Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Water SA
On-line version ISSN 1816-7950
Print version ISSN 0378-4738
Water SA vol.48 n.4 Pretoria Oct. 2022
http://dx.doi.org/10.17159/wsa/2022.v48.i4.3930
RESEARCH PAPER
Membrane fouling in thermophilic aerobic membrane distillation bioreactor treating hospital wastewater
Elif İnce; Mahir İnce; Handenur Yajar; Yasin Abdullah Uslu
Gebze Technical University, Department of Environmental Engineering, Kocaeli, Turkey
ABSTRACT
In the membrane distillation bioreactor (MDBR) process, flux increases with increasing feed temperature, but the presence of microorganisms limits the feed temperature. Also, the accumulation of cells and other substances on the membrane surface can affect the efficiency of MDBR. In this study, hospital wastewater was treated by thermophilic activated sludge MDBR. In the MDBR, the initial flux was 7.87 L-m-2-h-1 and the stable flux was 3.88 L-m-2-h-1. The particle size, zeta potential and hydrophobicity of the activated sludge in MDBR were 2.25 urn, -14 mV and 24%, respectively. In addition, EPS (extracellular polymeric substances) and SMP (soluble microbial products), having a significant effect on membrane fouling, were determined to be 201.50 mg-L-1 and 669.35 mg-L-1 in MDBR, respectively. Contact angle, FTIR (Fourier transform infrared), SEM (scanning electron microscope) and EDX (energy dispersive X-ray spectroscopy) measurements were also made on a virgin membrane and used membrane. Analysis of EDX, SEM and F-TIR showed that the membrane fouling was caused by CaCO3 and EPS.
Keywords: membrane distillation, bioreactor, membrane fouling, SEM, FTIR, EDX
INTRODUCTION
Temperature is one of the most significant parameters affecting microbial growth and hence has great importance in biological wastewater treatment systems. The growth of bacteria is quite low at low temperatures, but their growth rates increase with increasing temperature. Microorganisms can live at high temperatures, which do not denature their proteins. However, above 60°C, these proteins denature and microorganisms cannot grow. Microorganisms living at these high temperatures are called thermophilic and their metabolic rate is quite high. Therefore, they are preferred for rapid treatment of organic wastes compared to mesophilic microorganisms (Ince and Topaloglu, 2018).
The final performance of biological treatment systems depends on the degree of separation of the produced biomass from the mixed wastewater. In activated sludge systems, the environmental conditions in the reactor affect the efficiency of biomass separation. The settling properties of the sludge change, depending on the dissolved oxygen concentration, biodegradability of organic pollutants, and organic loading (Tchobanoglous and Burton, 1991). For these reasons, membrane technology is used for both solid/liquid separation and increasing reactor performance (Chaize and Huyard, 1991; Hai and Yamamoto, 2011; Lübbecke et al., 1995; Muller et al., 1995).
Membrane bioreactor (MBR) systems are treatment systems that combine the activated sludge process with the membrane separation process (DeCarolis and Adham, 2007). The reactor is operated similarly to a conventional activated sludge process, not requiring final settling tank and tertiary treatment operations such as sand filtration. The MBR process enables differerentiation between sludge retention time (SRT) and hydraulic retention time (HRT); thus, the sludge age can be easily adjusted.
Since the hydrophilic pollutants having a smaller diameter than the membrane pore size in wastewater treatment can easily pass through MF and UF membranes, the residence time of these pollutants in MBRs may be the same as the HRT. This situation is especially important for MBRs operated at high MLSS concentrations due to being operated at shorter HRT. Therefore, removal of contaminants in the effluent of MBR requires advanced treatment such as reverse osmosis (RO), nanofiltration (NF), UV oxidation or ozonation to obtaine high quality water, resulting in significant increase in investment and operation cost.
The membrane distillation bioreactor (MDBR) is a new technology that combines the bioreactor with membrane distillation (MD) for wastewater treatment. MD is a separation process that rejects 99.99% of macromolecules, colloids, cells, ions, and non-volatile substances and has the advantage of operating at lower temperatures and lower pressure compared to conventional distillation and pressure-driven membranes, respectively. Membranes used in MDBR are microporous and hydrophobic, allowing water vapour to pass and are not wetted by water. The effluent water quality obtained in a single system with MDBR is the same as the effluent quality of multiple treatment systems such as the conventional activated sludge system + MF + RO or MBR + RO.
The separation is achieved by the vapour pressure differences between the membrane surfaces. The thermophilic bioreactor provides good compatibility with the MD system as the temperature in the reactor promotes both bacterial production activity and MD separation. Also, energy efficiency of MDBR can be increased with using low-grade temperature sources, such as solar energy and waste heat (Tijing et al., 2015; Wijekoon et al., 2014). The inclusion of biomass, by combining thermophilic bioprocesses with the MD process, ensures the biological removal of organics and nutrients, leading to decrease in hydrophobicity of membranes over time. MDBR provides more time for the biodegradation of recalcitrant organic pollutants, due to organic retention time being completely independent from HRT.
To the best of our knowledge, there have been limited published studies on wastewater treatment in MBDR, with most dealing with petrochemical or synthetic wastewater (Khaing et al., 2010; Phattaranawik et al., 2008, 2009), there is no published study that has investigated the treatment of hospital wastewater by MDBR. Therefore, the aim of this study was to investigate the membrane fouling and decrease in the flux in the aerobic thermophilic MDBR treating hospital wastewater.
MATERIALS AND METHODS
Wastewater supply
Within the scope of the study, hospital wastewater was obtained from a hospital in Kocaeli, Turkey. The hospital has 725 beds (67 of which belong to the general intensive care unit). The total installed area of the hospital is 86 000 m2. In 2016, a monthly average of 2 688 patients were operated on in this hospital, 2 786 inpatients were served, and an average of 36 538 patients had an outpatient examination. In this study, the hospital wastewater used was supplied from the building connection channel before distributing it to the main/urban sewage channel. Since the hospital wastewater changes over the course of a day, and from day to day, this presents problems in replicating the methods and results obtained. Hence daily composite samples were obtained from the wastewater channel by the automatic sampler, within 2 h periods. These daily composite samples were collected in the cold room, and at the end of a week the daily composite samples were mixed in the same ratios and a weekly composite wastewater was obtained.
MDBR system
The experimental set-up consisted of a bioreactor with submerged direct contact distillation membrane (DCMD) module, feed tank, distillate tank, PLC (programmable logic controller) control unit and aeration system (Fig. 1). The air was continuously supplied using an air blower, at a flow rate of 10 L-min-1, to supply oxygen to the system to keep the thermophilic aerobic culture in the bioreactor in suspension, as well as to control of membrane fouling.
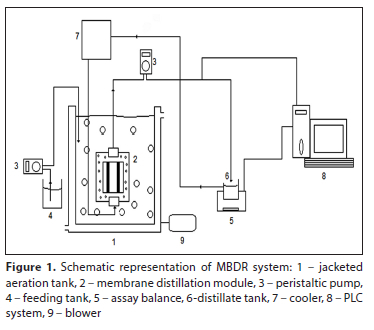
The temperature of mixed liquor and distillate were monitored with digital thermometers (Omron E5CN) with flexible thermocouples (type K). pH, electrical conductivity and dissolved oxygen (DO) of mixed liquor were measured with an online pH meter (Chemitec 50 series), online electrical conductivity meter (Chemitec 50 series) and online DO meter (Mettler Toledo O24050e), respectively. The distillate tank was placed on a scale of 6 100 g (AND EJ-6100) for observing the distillate flux. The distillate was circulated with the peristaltic pump from the submerged DCMD module into the distillate tank and then into the cooler. All online monitoring instruments were connected to a data acquisition system with PLC installed.
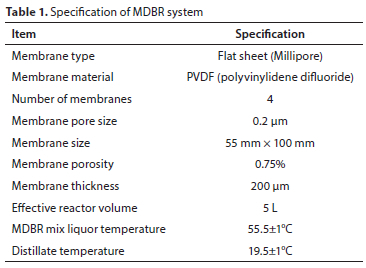
The specifications of the MDBR system is given in Table 1. To keep the operating temperature at 55.5±1°C, a constant temperature water circulator connection was established to the bioreactor. The temperature of the distillate circulating on the other side of the membrane was kept constant at 19.5±1°C by using a cooler (glass tube containing cold water channels). The effective volume of MDBR was 5 L. The double-faced flat-sheet membrane module was submerged in the bioreactor and the mixed liquor was in direct contact with the membrane surface. Figure 2 shows the membrane distillation module used in the system, both open and closed.
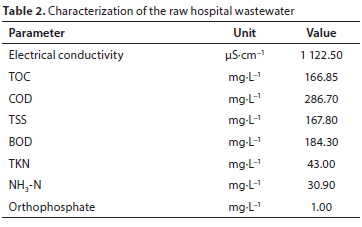
Raw hospital wastewater was fed to the reactors with a peristaltic pump controlled by PLC. The characterization of the hospital wastewater taken from a research hospital prior to discharging to the sewer line, is summarized in Table 2.
Sludge retention time (SRT) is a significant operational parameter for wastewater treatment plants, which refers to the residence time of biomass in the system. High SRTs provide not only an increase in mixed culture in the bioreactor, but also acclimatisation of microorganisms to recalcitrant and complex pollutants such as micropollutants. In addition, difficult biodegradable pollutants can be degraded by microorganisms at high SRT. Therefore, in this study, the SRT was adjusted to 30 days to accommodate the treatment of hospital wastewater containing micropollutants.
In MDBR, inorganics (salts) accumulate in the reactor over time. Therefore, an appropiate amount of sludge is disposed of from the system so that salt accumulation does not negatively affect microorganisms. This is achieved by calculating the mass balance concentration factor (CF), which is the ratio of SRT to HRT.

CF is an important parameter in terms of controlling the increase in salinity in the bioreactor (Lay et al., 2010). High CF leads to an increase in inorganic salts in the bioreactor, but also an increase in water recovery rate. Therefore, the optimum CF should be determined by taking into account the target water recovery rate and the salt accumulation in the bioreactor. In this study, both SRT (day) and the CF in the MDBR were adjusted to 30.
Sample analysis
TOC (total organic carbon) was measured by a TOC analyser (HACH IL 550 TOC-TN); COD (chemical oxygen demand), BOD (biochemical oxygen demand), TKN (total Kjeldahl nitrogen), ammonia and orthophosphate were measured by APHA (2005) 5220-D, APHA (2005)-5210 B, APHA (2005) 4500-Norg - B, APHA (2005) 4500-NH3 - B and APHA (2005) 4500-P - G, respectively. Electrical conductivity of mixed liquor and distillate were measured by a Seven-multimeter (Mettler Toledo, USA). Total suspended solids (TSS) were measured using a glass microfibre filter of 1.2 μm bystandard methods (APHA (2005) 2540 - D) (Clescerl et al., 1998). The EPS and SMP contents in the MDBR were determined by the formaldehyde extraction method (Tinggang et al., 2008). In particular, the sum of carbohydrate (C) and protein (P) was considered as total EPS (total EPS = EPSp + EPSc + SMPp + SMPc). The Lowry and the phenol sulfuric acid methods were used for protein and carbohydrate analysis, respectively (ince and Topaloglu, 2018). In this study, MATH (microbial adhesion to hydrocarbons) method was used for relative hydrophobicity analysis (Sanin et al., 2003). The principle of this method is the adhesion of microorganisms to a selected hydrocarbon, depending on the surface hydrophobicity. In this method, the surface hydrophobicity of microorganisms was determined with n-hexadecane. The solution was washed 2-3 times with Tris-HCl buffer (pH = 7.1) in order to minimize the errors stemming from the electrostatic effects found in the bacterial environment (Chang and Lee, 1998). After the washing process, 3 mL of bacterial suspension was put in a 10 mm UV cuvette and the first optic density (OD) value was read at 600 nm wavelength, then 0.3 mL of n-hexadecane was added, mixed for 2 min in a vortex device, and kept for 15 min at room temperature. The final OD value was read at 600 nm. The result was calculated according to Eq. 2 (Sanin et al., 2003):
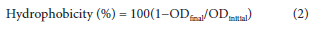
To determine the floc size distribution of thermophilic activated sludge taken from the MDBR system, a Malvern Brand Mastiserer 2000 was used. The membrane fouling layer on the membrane surface was analysed using a scanning electron microscope (SEM, FEI, PHILIPS, XL30 SFEG) coupled with an energy dispersive X-ray analyser (EDX, elemental analysis detector). After physical washing, the membranes were dried at 60°C for 1 day and the FTIR (Perkin Elmer) spectra of the membranes determined with a spectral resolution of 4 cm-1 in the range of 4 000-400 cm-1. Meanwhile, each sample spectrum was obtained by taking the average of 50 spectra.
RESULTS AND DISCUSSION
Distillate characterization
In this study, the MDBR system was operated continuously and pH and conductivity values of the bioreactor were measured online. The system pH was automatically controlled and fixed between 6.9 and 7.4. It is known that the electrical conductivity in the feed wastewater has an important effect on the MDBR product water quality. Gryta et al. (2006), reported that the possibility of wastewater treatment by the MD process was essentially dependent on the composition of the wastewater. The membranes used in the MD process are hydrophobic so as not to be wet by water (Zuo et al., 2016). However, organic compounds in wastewater reduce the contact angle, making it easier for wetting to occur (Wang and Chung, 2015). If the membrane (part or all of the surface) is wet, the salts in the feed are spread into the liquid that fills the wet pores. In this case, the electrical conductivity of the distillate increases systematically during the MD process.
A decrease in hydrophobicity of the membrane surface results in membrane wetting which leads to a decrease in permeate quality (or increase in effluent conductivity). In this study, as can be seen from Fig. 3, the electrical conductivity in the distillate increased slightly due to high rejection efficiency (99%) of MDBR. This high salt rejection rate is essential for the MDBR system to provide a good product quality for water reuse. Reducing membrane pore size is one of the effective ways to reduce wetting (Khaing et al., 2010). However, it is also recognised that flux decreases when membrane pore size is reduced. In order to address the wettability of membrane pores, the pore size must be such that it does not lead to any conflict between wettability and permeability.
Although electrical conductivity of the thermophilic activated sludge in the MDBR reached 42.01 mS-cm-1, its maximum value in the distillate was 135 |iS-cm-1. Phattaranawik et al. (2009) studied synthetic wastewater treatment with submerged MDBR system using both flat and tubular membranes. They reported that the electrical conductivity of the distillate from the tubular membrane (3.04 S-m-1) was greater than that from the flat membrane (2.26 S-m-1). The same researchers measured the electrical conductivity in the reactor as 4.02-4.5 S-m-1 in an earlier study using synthetic wastewater (Phattaranawik et al., 2008). Goh et al. (2013a) measured the electrical conductivity as 15 mS-cm-1 in the submerged MDBR. In another study by Goh et al. (2012), the electrical conductivity was 10.7±2.2 mS-cm-1 at an SRT of 10 days while it was 11.6±2.7 mS-cm-1 at an SRT of 30 days. Wijekoon et al. (2014) reported in their study investigating the removal of trace organic compounds from synthetic wastewater with the external MDBR system that the flux reached a constant value at the end of the first 10 days, and the electrical conductivity of the effluent was less than 5 μS-cm-1 during the entire study (about 40 days). This electrical conductivity value indicates that there is no wetting of the membrane throughout the study (Wijekoon et al., 2014). Khaing et al. (2010) reported that in a submerged MDBR for treatment of petrochemical industry wastewater the electrical conductivity of the effluent was 33.7-82.1 μS-cm-1 in the first 39 days. In the period of 40-105 days, the effluent electrical conductivity increased to 13.5-52.8 μS-cm-1 with increasing conductivity of the sludge, from to 11.3-18.5 mS-cm-1. Likewise the electrical conductivity of the wastewater fed to the reactor in this period varied from 1.2-1.6 mS-cm-1 (Khaing et al., 2010).
Another indication of membrane wetting is increase in TOC concentration of the distillate. In this study, no increase in the TOC concentration (<1 mg-L-1) of the distillate during operation also indicated that the membranes were not wetted. On the other hand, the slight increase in conductivity suggested that the free ammonia at the thermophilic temperature (55.5±1°C) passed through the MD membrane, and afterwards was converted to NH4+ form in the distillate due to the temperature of distillate (19.5±1°C).
Distillate flux
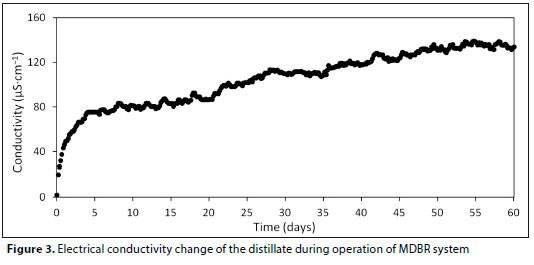
Figure 4 shows the profiles of the distillate flux throughout the study. The initial distillate flux was 7.87 L-m-2-h-1 (Day 1), but, as expected, it decreased gradually (from Day 2 to Day 15). Finally, it reached a steady state in the range of 3.44-3.91 L-m-2-h-1. As can be seen in Fig. 3, the stable flux was 3.59 L-m-2-h-1. This value was higher compared to other MDBR studies using a pore diameter of 0.2 μm (Phattaranawik et al., 2009; Wijekoon et al., 2014).
Phattaranawik et al. (2009) reported, using the same type of membrane, that the initial and final flux values were 12.7 L-m-2-h-1 and 1.9 L-m-2-h-1, respectively. They also stated for the same study, with PTFE membrane having pore size of 0.45 μm, the initial and final flux values were 20.1 L-m-2-h-1 and 3.6 L-m-2-h-1, respectively. In another study, it was reported that the distillate flux decreased from 4 L-m-2-h-1 to about 2 L-m-2-h-1 in the first 3 days and became approximately stable at 1.2 L-m-2-h-1 in the external MDBR with membrane of 0.22 um pore diameter (Wijekoon et al., 2014). In studies using MDBR for wastewater treatment, a reduction of more than 50% in the flux were observed in the first few days (Jacob et al., 2015; Phattaranawik et al., 2008). However, in this study, a decrease by 50% was observed at the end of 15 days, and in the following days the flux became stable. This flux declination was due to membrane fouling which was stemmed from adsorbing or adhering of dissolved or particulate substances on the membrane surface or into pores (Gryta, 2008; Li and Yang, 2007). The fouling layer prevents the transfer of heat to the membrane surface, resulting in reduction of interfacial feed temperature and vapour pressure. Hence, a decrease in the flux was observed (Gryta, 2008; Khaing et al., 2010).
Mixed liquor analysis in MDBR
In this study, while the carbohydrate and protein content of EPS in the MDBR were 76.1 mg-L-1 and 125.4 mg-L-1, respectively, the carbohydrate and protein content of SMP were measured as 150.55 mg-L-1 and 518.8 mg-L-1, respectively. In addition, protein and carbohydrate fraction (as P/C) was 1.65 in EPS and 3.44 in the SMP. In this study, EPS and SMP analysis were also performed in the distillate after 60 days and it was found that the distillate did not contain carbohydrates and proteins as expected. In recent studies, it has been reported that increasing salinity in the bioreactor affects the physical and biochemical properties of activated sludge (Qiu and Ting, 2014). For example, the salinity increase in the bioreactor can increase EPS and SMP concentrations, which changes the rheological properties of activated sludge, resulting in a decrease in oxygen transfer efficiency and dramatic increase in membrane fouling (Lay et al., 2010). Increasing salinity might also lead to changes in microbial culture in activated sludge, deceleration of microbial kinetics and even inhibition of microbial growth (Nawaz et al., 2013; Qiu and Ting, 2014). Another reason for low concentrations of EPS and SMP in the MDBR, was that salt concentration was not allowed to increase too much, by maintaining an optimal CF. Otherwise, salinity levels could reach values which negatively affect the metabolism of thermophilic activated sludge, resulting in increasing EPS and SMP concentrations.
Particle size and zeta potential analysis were also performed on the mixed liquor samples taken from the MDBR and the average particle size of the thermophilic activated sludge was found to be 2.25 μm (Fig. 5).
In other studies the particle sizes observed in MDBR remained in the lower range of 0.3-30 μm (Goh et al., 2015; Goh et al., 2013b). The particle sizes obtained in this study were quite low (maximum 2.28 μm). Therefore, small particle size increased the contact surface between the pollutants and microorganisms, contributing to biodegradation efficiency. Smaller particle size (compared to mesophilic processes) observed in the thermophilic process reduced hydrophobic interactions, resulting in aggregation and fragmentation in flocs and increasing sensitivity to shear forces. Goh et al. (2013a) carried out particle size analysis for two different activated sludges in external MDBR with vacuum evaporation and cross flow. In the vacuum evaporation study, while the average particle size of the low-hydrophilic activated sludge was 17.1 um, it was measured as 2.9 μm in the high-hydrophilic sludge. As for the cross-flow study, the average particle size of the low-hydrophilic sludge was 2.5 um, while the particle size was measured as 1.2 um in the high-hydrophilic sludge (Goh et al., 2013b). In this study, the zeta potential of the thermophilic mixed liquor samples taken from the MDBR was found to be -14.09 mV. Characterization of activated sludge included particle size, zeta potential, cell surface hydrophobicity and EPS production rate. As cell hydrophobicity and carbohydrate fraction in EPS increases, the sludge floc size increases. Cell surface hydrophobicity is the most important driving force to form flocs (Tay et al., 2001; Zhang et al., 2005). High cell hydrophobicity leads to strong interaction between cells, resulting in compact microbial flocs. The relative hydrophobicity value of the mixed liquor in the MDBR was measured at 25.54%. Cell surface characteristics of microorganisms are important for floc formation and solid-liquid separation in the activated sludge process (Xie et al., 2007). The cell surface structure gives information about hydrophobicity and surface charge (Urbain et al., 1993; Xie et al., 2010). The surface charge and zeta potential have great importance for the activated sludge form and stability. Generally, microorganisms have a negative surface charge and negative zeta potential (Daffonchio et al., 1995; Liu and Fang, 2002; Xie et al., 2010). Furthermore, many studies have shown that the protein content in EPS is greater than the carbohydrate content in the activated sludge system and that protein in EPS plays an important role in flocculation (Higgins and Novak, 1997; Liao et al., 2001; Liu and Fang, 2002). The relationship between positively charged proteins and negatively charged carbohydrates affects the zeta potential and hydrophobicity of activated sludge flocs (Liao et al., 2001; Xie et al., 2010). A high P/C ratio leads to less negative charge.
Membrane fouling
The fouling layer leads to heat transfer resistance and probably results in mass transfer resistance, thus explaining the lower flux observed. The contact angles were measured on the virgin and used membrane taken from the MDBR. While the contact angle measured on the virgin membrane was 131.34°, for the used membrane it was 90.10°. It is understood from these values that the hydrophobic property of the membrane decreased over the operation time. Eykens et al. (2017) used three different concentrations of sodium dodecyl sulfate (SDS) solution to investigate effects of water containing oil and surfactant on wettability of three different MD membranes (PE, PES and PTFE) (Eykens et al., 2017). They reported that, as the concentration of SDS increased, the contact angle value for the PE membrane decreased significantly. But in the other two membranes, only slight decreases in the contact angle were observed. Goh et al. (2013b) used PVDF membranes with a contact angle of 126.33±6.15° in their study comparing MD and MDBR systems. They reported that the contact angles of the fouled membranes from MD and MDBR were 32.9° and 59.1°, respectively (Goh et al., 2013b).
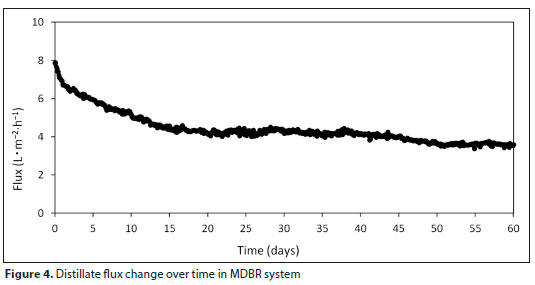
FTIR analysis was performed on virgin and used membranes (Fig. 6) in MDBR, which were evaluated comparatively.
The peaks in the 3 400-3 300 cm-1 band show the O-H and N-H bonds (Goh et al., 2013). These peaks are generally an indication of the presence of humic substances, carbohydrates and proteins (Goh et al., 2013). This clearly reveals that the used membrane in the system was fouled by protein and carbohydrate. The peaks seen in the 2 800-3 000 cm-1 band indicate fatty acids attached to the membrane surface (Schmitt and Flemming, 1998). The peaks seen in the 950-1 480 cm-1 band arise from the presence of carbohydrates (Goh et al., 2013b). In the FTIR measurements made on the used membrane, peaks in the 1 600 cm-1 band indicating C=O were also found. These peaks originate from amides (Schmitt and Flemming, 1998). The peak in the 1 100-1 090 cm-1 band shows the bond C=C. In addition, peaks were determined for the C-N, PO43-, C-Cl, and CO32- bonds at 1 180 cm-1, between 1 000-1 100 cm-1, 700615 cm-1, and 870-880 cm-1, respectively. Figure 6 also shows double peaks in the band 650-850 cm-1 for the aromatic C-H bond, which could indicate an aromatic structure on the membrane.
In this study, SEM and EDX analysis were performed on both virgin and used membranes. EDX analysis revealed that significant amounts of Ca, C, O, Na, Fe, F, Mg and Cl were present on the used membrane surface (Fig. 7). Since most of the elements in the fouling layer formed on the membrane were Ca, C and O, it was thought that scaling by CaCO3 formed on the membrane surface and/or pores. Considering the contact angle measurement, SEM images and FTIR results, the fouling on the surface of the used membrane constituted EPS and SMP, and scaling of CaCO3. While the bio-fouling originating from EPS has a compact structure which adversely affects mass transfer, CaCO3 precipitate forms a porous structure that alleviates this negative effect. Therefore, there was not much decrease in the flux.
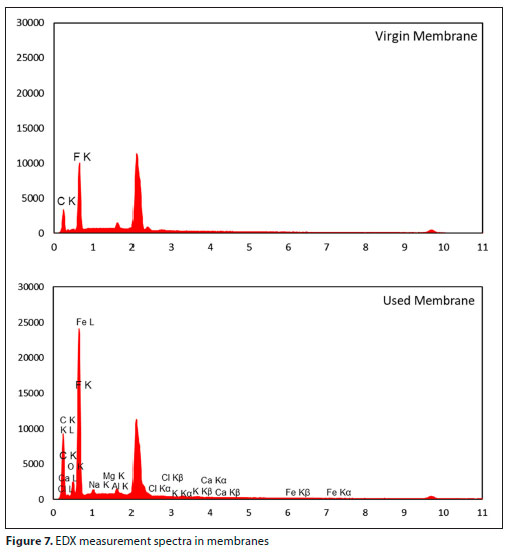
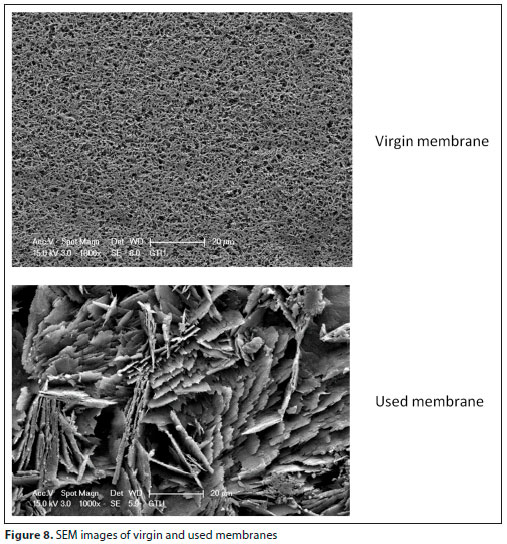
SEM images (Fig. 8) showed that biofilm growth on the membrane surface was limited (Gryta, 2016). However, bio-fouling originating from EPS and CaCO3 accumulation was also present. The SEM image of the membrane (Fig. 8) after the operation of the MDBR system shows a non-uniform coating on the PVDF membrane pores. After 60 days of operation, the fouling layer evidently did not result in significant mass or heat transfer resistance (Fig. 3). The flux in the MDBR process is influenced not just by the fouling layer thickness but also the characteristics of the fouling layer (e.g., structure, composition, coverage, pore blocking). Pore blocking by scalants and protein crystallization has been known to reduce the effective membrane area in MDBR processes, resulting in flux decline (Goh et al., 2013b). It is likely that the bulk of the biofilm may have played a minor role in contributing to the hydraulic resistance while the pore coverage may have accounted for the significant decrease in distillate flux for the MDBR. Khaing et al. (2010) reported that, apart from a significant amount of Ca, the precipitate on the membrane also contained C, O, Na, Mg, and Si, according to SEM and EDX analyses (Khaing et al., 2010).
CONCLUSIONS
In this study, membrane fouling in the MDBR used for treatment of hospital wastewater was investigated. Although the conductivity of thermophilic activated sludge in MDBR was 42.01 mS-cm-1 at the end of 60 days, the maximum conductivity value measured in the distillate was 135 |iS-cm-1. The floc size of the thermophilic activated sludge taken from the reactor was 2.25 μm and the average zeta potential was measured as -14.09 mV. The EDX showed that scaling by CaCO3 occurred on the membrane surface and/or pores. In addition, considering the contact angle measurement, SEM images and FTIR results indicated bio-fouling originating from EPS, having a compact structure which adversely affects mass transfer, while CaCO3 precipitates form a porous structure, alleviating this negative effect. MDBRs hold promise for the future, for high quality water recovery from wastewater by only one process and efficient operation due to low membrane fouling, especially for industries that release waste heat.
ACKNOWLEDGEMENT
This work was supported by The Scientific and Technological Research Council of Turkiye (Grant number 115Y277).
REFERENCES
CHAIZE S and HUYARD A (1991) Membrane bioreactor on domestic wastewater treatment sludge production and modeling approach. Water Res. 23 1591-1600. https://doi.org/10.2166/wst.1991.0613 [ Links ]
CHANG IS and LEE CH (1998) Membrane filtration characteristics in membrane-coupled activated sludge system - the effect of physiological states of activated sludge on membrane fouling. Desalination. 120 (3) 221-233. https://doi.org/10.1016/S0011-9164(98)00220-3. [ Links ]
CLESCERL LS, GREEN AE and EATON AD (1998) Standard Methods for the Examination of Water and Wastewater (20th edn). American Public Health Association, Washington DC. [ Links ]
DAFFONCHIO D, THAVEESRI J and VERSTRAETE W (1995) Contact angle measurement and cell hydrophobicity of granular sludge from upflow anaerobic sludge bed reactors. Applied and Environ. Microbiol. 61 (10) 3676-3680. https://doi.org/10.1128/aem.61.10.3676-3680.1995 [ Links ]
DECAROLIS JF and ADHAM S (2007) Performance investigation of membrane bioreactor systems during municipal wastewater reclamation. Water Environ. Res. 79 (13) 2536-2550. https://doi.org/10.2175/106143007x212184. [ Links ]
EYKENS L, DE SITTER K, DOTREMONT C, DE SCHEPPER W, PINOY L and VAN DER BRUGGEN B (2017) Wetting resistance of commercial membrane distillation membranes in waste streams containing surfactants and oil. Appl. Sci. 7 (2). https://doi.org/10.3390/app7020118 [ Links ]
GOH S, ZHANG Q, ZHANG J, LIU Y and FANE AG (2012) Effects of sludge retention time (SRT) on the performance of high-retention membrane distillation bioreactor (MDBR). Procedia Eng. 44 1831-1834. https://doi.org/10.1016/Lproeng.2012.08.966 [ Links ]
GOH S, ZHANG Q, ZHANG J, MCDOUGALD D, KRANTZ WB, LIU Y and FANE AG (2013a) Impact of a biofouling layer on the vapor pressure driving force and performance of a membrane distillation process. J. Membr. Sci. 438 140-152. https://doi.org/10.1016/j.memsci.2013.03.023 [ Links ]
GOH S, ZHANG J, LIU Y and FANE AG (2013b) Fouling and wetting in membrane distillation (MD) and MD-bioreactor (MDBR) for wastewater reclamation. Desalination. 323 39-47. https://doi.org/10.1016/j.desal.2012.12.001 [ Links ]
GOH S, ZHANG J, LIU Y and FANE AG (2015) Membrane distillation bioreactor (MDBR) - a lower green-house-gas (GHG) option for industrial wastewater reclamation. Chemosphere. 140 129-142. https://doi.org/10.1016/Lchemosphere.2014.09.003 [ Links ]
GRYTA M (2008) Fouling in direct contact membrane distillation process. J. Membr. Sci. 325 (1) 383-394. https://doi.org/10.1016/j.memsci.2008.08.001 [ Links ]
GRYTA M (2016) The application of membrane distillation for broth separation in membrane bioreactors. J. Membr. Sci. Res. 2 (4) 193-200. https://doi.org/10.22079/jmsr.2016.21950 [ Links ]
GRYTA M, TOMASZEWSKA M and KARAKULSKI K (2006) Wastewater treatment by membrane distillation. Desalination. 198 (1-3) 67-73. https://doi.org/10.1016/Ldesal.2006.09.010 [ Links ]
HAI FI and YAMAMOTO K (2011) Membrane biological reactors. Treatise Water Sci. 4 571-613. https://doi.org/10.1016/B978-0-444-53199-5.00096-8 [ Links ]
HIGGINS MJ and NOVAK JT (1997) Characterization of exocellular protein and its role in bioflocculation. J. Environ. Eng. 123 (5) 479-485. https://doi.org/10.1061/(ASCE)0733-9372(1997)123:5(479) [ Links ]
INCE M and TOPALOGLU A (2018) Impacts of sludge retention time on membrane fouling in thermophilic MBR. Membr. Water Treat. 9 (4) 245-253. https://doi.org/10.12989/mwt.2018.9.4.245 [ Links ]
JACOB P, PHUNGSAI P, FUKUSHI K and VISVANATHAN C (2015) Direct contact membrane distillation for anaerobic effluent treatment. J. Membr. Sci. 475 330-339. https://doi.org/10.1016/j.memsci.2014.10.021 [ Links ]
KHAING TH, LI J, LI Y, WAI N and WONG FS (2010) Feasibility study on petrochemical wastewater treatment and reuse using a novel submerged membrane distillation bioreactor. Sep. Purif. Technol. 74 (1) 138-143. https://doi.org/10.1016/j.seppur.2010.05.016 [ Links ]
LAY WCL, LIU Y and FANE AG (2010) Impacts of salinity on the performance of high retention membrane bioreactors for water reclamation: A review. Water Res. 44 (1) 21-40. https://doi.org/10.1016/j.watres.2009.09.026 [ Links ]
LI XY and YANG SF (2007) Influence of loosely bound extracellular polymeric substances (EPS) on the flocculation, sedimentation and dewaterability of activated sludge. Water Res. 41 (5) 1022-1030. https://doi.org/10.1016/jwatres.2006.06.037 [ Links ]
LIAO BQ, ALLEN DG, DROPPO IG, LEPPARD GG and LISS SN (2001) Surface properties of sludge and their role in bioflocculation and settleability. Water Res. 35 (2) 339-350. https://doi.org/10.1016/S0043-1354(00)00277-3 [ Links ]
LIU H and FANG HHP (2002) Extraction of extracellular polymeric substances (EPS) of sludges. J. Biotechnol. 95 (3) 249-256. https://doi.org/10.1016/S0168-1656(02)00025-1 [ Links ]
LÜBBECKE S, VOGELPOHL A and DEWJANIN W (1995) Wastewater treatment in a biological high-performance system with high biomass concentration. Water Res. 29 (3) 793-802. https://doi.org/10.1016/0043-1354(94)00215-S [ Links ]
MULLER EB, STOUTHAMER AH, VAN VERSEVELD HW and EIKELBOOM DH (1995) Aerobic domestic waste water treatment in a pilot plant with complete sludge retention by cross-flow filtration. Water Res. 29 (4) 1179-1189. https://doi.org/10.1016/0043-1354(94)00267-B [ Links ]
NAWAZ MS, GADELHA G, KHAN SJ and HANKINS N (2013) Microbial toxicity effects of reverse transported draw solute in the forward osmosis membrane bioreactor (FO-MBR). J. Membr. Sci. 429 323-329. https://doi.org/10.1016/j.memsci.2012.11.057 [ Links ]
PHATTARANAWIK J, FANE AG, PASQUIER ACS and BING W (2008) A novel membrane bioreactor based on membrane distillation. Desalination. 223 (1-3) 386-395. https://doi.org/10.1016ZJ.DESAL.2007.02.075 [ Links ]
PHATTARANAWIK J, FANE AG, PASQUIER ACS, BING W and WONG FS (2009) Experimental study and design of a submerged membrane distillation bioreactor. Chem. Eng. Technol. 32 (1) 38-44. https://doi.org/10.1002/ceat.200800498 [ Links ]
QIU G and TING YP (2014) Direct phosphorus recovery from municipal wastewater via osmotic membrane bioreactor (OMBR) for wastewater treatment. Bioresour. Technol. 170 221-229. https://doi.org/10.1016/j.biortech.2014.07.103 [ Links ]
SANIN SL, SANIN FD and BRYERS JD (2003) Effect of starvation on the adhesive properties of xenobiotic degrading bacteria. Process Biochem. 38 (6) 909-914. https://doi.org/10.1016/S0032-9592(02)00173-5 [ Links ]
SCHMITT J and FLEMMING H (1998) FTlR-spectroscopy in microbial and material analysis. Int. Biodeterior. Biodegrad. 41 1-11. https://doi.org/10.1016/S0964-8305(98)80002-4 [ Links ]
TAY JH, LIU QS and LIU Y (2001) The role of cellular polysaccharides in the formation and stability of aerobic granules. Lett. Appl. Microbiol. 33 (3) 222-226. https://doi.org/10.1046/j.1472-765X.2001.00986.x [ Links ]
TCHOBANOGLOUS G and BURTON FL (1991) Wastewater Engineering Treatment, Disposal, Reuse. McGraw Hill, Metcalf and Eddy, New York. [ Links ]
TIJING LD, WOO YC, CHOI JS, LEE S, KIM SH and SHON HK (2015) Fouling and its control in membrane distillation-A review. J. Membr. Sci. 475 215-244. https://doi.org/10.1016/j.memsci.2014.09.042 [ Links ]
TINGGANG L, RENBI B and JUNXIN L (2008) Distribution and composition of extracellular polymeric substances in membrane-aerated biofilm. J. Biotechnol. 135 (1) 52-57. https://doi.org/10.1016/j.jbiotec.2008.02.011 [ Links ]
URBAIN V, BLOCK JC and MANEM J (1993) Bioflocculation in activated sludge: an analytic approach. Water Res. 27 (5) 829-838. https://doi.org/10.1016/0043-1354(93)90147-A [ Links ]
WANG P and CHUNG TS (2015) Recent advances in membrane distillation processes: Membrane development, configuration design and application exploring. J. Membr. Sci. 474 39-56. https://doi.org/10.1016/j.memsci.2014.09.016 [ Links ]
WIJEKOON KC, HAI FI, KANG J, PRICE WE, GUO W, NGO HH, CATH TY and NGHIEM LD (2014) A novel membrane distillation-thermophilic bioreactor system: Biological stability and trace organic compound removal. Bioresour. Technol. 159 334-341. https://doi.org/10.10167j.biortech.2014.02.088 [ Links ]
XIE B, GU J and LU J (2010) Surface properties of bacteria from activated sludge in relation to bioflocculation. J. Environ. Sci. 22 (12) 1840-1845. https://doi.org/10.1016/S1001-0742(09)60329-6 [ Links ]
XIE RJ, GOMEZ MJ and XING YJ (2007) Field investigation of advanced oxidation of secondary effluent from municipal wastewater treatment plant. J. Environ. Sci. Health A. 42 (13) 2047-2057. https://doi.org/10.1080/10934520701629823 [ Links ]
ZHANG T, KE SZ, LIU Y and FANG HP (2005) Microbial characteristics of a methanogenic phenol-degrading sludge. Water Sci. Technol. 52 (1-2) 73-78. https://doi.org/10.2166/wst.2005.0500 [ Links ]
ZUO J, BONYADI S and CHUNG TS (2016) Exploring the potential of commercial polyethylene membranes for desalination by membrane distillation. J. Membr. Sci. 497 239-247. https://doi.org/10.1016/j.memsci.2015.09.038 [ Links ]
 Correspondence:
Correspondence:
Elif ince
Email: e.senturk@gtu.edu.tr
Received: 6 July 2021
Accepted: 22 September 2022














