designed to predict the performance of proposed stormwater treatment processes and technologies subjected to varying conditions with the overall aim of protecting receiving water bodies from pollution (Wong et al., 2006). One aim of characterizing and modelling the pollutants in stormwater runoff is to develop suitable options that can lower the concentrations of pollutants or minimize runoff volume, culminating in the overall reduction in the pollutant load that is received by the streams or rivers (Charters, 2016).
Several adsorption isotherm models have been developed and used to describe the behaviour of adsorbate on adsorbent materials in the past. Some of these models are presented in Table 1. The terms in the equations have been defined in the different sources cited from.
Adsorption isotherm models are functions that relate the change in the quantity of adsorbate adsorbed on the surface of the adsorbents, and the quantity of adsorbate left in liquid phase under equilibrium, with respect to variation in pressure at constant temperature (Piccin et al., 2017). Linear forms of the Langmuir and Freundlich isotherm models have been successfully used to describe the adsorption of ions from single and mixed metal solutions onto soils or other natural adsorbents under constant temperature (Echeverría et al., 1998, Christophi and Axe, 2000; Gulbaz et al., 2015; Song and Liu, 2013; Thuy Chung et al., 2015; Wu et al., 2014). It has been reported in literature that Langmuir and Freundlich isotherm models are optimum adsorption models when compared to others such as Temkin, Sips, Hill-Deboer, etc. (Wang and Guo, 2020; Zhuang et al., 2020; Manaa et al., 2020). The Langmuir and Freundlich models are frequently used due to the simplicity of their application in linear regression modelling (Wang and Guo, 2020). These models were therefore selected for further investigation in this research.
The use of only the coefficient of determination, R2, to describe the suitability/fitness of isotherm models in describing an adsorption process has been reported to be insufficient, since this parameter considers the difference between the theoretical and experimental data in linear plots (Hami et al., 2021). Appropriate isotherm models to describe adsorption can be determined by using error functions to validate the linearized isotherm equations with experimental results (Hami et al., 2021; Balarak and Salari, 2019). Optimal adsorption isotherm models are generally characterized by high R2 values and low values of error functions. Common error functions that have been used by researchers include: residual sum of squares (RSS), sum of absolute errors (SAE), hybrid function fractional error (Hybrid), average relative error (ARE), non-linear chi-square test (^2), root mean square error (RMSE) among others (Hami et al., 2021, Amtul et al., 2017).
This paper reports on the modelling of adsorption of iron and lead from automobile workshop stormwater using Langmuir and Freundlich isotherm models. The aims of the study were:
• To predict the removal of iron and lead from automobile stormwater runoff onto combined filters of GAC-RH and GR-GAC as well as to determine the fitof the predicted data to the isotherm models, using the model parameters and error functions
• To determine the effect of adsorbent (GAC-RH and GR-GAC) dosage on the adsorption capacities of the adsorbents for the removal of iron and lead from automobile stormwater runoff
• To determine the adsorbent removal efficiencies for removal of iron and lead from automobile stormwater runoff
• To determine the effect of contact time on the efficiencies of iron and lead removal using GAC-RH and GR-GAC combined filters
The use of these low-cost combined adsorbents as combined filters and the use of these isotherm models to predict iron and lead adsorption from automobile workshop stormwater, along with the metals' effect on quality of urban runoff, have hitherto been neglected, and are the major novel contributions of this study.
MATERIALS AND METHODS
Figure 1 shows the adsorption mechanism. The selected adsorbents on which the adsorption took place were granular activated carbon (GAC), rice husk (RH) and river gravel (RG). Iron and lead were the adsorbates. The reverse of this process, called desorption, was not however carried out in this research. The combined GAC-RH and GR-GAC filter systems were modelled as a series of compartments by using theoretical removal mechanisms for each compartment, as shown in Fig. 1. The compartments were modelled as sites/aspects of the adsorptive filtration processes.
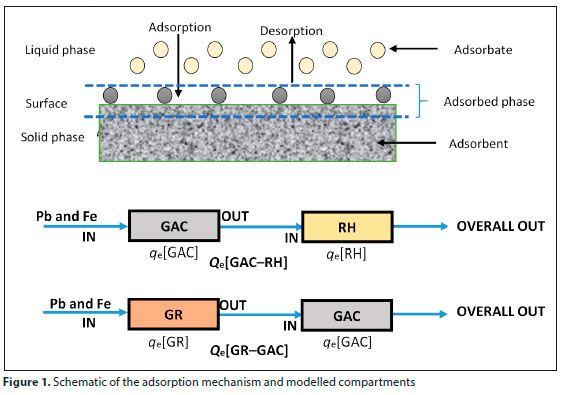
The concentrations retained in the adsorbent phase (Qe) were determined using:
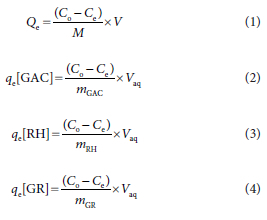
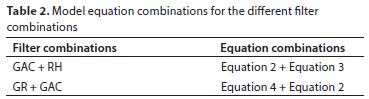
where qe[GAC] is the adsorption capacity of GAC adsorbent (mg/g), qe[RH] is the adsorption capacity of RH adsorbent (mg/g), qe[GR] is the adsorption capacity of GR adsorbent (mg/g), Vaq is the volume of the solution containing metal used (L), Co is the influent concentration (mg/L), Ce is the effluent concentration (mg/L), mGAC is the dosage of GAC (g), mGR is the dosage of GR (g) and mRH is the dosage of RH (g). The model equations for the different filter combinations are shown in Table 2.
Stormwater runoff samples, which served as the adsorbate solutions, were obtained from two selected automobile workshops in Nigeria. The characterization of the untreated and treated stormwater runoff samples from the selected automobile workshops has been presented in Ataguba and Brink (2021), along with preparation of the adsorbents used in this research. Laboratory tests were carried out in accordance with APHA (2017) and average values obtained were used for analyses. The laboratory room temperature was maintained at 25°C and the initial average stormwater pH was 9.56. Also the initial concentrations of the adsorbate, iron and lead, were noted as 35.5 mg/L and 1.65 mg/L, respectively. No thermodynamic study was carried out. Adsorbent dosage and contact time effects on the adsorptive removal of iron and lead on prepared GAC-RH and GR-GAC filter beds were tested as batch sorption experiments in a series of capped 250 mL Erlenmeyer flasks at room temperature of 25±1°C with adsorbent dosage range of 0.5 g to 8.0 g. The detailed experimental procedure for adsorption equilibrium determination has been described in Worch (2021). The adsorption capacities of each adsorbent combination at equilibrium were mathematically analysed using Eqs 1-4 and Table 2. This was performed for the different adsorbent dosages. Briefly, the adsorbents were prepared as crushed granular activated carbon, rice husk and gravel samples to allow accommodation in flasks. Suspensions were magnetically agitated at constant speed (200 r/min) until equilibrium was reached. The process was repeated for the different adsorbent dosages as mentioned above and contact time in the range of 5-80 min. After equilibrium was attained, the resulting solutions were centrifuged at 4 000 r/min and the supernatant analysed using ICE 3000 Series AA Spectrometer (flame atomic absorption spectrometry technique). All tests were performed in the Water Quality Laboratory at the National Geosciences Research Laboratories in Kaduna, Nigeria.
The Langmuir isotherms were obtained from the plot of 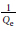 vs
vs 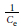 with a linearized equation:
with a linearized equation:
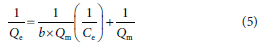
Similarly, the Freundlich isotherms were obtained from the plot of ln(Qe) vs ln(Ce) with a linearized equation:
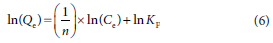
where Qm = maximum adsorption capacity (mg/g), b = constant related to affinity of binding sites (L/mg), KF = Freundlich isotherm constant related to the adsorption capacity (mg/g), n = Freundlich isotherm constant related to the adsorption intensity (L/g)
The concentrations Co and Ce at the optimum adsorbent dosage were used to compute the removal efficiencies for the adsorbents given by Eq. 7.
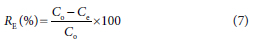
The metal removal efficiencies, RE, were used to evaluate the performance of the adsorption processes using the two different combined adsorbents (filters). The study also sought to establish the relationship between the dosages of the different adsorbents and their modelled adsorption capacities, as shown in Figs 10 and 11. Also, for each contact time, the metal removal efficiencies were computed using Eq. 7 and graphs plotted as shown in Figs 12 and 13.
The favourableness/fitness of the predicted data using Langmuir and Freundlich isotherm models were also determined from the equilibrium parameters RL and n, respectively. For Langmuir, the Langmuir isotherm constant related to the adsorption capacity in mg/g RL =  while Freundlich constants n and KF were obtained from the slope and intercept of ln Qe vs ln Ce plots. The nature of the adsorption process based on the Langmuir isotherm model is defined by any of the following relationships (Hamzaoui et al., 2018): RL > 1 (unfavourable), RL = 1 (linear), 0 < RL < 1 (favourable) or RL = 0 (irreversible). Similarly, the nature of the adsorption process based on the Freundlich isotherm model (Hamzaoui et al., 2018) is defined as n > 1 (adsorption is a physical process), n < 1 (adsorption is a chemical process or chemisorption) or n = 1 (adsorption is linear).
while Freundlich constants n and KF were obtained from the slope and intercept of ln Qe vs ln Ce plots. The nature of the adsorption process based on the Langmuir isotherm model is defined by any of the following relationships (Hamzaoui et al., 2018): RL > 1 (unfavourable), RL = 1 (linear), 0 < RL < 1 (favourable) or RL = 0 (irreversible). Similarly, the nature of the adsorption process based on the Freundlich isotherm model (Hamzaoui et al., 2018) is defined as n > 1 (adsorption is a physical process), n < 1 (adsorption is a chemical process or chemisorption) or n = 1 (adsorption is linear).
In this research, two statistical error functions were used in addition to the coefficient of determination (R2) to determine the suitability/fitness of the adsorption isotherm models under consideration. These error functions were the Chi-square test (X2) and the RMSE. The error function analyses and the model equations were obtained using Microsoft Excel.
RESULTS AND DISCUSSION
Figures 2 to 9 show measured and predicted results using the Langmuir and Freundlich isotherms. Model parameters including RMSE, Chi-square test and R2 values from the analyses of the results have been presented in Table 3.
From the results represented in Figs 2-5, it was observed that the linearized Langmuir and Freundlich models predicted the removal of lead using the GR-GAC treatment system with R2 values of 0.889 and 0.814, respectively. Iron adsorption prediction for the GR-GAC system had a better fit to experimental data for the Langmuir model than the Freundlich model, with R2 values of 0.842 vs 0.645, respectively.
Lead adsorption for the GAC-RH system (Figs 6-9) gave R2 values of 0.862 and 0.634 for the Langmuir and the Freundlich isotherm models, respectively, whereas iron adsorption for the Langmuir and Freundlich isotherm models gave R2 values of 0.969 and 0.916, respectively.
Table 3 summarizes the corresponding Langmuir and Freundlich isotherm parameters, their correlation coefficients (R2) and related chi-square values as well as standard errors for each parameter. Although both models predicted the removal of iron and lead to differing degrees, the Langmuir model consistently gave a better fit to experimental data than the Freundlich model. From the Freundlich isotherm model parameters in Table 3, the adsorption processes were found to be chemisorption as all values of n were less than 1. Also, the Freundlich isotherm model gave a good fit to measured data with low errors and high R2-values. On the other hand, Table 3 showed that the adsorption processes predicted by the Langmuir isotherm were found to be favourable with values of RL less than 1; this model also gave a better fit with higher R2 values and low errors. This trend was also observed by Desta (2013) who found that the Langmuir isotherm model predicted the adsorption of nickel from textile wastewater onto teff straw, with R2 = 0.998 and RL values between 0.298 and 0.986 indicating favourable adsorption, giving a better fit to the data than the Freundlich isotherm model with R2 = 0.748, and with n values ranging between 1 and 10.
GR-GAC and GAC-RH removal efficiencies
Table 3 shows that the GAC-RH combined filter had percentage concentration removal efficiencies for iron and lead of 53.6% and 48.2%, respectively. The GR-GAC combined filter had lower percentage concentration removals for iron and lead, at 35.0% and 24.5%, respectively. The GAC-RH combined filter therefore performed better in the removal of iron and lead than the GR-GAC combined filter. This result was also reported in Ataguba and Brink (2021) where the systems were tested in real-world applications at automobile workshops in different areas in Nigeria. Here, the authors reported an average percentage removal efficiency using GAC-RH combined filter of 45.8% for iron and 41.9% for lead. It is presumed that the GAC-RH combined filter gave higher removal efficiencies due to the larger combined surface area (attachment sites) available for adsorption when compared to the GR-GAC combined filter.
Effect of adsorbent dosage on adsorption capacities
Figures 10 to 13 show the relationships between the modelled adsorption capacity of the adsorbents and the dosage of the adsorbents. From Fig. 10, it was found that the uptake of iron by the GAC-RH filter decreased from 1.6 mg/g to 0.18 mg/g with increase in the dosage of the adsorbents from 0.5 g to 8 g, respectively. This has shown that the optimum dosage of 0.5 g each of GAC and RH resulted in the highest iron uptake, of 1.6 mg/g. Similarly, from Fig. 11, the uptake of lead by the GAC and RH decreased from 0.06 mg/g to 0.008 mg/g with increase in the dosage of the adsorbents from 0.5 g to 8 g, respectively. The optimum dosage of 0.5 g each of GAC and RH resulted in the highest lead uptake, of 0.06 mg/g.
Furthermore, it was found, as shown in Fig. 12, that the uptake of iron by the GR and GAC decreased from 0.98 mg/g to 0.1 mg/g with increase in the dosage of the adsorbents from 0.5 g to 8 g, respectively. This implied that the optimum dosage of 0.5 g each of GR and GAC resulted in the highest iron uptake, of ~1 mg/g. Similarly, from Fig. 13, the uptake of lead by the GR and GAC decreased from 0.019 mg/g to 0.0038 mg/g with increase in the dosage of the adsorbents from 0.5 g to 8 g, respectively. These results showed the optimum dosage of 0.5 g each of GR and GAC which resulted in the highest iron uptake, of ~0.02 mg/g.
Some studies reported in the literature have noted a similar trend and have attributed this to an aggregation of adsorbents, which reduced the surface area of the adsorbent available for adsorption to take place (Alghamdi et al., 2019; Radnia et al., 2012; Gorzin and Abadi, 2018).
Effect of contact time on the removal efficiency of the metals using the treatment technologies
Figures 14 and 15 show the effect of contact time on the removal efficiency for iron and lead using GAC-RH and GR-GAC treatment technologies, respectively. It can be observed that the two combined filter systems had higher removal efficiency for iron removal than lead removal. The removal efficiency for iron, as shown in Figs 14 and 15, increased steadily with increase in the contact time, from 5 min up to the optimum 80 min, using both GAC-RH and GR-GAC filters. A similar trend was observed in a study by Gorzin and Abadi (2018), where for chromium ion adsorption onto activated carbon the removal efficiency increased up to a contact time of 180 min and then gradually decreased to equilibrium. However, a slight difference noted in the current study was that there was a gradual increase in iron and lead removal up to equilibrium. This difference may be attributed to the fact that combined filter systems were used in this treatment.
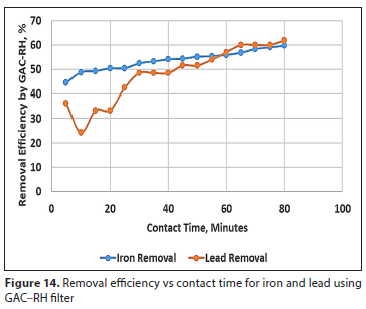
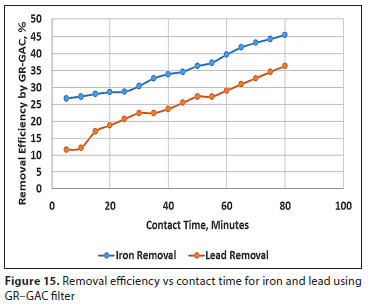
The removal efficiency for lead using GR-GAC (Fig. 15) showed a gradual increase with increasing contact time. However, there was a decrease in removal efficiency from 37% to 24% between 5 and 10 min. After this period, a gradual rise in removal efficiency was observed to 80 min of contact time. For the GAC-RH filter in Fig. 14, there was a sharp reduction in removal efficiency from 36% to 24% between contact times of 5-10 min. This may possibly be attributed to intra-particle diffusion of the lead ions into the GAC-RH pores, as reported by Kumar et al. (2008).
It has been reported in the literature that optimum adsorbent dosage for metal removal generally ranges between 0.1 g and 3.5 g (Ho, 2008; Karthikeyan et al., 2007; Melese et al., 2020). Similarly, optimum contact times required for metal removal have varied between 30 min and 180 min (Melese et al., 2020; Malakootian et al., 2009; Desta, 2013).
From the results obtained in this study, optimum dosage of 0.5 g each of GAC and RH resulted in maximum removal efficiencies of 60% and 62% (Fig. 14) for iron and lead, respectively, at equilibrium contact time of 80 min. Similarly, for the same optimum dosage of 0.5 g of GR and GAC, maximum removal efficiencies of 46% and 37% (Fig. 15) were achieved for iron and lead, respectively, at the same equilibrium contact time of 80 min.
Comparison of adsorption model performance for related research
Naiya et al. (2009) have reported the suitability of rice husk ash for the treatment of wastewater, with a 96.83% removal efficiency for lead using rice husk ash. This research also reported that the Freundlich isotherm model (with R2 = 0.99) performed better than the Langmuir isotherm model (with R2 = 0.94) in predicting the adsorption of lead onto rice husk ash. In the work carried out by Karnib et al. (2014), it was reported that the Freundlich isotherm model predicted the adsorption of lead from water onto activated carbon (with R2 = 0.97) better than the Langmuir isotherm model (with R2 = 0.70). Sizirici et al. (2017) reported that gravel can be used as adsorbent or filter to remove metals from polluted water.
From the adsorption modelling carried out, the Freundlich model predicted the adsorption of copper, iron and zinc from landfill leachate (with R2 of 0.94, 0.71, and 0.71, respectively) similar to the Langmuir model (with R2 of 0.97, 0.43 and 0.70, respectively). It is worthwhile to note that in this research, combined filter systems were developed, whereas the published works cited above were focused on singular filter materials.
Different researchers have reported on the effects of, inter alia, temperature, pH, concentration and contact time on adsorption. Senthil et al. (2010) reported that the rate of adsorption increases at lower concentration due to the fact that a larger surface area of the adsorbent is available for the process. Previous studies have also shown that adsorption capacity of the adsorbent increases with increasing pH of the adsorbent and decreases at lower pH (Ushakumary, 2013; Kaakani, 2012). Temperature gradient has been found to affect the solubility of the adsorbate, depending on the type of adsorbent used (Singh et al., 2021). It has been reported that an increase in temperature decreased the adsorption of copper ions and resulted in desorption from the surface of rice husk ash (Kashif et al., 2016). It has also been reported in the literature that the efficiency of the adsorbent in removing metal ions increases with increase in the dosage of adsorbent used (Jeyakumar and Chandrasekaran, 2014; Salihi et al., 2017). Previous publications have also reported that the uptake of metal ions increases with time until an optimum value is reached, after which there is no more uptake of the metal ions (Mohammad et al., 2011; Azouaou et al., 2010; Martinez et al., 2006). At this optimum contact time, the maximum adsorption rate is attained (Ugwu et al., 2020).
Table 4 compares the model parameters and removal efficiencies obtained for the current study with other published work. This shows that even though the removal efficiencies of GAC-RH and GR-GAC were lower than for the other adsorbents in previous studies, GAC-RH performed well in the removal of iron and lead, with removal efficiencies of 54% and 48%, respectively. However, GR-GAC performed below average. This could be due to the fact that the surface area available for adsorption is not large enough to adsorb much of the adsorbate. Perhaps, chemical optimization of GAC-RH may enhance the removal efficiency of the adsorbent. It could also be observed that the Langmuir isotherm derived for this research showed a similar trend to that in the cited work in Table 4. However, the Freundlich isotherm derived for this research differed from the cited work as the n values obtained here showed that the nature of the adsorption processes predicted by the Freundlich isotherm model is chemisorption.
This study has reported R2 values of no less than 0.6, which showed a reasonable fit to the isotherm models. Further modification/optimization of the GAC-RH combined filter may improve adsorption capacity and removal efficiency of these combined adsorbents.
CONCLUSIONS
The Langmuir and Freundlich isotherm models were applied to two low-cost combined filter systems, GAC-RH and GR-GAC, to predict the removal of iron and lead from automobile workshop stormwater runoff. The Langmuir model generally gave a better fit to measured data (R2 ranging from 0.842 to 0.963) when compared to the Freundlich model (R2 ranging from 0.634 to 0.916). The Langmuir isotherm model predicted the adsorption processes involved in this research as favourable, with RL < 1. The Freundlich isotherm models predicted the adsorption processes as chemisorption with n < 1.
The adsorbent dosage and contact time influenced the adsorption capacity of the adsorbents used (granular activated carbon, rice husk and gravel) as well as the metal removal efficiencies of the filter systems. The study revealed that increased dosages of the GAC-RH and GR-GAC resulted in a decrease in the uptake of iron and lead, with optimum adsorbent dosage of 0.5 g. Also, the efficiencies of iron and lead removal by GAC-RH and GR-GAC increased with increase in the contact time, with optimum contact time of 80 min. Furthermore, it was found that the combined GAC-RH filter system produced higher removal efficiencies for iron and lead (54% and 48%, respectively) in comparison to the combined GR-GAC filter system (35% and 25%, respectively). The results have indicated that combined low-cost filters (GAC-RH and GR-GAC) can adsorb metals from polluted stormwater runoff from automobile workshops.
Further research towards modifying/optimizing the combined GAC-RH to increase its metal adsorption capacity and removal efficiency is warranted. Also, investigation of the performance of these models in the removal of oil and grease in automobile workshop stormwater using the same or modified/optimized combined filter systems is warranted.
ACKNOWLEDGEMENT
This research was funded by the Tertiary Education Trust Fund, Nigeria.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest regarding the publication of this manuscript.
REFERENCES
AGUNWAMBA JC (2001) Waste Engineering and Management Tools. Immaculate Publishers Ltd., Enugu. [ Links ]
ALGHAMDI AA, AL-ODAYNI AB, SAEED WS, AL-KAHTANI A, ALHARTHI FA and AOUAK T (2019) Efficient adsorption of lead (II) from aqueous phase solutions using polypyrrole-based activated carbon. Materials. 12. https://doi.org/10.3390/ma12122020 [ Links ]
AMTUL Q, SYED AK and SAEEDA NA (2017) Equilibrium modelling for adsorption of aqueous Cd(II) onto turmeric: linear versus nonlinear regression analysis. Mor. J. Chem. 2 362-370. [ Links ]
APHA (2017) Standard Methods for the Examination of Water and Wastewater (23rd edn). American Public Health Association, Washington D.C. [ Links ]
ARGUE JR and PEZZANITI D (2005) Infiltration systems. In: Wong THF (ed.) Australian Runoff Quality: A Guide to Water Sensitive Urban Design. Engineers Australia, Sydney. [ Links ]
ATAGUBA CO and BRINK I (2021) Design and construction of laboratory-scale activated carbon, gravel and rice husk filter columns for the treatment of stormwater runoff from automobile workshops. J. S. Afr. Inst. Civ. Eng. 63 (1) 61-66. https://doi.org/10.17159/2309-8775/2021/v63n1a6 [ Links ]
AYAWEI N, ANGAYE SS, WANKASI D and DIKIO ED (2017) Synthesis, characterization and application of Mg/Al layered double hydroxide for the degradation of Congo red in aqueous solution. Open J. Phys. Chem. 5 (03) 56-70. https://doi.org/10.4236/ojpc.2015.53007 [ Links ]
AZOUAOU N, SADAOUI Z, DJAAFRI A and MOKADDEM H (2010) Adsorption of cadmium from aqueous solution onto untreated coffee grounds: Equilibrium and kinetics and thermodynamics. J. Hazardous Mater. 184 126-134. https://doi.org/10.1016/j.jhazmat.2010.08.014 [ Links ]
BAHGAT M, DEWEDAR MA and ZAYED A (1999) Sand-filters used for wastewater treatment: buildup and distribution of microorganisms. Water Res. 33 (8) 1949-1955. https://doi.org/10.1016/S0043-1354(98)00290-5 [ Links ]
BALARAK D and SALARI AA (2019) Error analysis of adsorption isotherm models for sulfamethazine onto multi walled carbon nanotubes. J. Pharmaceut. Res. Int. 25 (6) 1-10. https://doi.org/10.9734/jpri/2018/v25i630121 [ Links ]
BROWN JN and PEAKE BM (2006) Sources of heavy metals and polycyclic aromatic hydrocarbons in urban stormwater runoff. Sci. Total Environ. 359 (1-3) 145-155. https://doi.org/10.1016/j.scitotenv.2005.05.016 [ Links ]
CHARTERS F (2016) Characterizing and modelling urban runoff quality for improved stormwater management. PhD Dissertation, University of Canterbury, Christchurch. [ Links ]
CHRISTOPHI CA and AXE L (2000) Competition of Cd, Cu, and Pb adsorption on goethite. J. Environ. Eng. 126 (1) 66-74. https://doi.org/10.1061/(ASCE)0733-9372(2000)126:1(66) [ Links ]
DABROWSKI A (2001) Adsorption-from theory to practice. Adv. Colloid Interf. Sci. 93 (1-3) 135-224. https://doi.org/10.1016/S0001-8686(00)00082-8 [ Links ]
DAS B and MONDAL N (2011) Calcareous soil as a new adsorbent to remove lead from aqueous solution: equilibrium, kinetic and thermodynamic study. Env. Res. Technol. 1 515-530. [ Links ]
DESTA MB (2013) Batch sorption experiments: Langmuir and Freundlich isotherm studies for the adsorption of textile metal ions onto teff straw (Eragrostistef) agricultural waste. J. Thermodyn. 2013 6 pp. https://doi.org/10.1155/2013/375830. [ Links ]
ECHEVERRÍA JC, MORERA MT, MAZKIARÁN C and GARRIDO JJ (1998) Competitive sorption of heavy metal by soils. Isotherms and fractional factorial experiments. Environ. Pollut. 101 (2) 275-284. https://doi.org/10.1016/S0269-7491(98)00038-4 [ Links ]
ELMORSI TM (2011) Equilibrium isotherms and kinetic studies of removal of methylene blue dye by adsorption onto miswak leaves as a natural adsorbent. J. Environ. Protect. 2 (6) 817-827. https://doi.org/10.4236/jep.2011.26093 [ Links ]
FOST SD and ALY MO (1981) Adsorption Processesfor Water Treatment. Betterworth Publications, Stoneharm, Massachusetts. [ Links ]
FUF and WANG Q (2011) Removal of heavy metal ions from wastewaters: a review. J. Environ. Manage. 92 (3) 407-418. https://doi.org/10.1016/j.jenvman.2010.11.011 [ Links ]
FUERHACKER M, HAILE TM, MONAI B and MENTLER A (2011) Performance of a filtration system equipped with filter media for parking lot runoff treatment. Desalination. 275 118-125. https://doi.org/10.1016/j.desal.2011.02.041 [ Links ]
GORZIN F and ABADI BR (2018) Adsorption of Cr(VI) from aqueous solution by adsorbent prepared from paper mill sludge: Kinetics and thermodynamics studies. Adsorp. Sci. Technol. 36 (1-2) 149-169. https://doi.org/10.1177/0263617416686976 [ Links ]
GULBAZ S, KAZEZYILMAZ-ALHAN CM and COPTY NK (2015) Evaluation of heavy metal removal capacity of bioretention systems. Water Air Soil Pollut. 226 (11). https://doi.org/10.1007/s11270-015-2640-y [ Links ]
GUYER JP (2017) An Introduction to Low Impact Development. Guyer Partners, California. [ Links ]
HAMI HK, ABBAS RF, MAHDI AS and MARYOOSH AA (2021) An overview of using error function in adsorption isotherm modeling. Muthanna J Pure Sci. 8 (1). https://doi.org/10.52113/2Z08.01.2021/22-30 [ Links ]
HAMZAOUI M, BESTANI B and BENDERDOUCHE N (2018) The use of linear and nonlinear methods for adsorption isotherm optimization of basic green 4-dye onto sawdust-based activated carbon. Mater. Environ. Sci. 9 (4) 1110-1118. [ Links ]
HATT BE, STEINEL A, DELETIC A and FLETCHER TD (2011) Retention of heavy metals by stormwater filtration systems: Breakthrough analysis. Water Sci. Technol. 64 1913-1919. https://doi.org/10.2166/wst.2011.188 [ Links ]
HO YS (2008) Citation review of Lagergren kinetic rate equation on adsorption reactions. Scientometrics. 59 (1) 171-177. https://doi.org/10.1023/B:SCIE.0000013305.99473.cf [ Links ]
JEYAKUMAR RPS, and CHANDRASEKARAN V (2014) Adsorption of lead (II) ions by activated carbons prepared from marine green algae: equilibrium and kinetics studies. Int. J. Ind. Chem. 5 (10). https://doi.org/10.1007/s40090-014-0010-z [ Links ]
KAAKANI MW (2012) Heavy metal removal from wastewater using novel adsorbent. Masters thesis, American University of Sharjah. [ Links ]
KARNIB M, KABBANI A, HOLAIL H and OLAMA Z (2014) Heavy metals removal using activated carbon, silica and silica activated carbon composite. Energ. Procedia. 50 113-120. https://doi.org/10.1016/j.egypro.2014.06.014 [ Links ]
KARTHIKEYAN S, BALASUBRAMANIAN R and IYER CSP (2007) Evaluation of the marine algae Ulvafasciata and Sargassum sp. for the biosorption of Cu(II) from aqueous solutions. Bioresour. Technol. 98 (2) 452-455. https://doi.org/10.1016/j.biortech.2006.01.010 [ Links ]
KASHIF I, DILDAR A and NASEEM Z (2016) Factors affecting copper removal from simulated wastewater using rice husk ash as adsorbent. Int. J. Res. Granthaalayah. 4 (2) 52-61. https://doi.org/10.29121/granthaalayah.v4.i2.2016.2812 [ Links ]
KAVAND M, SOLEIMANI M, KAGHAZCHI T and ASASIAN N (2016) Competitive separation of lead, cadmium, and nickel from aqueous solutions using activated carbon: response surface modeling, equilibrium, and thermodynamic studies. Chem. Eng. Comm. 203 (1) 123-135. https://doi.org/10.1080/00986445.2014.962691 [ Links ]
KHAN NA, IBRAHIM S and SUBRAMANIAM P (2004) Elimination of heavy metals from wastewater using agricultural wastes as adsorbents. Malaysian J. Sci. 23 43-51. [ Links ]
KISELER AVC (1958) Vapour adsorption in the formation of adsorbate-molecule complexes on the surface. KolloidZhur. 20 338-348. [ Links ]
KUMAR PS, RAMALINGAMB S, KIRUPHAC SD, MURUGESANC A and VIDHYAREVICSIVANESAM S (2011) Adsorption behaviour of nickel (II) onto cashew nut shell: equilibrium, thermodynamics, kinetics, mechanism and process design. Chem. Eng J. 167 122-131. https://doi.org/10.1016/jxej.2010.12.010 [ Links ]
KUMAR R, BISHNOI NR and BISHNOI K (2008) Biosorption of chromium (VI) from aqueous solution and electroplating wastewater using fungal biomass. Chem. Eng. J. 135 202-208. https://doi.org/10.1016/j.cej.2007.03.004 [ Links ]
LARM T and WAHLSTEN A (2019) Modelling different types of storm-water treatment facilities considering irreducible concentrations. In: Mannina G (ed) New Trends in Urban Drainage Modelling. UDM 2018. Green Energy and Technology. Springer, Cham. https://doi.org/10.1007/978-3-319-99867-1_28 [ Links ]
LAURENSON G, LAURENSON S, BOLAN N, BEECHAM S and CLARK I (2013) The role of bioretention systems in the treatment of stormwater. Adv. Agron. 120 223-274. https://doi.org/10.1016/B978-0-12-407686-0.00004-X [ Links ]
LI H and DAVIS AP (2008) Heavy metal capture and accumulation in bioretention media. Environ. Sci. Technol. 42 5247-5253. https://doi.org/10.1021/es702681j [ Links ]
MALAKOOTIAN M, NOURI J and HIWA H (2009) Removal of heavy metals from paint industry's wastewater using Leca as an available adsorbent. Int. J. Environ. Sci. Technol. 6 2-6. https://doi.org/10.1007/BF03327620 [ Links ]
MANAA MB, ISSAOUI N, BOUAZIZ N and LAMINE AB (2020) Combined statistical physics models and DFT theory to study the adsorption process of paprika dye on TiO2 for dye sensitized solar cells. J. Mater. Res. Technol. 9 1175-1188. https://doi.org/10.1016/j.jmrt.2019.11.045 [ Links ]
MARTINEZ ML, TORRES MM, GUZMAN CA and MAESTRI DM (2006) Preparation and characteristics of activated carbon from olive stones and walnut shells. Indust. Crops Products. 23 23-28. https://doi.org/10.1016/j.indcrop.2005.03.001 [ Links ]
MELESE T, CHALA K, AYELE Y and ABDISA M (2020) Preparation, characterization of raw corncob adsorbent for removal of heavy metal ions from aqueous solution using batch method. Afr. J. Pure Appl. Chem. 14 (4) 81-90. [ Links ]
MOHAMMAD MMR, PARISA R, ATEFEH A and ALI RK (2011) Kinetics and equilibrium studies on biosorption of cadmium, lead and nickel ions from aqueous solutions by intact and chemically modified brown algae. J. Hazardous Mater. 185 401-407. https://doi.org/10.1016/j.jhazmat.2010.09.047 [ Links ]
NAIYA TK, BHATTACHARYA AK, MANDAL S and DAS SK (2009) The sorption of lead (II) ions on rice husk ash. J. Hazardous Mater. 163 1254-1264. https://doi.org/10.1016/j.jhazmat.2008.07.119 [ Links ]
NAP (2009) Urban Stormwater Management in the United States. The National Academies Press, Washington DC. [ Links ]
NASSAR MM, EWIDA KT, EBRAHIEM EE, MAGDY YH and MHEAEDI MH (2004) Adsorption of iron and manganese using low cost materials as adsorbents. J. Environ. Sci. Health A. 39 (2) 421-434. https://doi.org/10.1081/ese-120027533 [ Links ]
PICCIN JS, CADAVAL TRS, DE PINTO LAA and DOTTO GL (2017) Adsorption isotherms in liquid phase: experimental, modeling, and interpretations. Chapter 2. In: Bonilla-Petriciolet A, Mendoza-Castillo DI and Reynel-Ávila HE (eds) Adsorption Processesfor Water Treatment and Purification. Springer International Publishing, Switzerland. https://doi.org/10.1007/978-3-319-58136-1_2 [ Links ]
PITCHER SK, SLADE RCT and WARDS NI (2004) Heavy metal removal from motorway stormwater using zeolites. Sci. Total Environ. 334-335 161-166. https://doi.org/10.1016/j.scitotenv.2004.04.035 [ Links ]
PODDER MS and MAJUMDER CB (2016) Studies on removal of As(III) and As(V) onto GAC/MnFe204 composite: isotherm studies and error analysis. Composite Interf. 23 (4) 327-372. https://doi.org/10.1080/09276440.2016.1137715 [ Links ]
RADNIA H, GHOREYSHI AA, YOUNESI H and NAJAFPOUR GD (2012) Adsorption of Fe(II) ions from aqueous phase by chitosan adsorbent: Equilibrium, kinetic, and thermodynamic studies. Desal. Water Treat. 50 348-359. https://doi.org/10.1080/19443994.2012.720112 [ Links ]
REDDY KR, XIE T and DASTGHEIBI S (2014) Mixed-media filter system for removal of multiple contaminants from urban storm water: large-scale laboratory testing. J. Hazardous Toxic Radioact. Waste. 18 (3) 4001-4011. https://doi.org/10.1061/(ASCE)HZ.2153-5515.0000226 [ Links ]
SALIHI UI, KUTTY SRM and ISA MH (2017) Adsorption of lead ions onto activated carbon derived from sugarcane bagasse. IOP Conf. Ser.: Mater. Sci. Eng. 201 012034. https://doi.org/10.1088/1757-899X/201/1/012034 [ Links ]
SCHOLES L, REVITT MD and ELLIS JB (2008) A systematic approach for the comparative assessment of stormwater pollutant removal potentials. J. Environ. Manage. 88 467-478. https://doi.org/10.1016/j.jenvman.2007.03.003 [ Links ]
SENTHIL KP, RAMALINGAM S, SENTHAMARAI C, NIRANJANA AM, VIJAYALAKSHMI P and SIVANESAN S (2010) Adsorption of dye from aqueous solution by cashew nut shell: Studies on equilibrium isotherm, kinetics and thermodynamics of interactions. Desalination. 261 52-60. https://doi.org/10.1016/j.desal.2010.05.032 [ Links ]
SINGH P, SINGH TS and PANT KK (2001) Removal of arsenic from drinking water using activated alumina. Res. J. Chem. Environ. 5 25-28. [ Links ]
SIZIRICI B, YILDIZ I, ALYAMMAHI A, OBAIDALLA F, ALMEHAIRBI M, ALKHAJEH S and ALHAMMADI TA (2018) Adsorptive removal capacity of gravel for metal cations in the absence/presence of competitive adsorption. Environ. Sci. Pollut. Res. 25 7530-7540. https://doi.org/10.1007/s11356-017-0999-6 [ Links ]
SONG Y and LIU H (2013) A comparative study on the adsorption and desorption of nitrogen and phosphorus by three matrices of eco-river channel. Res. J. Appl. Sci. Eng. Technol. 5 2734-2739. https://doi.org/10.19026/rjaset.5.4799 [ Links ]
THUY CN, LOGANATHAN P, TIEN VN, VIGNESWARAN S, KANDASAMY J and NAIDU R (2015) Simultaneous adsorption of Cd, Cr, Cu, Pb, and Zn by an iron-coated Australian zeolite in batch and fixed-bed column studies. Chem. Eng. J. 270 393-404. https://doi.org/10.1016/j.cej.2015.02.047 [ Links ]
UGWU EI, TURSUNOV O, KODIROV D, SHAKER LM, AMIERY AAA, YANGIBAEVA I and SHAVKAROV F (2020) Adsorption mechanisms for heavy metal removal using low cost adsorbents: A review. IOP Conf. Series: Earth Environ. Sci. https://doi.org/10.1088/1755-1315/614/1/012166 [ Links ]
UNEP (2004) Environmentally sound technologies in wastewater treatment for the implementation of the UNEP Global Programme of Action (GPA). In: Guidance on Municipal Wastewater. United Nations Environment Programme. [ Links ]
USHAKUMARY ER (2013) Waste water treatment using low cost natural adsorbents. PhD dissertation, Cochin University of Science and Technology. [ Links ]
VIJAYARAGHAVAN K, PADMESH TVN, PALANIVELU K and VELAN M (2006) Biosorption of nickel(II) ions onto Sargassum wightii: application of two-parameter and three-parameter isotherm models. J. Hazardous Mater. 133 (1-3) 304-308. https://doi.org/10.1016/j.jhazmat.2005.10.016 [ Links ]
WANG J and GUO X (2020) Adsorption isotherm models: Classification, physical meaning, application and solving method. Chemosphere. 258 127279. https://doi.org/10.1016/j.chemosphere.2020.127279 [ Links ]
WANG Z, BARFORD JP, HUI CW and MCKAY G (2015) Kinetic and equilibrium studies of hydrophilic and hydrophobic rice husk cellulosic fibers used as oil spill sorbents. Chem. Eng. J. 281 961-969. https://doi.org/10.1016/jxej.2015.07.002. [ Links ]
WONG THF, FLETCHER TD, DUNCAN HP and JENKINS GA (2006) Modelling urban stormwater treatment - a unified approach. Ecol. Eng. 27 (1) 58-63. https://doi.org/10.1016/j.ecoleng.2005.10.014 [ Links ]
WORCH E (2021) Adsorption Technology in Water Treatment: Fundamentals, Processes, and Modeling. Walter de Gruyter GmbH & Co. KG, Berlin, Germany. 345 pp. [ Links ]
WU M, LI Q, TANG X, HUANG Z, LIN L and SCHOLZ M (2014) Arsenic(V) removal in wetland filters treating drinking water with different substrates and plants. Int. J. Environ. Anal. Chem. 94 (6) 618-638. https://doi.org/10.1080/03067319.2013.864647. [ Links ]
ZHANG X, YAN L, LI J and YU H (2020) Adsorption of heavy metals by L-cysteine intercalated layered double hydroxide: Kinetic, isothermal and mechanistic studies. J. Colloid Interf. Sci. 562 149-158. https://doi.org/10.1016/j.jcis.2019.12.028 [ Links ]
 Correspondence:
Correspondence:
Clement Oguche Ataguba
Email: clematrix2008@gmail.com
Received: 31 December 2021
Accepted: 22 September 2022














