Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Water SA
On-line version ISSN 1816-7950
Print version ISSN 0378-4738
Water SA vol.48 n.3 Pretoria Jul. 2022
http://dx.doi.org/10.17159/wsa/2022.v48.i3.3892
RESEARCH PAPER
Recovery of enteroviruses and poliovirus in Harare sewage using the bag-mediated filtration system at the introduction of the inactivated polio vaccine in Zimbabwe
Vurayai RuhanyaI; Nicolette A ZhouII; Chipo BerejenaI; George NyandoroI; Paradzai ChibukiraI; Arnold MukaratirwaI; Simon Takawira MuserereIII; Kudzai MasundaIII; John Scott MeschkeII
IDepartment of Medical Microbiology, University of Zimbabwe, Harare, Zimbabwe
IIDepartment of Environmental and Occupational Health Sciences, University of Washington, Seattle, USA
IIIHarare City, Old Mutual House, Corner Speke Avenue & Sam Nujoma Street, Harare, Zimbabwe
ABSTRACT
Environmental surveillance is a sensitive method for detecting circulating virus in the absence of clinical cases and is important for monitoring progress for poliovirus (PV) eradication. This study used the bag-mediated filtration system (BMFS) to determine PV and enterovirus (EV) prevalence in sewage at the transition from oral polio vaccine type 2 (OPV2) use to inactivated polio vaccine (IPV) use in Zimbabwe, and examined the correlation between environmental surveillance results and vaccination coverage of OPV. A total of 18 BMFS samples from 6 sampling sites were analysed for the presence of EV and PV via direct RT-qPCR, direct ITD (intratypic differentiation), and the WHO algorithm. EV prevalence in Harare wastewater was 88.9% (16/18) using direct RT-PCR, 61.1% (11/18) using direct ITD, and 77.8% (14/18) using the WHO algorithm. Of the 18 samples analysed using the WHO algorithm, 10 samples (55.6%) were positive for Sabin-like PV type 3 (SL3). Of these 10 samples, 2 were also positive for non-polio enteroviruses (NPEV), resulting in a total of 6 (33.3%) samples positive for NPEV and 4 negative. The sensitivity of isolation in detecting EVs in sewage was 92.9% when comparing direct RT-qPCR results to the WHO algorithm. Using direct ITD, two high-density, low-income sampling sites were negative for SL3 and one low-density, high-income sampling point was negative for SL3 using the WHO algorithm. There was a strong association between relative EV concentration and the number of OPV3 vaccine recipients (r = 0.8590; p = 0.0284) in sampled areas. This study demonstrated the ability of BMFS to detect PVs circulating in Harare wastewater at the beginning of the OPV-IPV switch and can be used to monitor potential reintroduction of wild PV or vaccine-derived PVs from endemic areas.
Keywords: BMFS, enteroviruses, poliovirus, wastewater, environmental surveillance
INTRODUCTION
The World Health Organization Regional Office for Africa (WHO-AFRO) has made tremendous progress towards eradication of wild poliovirus (WPV) through the Polio Eradicationand Endgame Strategic plan. Cases of WPV types 2 and 3 (WPV2 and WPV3) were last reported in 1998 and 2012, respectively, and WPV type 1 reported in Nigeria in 2014 has been the last in the African Region (Okeibunor et al., 2017), leading to the WHO African Region being declared wild polio-free in 2020 (WHO, 2020). WPV2 was declared eradicated in 2015 (GPEI, 2015) and different parts of the world are at various stages of switching from the trivalent oral polio vaccine (tOPV) to bivalent OPV (bOPV). The use of OPV2 in WPV2-free areas creates the risk of sporadic vaccine-associated paralytic polio (VAPP) due to OPV2 in vaccinated children or unvaccinated contacts. During replication and transmission, vaccine stains can undergo mutations resulting in vaccine-derived polioviruses (VDPVs) which have reverted to increased neurovirulence. Monitoring data from the Global Polio Eradication Initiative (GPEI) indicates that over 90% of paralytic cases between 2000 and 2012 were due to circulating VDPVs and 40% of VAPP cases were derived from OPV2. This justifies the removal of OPV2 from trivalent OPV and subsequent introduction of the inactivated polio vaccine (IPV) in routine immunization.
In Zimbabwe, poliomyelitis was controlled mostly through the use of the tOPV until April 2019 when IPV was introduced in addition to the bOPV. No WPV had been isolated in Zimbabwe since 1990 using clinical surveillance for acute flaccid paralysis (AFP). In the context of polio eradication, IPV is introduced prior to the cessation of OPV to minimize the spread of virus to susceptible population (Maes et al., 2017). Therefore, in the transitional period, there is a need to determine whether there is silent circulation of PV in the population due to importation or VDPVs (Baicus, 2012). Environmental surveillance is a sensitive method for detecting low level and silent circulation of PV (Hovi et al., 2012; Asghar et al., 2014; Lopalco, 2017). Moreover, PV vaccine strains are detected frequently in environmental waters as they are shed in faeces after administration of the live attenuated OPV (Laassri et al., 2005). Environmental surveillance then becomes critical for monitoring a decline in, or disappearance of, the vaccine Sabin-like (SL) PV strains, which is particularly relevant to ongoing polio endgame vaccine policy changes.
However, the low level concentration of PV and other enteroviruses in environmental samples poses a challenge in the recovery of the viruses from these matrices for detection. The process can be complex as large volumes are concentrated via a primary and/or secondary concentration method for detection by the WHO algorithm, which involves cell culture amplification and intratypic differentiation (ITD) by real-time reverse transcription polymerase chain reaction (rRT-PCR). We employed the easy-to-use bag mediated filtration system (BMFS), which has an electricity-free primary concentration step and a skimmed milk flocculation secondary concentration step (Fagnant et al., 2014, 2018) to determine the prevalence of PVs in sewage water from 6 locations in Harare, Zimbabwe, at the beginning of the OPV-IPV transitional period. The rationale was to determine the presence or absence of silent PV circulation in the population and assess whether environmental surveillance can be used to evaluate OPV at the onset of IPV-OPV transitional period. Additionally, correlation between environmental surveillance results and vaccine coverage was examined using OPV, which is used to assess the performance of vaccination delivery systems (Mosser et al., 2019).
METHODS
Site selection
Wastewater samples were collected from 19 April 2019 to 9 May 2019 at 6 sites in Harare, Zimbabwe (n = 18). Three sampling sites, namely, Budiriro, Budiriro West, and Shelter Zimbabwe were in high-density suburbs (low-income residence), while the other three sites, Avonlea, Northeastern and New Marlborough, were in low-density suburbs (high-income residence). All 18 samples were collected from manholes close to the pump stations. Permission to conduct this study was granted by the Harare City Council Department of Water and the Department of Health.
Sample collection and processing
BMFS, a recently described method for the recovery and concentration of viruses from water, was used (Fagnant et al., 2018; Zhou et al., 2018; Van Zyl et al., 2019). This study utilized BMFS v2. Filtration was performed at the sampling sites, and filters were transported to the University of Zimbabwe Virology Laboratory for elution and secondary concentration. Filters were processed by two 15-min elutions with a 150-mL eluent volume for a total of 300 mL entering secondary concentration (Fagnant et al., 2017). Secondary concentration was performed via skimmed milk flocculation (Fagnant et al., 2017) and the final pellets were resuspended in a volume of 10 mL PBS. Resuspended BMFS pellets were chloroform extracted.
Vaccination data
To determine the relationship between polio vaccine uptake and enteroviruses detected in sewage, vaccine data for the pump station catchment areas during the sampling period were retrieved from Harare City council records. Immunization data were collected for OPV1, OPV2, and OPV3.
Sample assay
Samples were analysed by three methods: direct RT-qPCR for enterovirus (direct EV RT-qPCR), direct rRT-PCR using ITD for enterovirus and PV (direct EV or PV ITD), and by the WHO Poliovirus Isolation Algorithm (WHO algorithm).
Direct RT-qPCR for enterovirus and poliovirus
To prepare samples for analysis by direct RT-qPCR, secondary concentrate aliquots were extracted via Qiagen All Prep Power Fecal DNA/RNA Kit according to the manufacturer's instructions (Qiagen, Hilden, Germany). The input to the extraction kit was 200 µL of the resuspended secondary concentration pellet, and the elution volume was 60 µL. Enteroviruses were detected using published primers (Schwab et al., 1995) with the following sequences: forward primer 5'CCT CCG GCC CCT GAA TG3', reverse primer 5'ACC GGA TGG CCA ATC CAA3', and probe FAM-TAC TTT GGG TGT CCG TGT TTC-BHQ using 5 µL RNA in a 20 µL reaction (hereafter, direct EV RT-qPCR) using qScript XLT 1-Step RT-qPCR Tough Mix (Quantabio, Beverly, MA, USA). RNA extracts were also analysed directly using the PanEnterovirus and SL PV type 3 (SL3) assays from the Poliovirus ITD 5.0 rRT-PCR Kit (Centers for Disease Control and Prevention [CDC], Atlanta, GA, USA) using 1 µL RNA in a 20 µL reaction (Gerloff et al., 2018) (hereafter, direct EV or PV ITD).
WHO algorithm
Samples were analysed using the WHO algorithm by virus isolation using L20B and human rhabdomyosarcoma (RD) cells followed by ITD using rRT-PCR (WHO, 2015). Samples positive for cytopathic effects on L20B and RD cells were screened by ITD on an Applied Biosystems 7500 Thermocycler (Applied Biosystems, CA, USA) (Gerloff et al., 2018) for SL PV types 1, 2, and 3, WPV1 and 3, and VDPVs.
Statistical analysis
Correlations between environmental EVs/PVs and OPV coverage in sampled areas were completed using Pearson correlation and the respective proportionate distribution comparisons in Stata 12 statistical package. Results were presented in visual graphics and simplified tables.
RESULTS
Detection of enteroviruses
The overall prevalence of enteroviruses in Harare wastewater was 89.9% when measured via direct EV RT-qPCR and 61.1% when measured via direct EV ITD (Table 1). Four suburbs, namely, Budiriro, Budiriro West, Shelter Zimbabwe, and New Malborough, yielded 100% positivity of enterovirus using direct EV RT-qPCR, while Avonlea and North-Eastern had prevalence of 66.7% each. Considering locations, all 9 samples from the high-density suburbs were positive for enteroviruses. Seven of the nine (77.8%) samples from low densities were positive for enteroviruses. Using the direct EV ITD method, all 9 samples from the low density suburbs were positive for enteroviruses, while 22.2% of sample from high-density sites were positive. The difference in results between the two detection methods could be due to the primers and probes used, the difference in reaction input volume, and the inherent variability and low virus concentration in environmental samples.
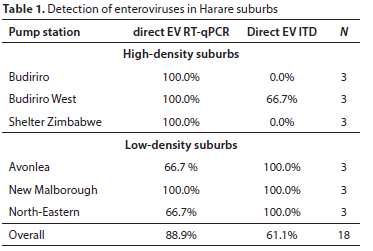
The Ct values measured via direct EV RT-qPCR or direct EV ITD represent the relative concentration of enteroviruses at the different sites (Table 2). The direct EV RT-qPCR results showed that on average the high-density suburbs had lower enterovirus concentrations than the low-density suburbs, with the highest enterovirus concentration in Avonlea and lowest in Budiriro.
The WHO algorithm for virus isolation was performed on the environmental samples to determine the prevalence of viable enteroviruses (Fig. 1). The prevalence of enteroviruses in high density suburbs was 100% when measured via the WHO algorithm and by direct EV RT-qPCR. In the low density suburbs however, the prevalence dropped from 77.8% when measured by direct EV RT-qPCR to 55.6% when measured by the WHO algorithm, with the prevalence remaining at 66.7% in both Avonlea and North-Eastern suburb and dropping from 100% to 33.3% at Marlborough.
The concentration of enteroviruses was high in these samples (Ct values were low) and direct EV RT-qPCR results significantly correlated with absolute numbers of monovalent OPV1 (0.8585, p = 0.0286), OPV2 (0.8250, p = 0.0432) and OPV3 (0.8590, p = 0.0284) vaccine recipients.
PV detection in BMFS samples analysed by the WHO algorithm and direct ITD
Using the WHO algorithm, the overall prevalence of PV was 55.6% and the prevalence of non-polio enteroviruses (NPEV) was 33.3%, with 11% of samples detecting both PV and NPEV (Fig. 2, Table 3). No virus was detected in 22.2% of samples. Four (67%) of the six NPEVs were detected in wastewater samples from high-density suburbs.
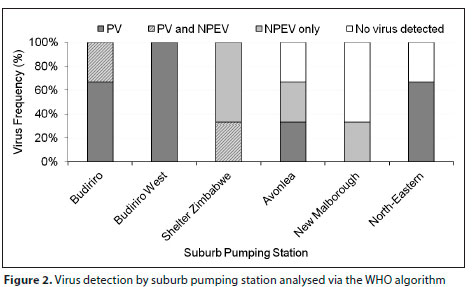
Using direct PV ITD, SL3 was detected in four of the six sampling locations with overall prevalence of 44.4% (Table 3). A summary of the ITD results, both direct PV ITD and following cell culture (WHO algorithm), is shown in Table 3. No VDPVs, WPVs, SL1, or SL2 were detected in the sampled locations. Using the WHO algorithm as the gold standard for detection of enteroviruses, the sensitivity of direct EV RT-qPCR was 92.9% and specificity was 25% while the sensitivity of direct EV ITD was 50% and specificity was 0% (Table A1, Appendix). Additionally, using the WHO algorithm as the gold standard for detection of SL3, the sensitivity of direct PV ITD was 50% and specificity was 62.5%.
DISCUSSION
Zimbabwe is regarded as free from WPV, since the last clinical cases were isolated in 1991 (GPEI, no date; CDC, 2003). In line with the GPEI, which has certified WPV serotype eradication in 2015, Zimbabwe is at transitional phase of OPV-2 cessation and introduction of IPV. The rationale for introduction of IPV prior to the cessation of OPV-2 is to interrupt the possibility of continued silent circulation and potential risk of VDPV2 or WPV2 outbreaks. Therefore, a systematic study of sewage samples is important for identifying the possibility of silent circulation which could arise from importations by travel to and from endemic countries. This study has demonstrated the ability to utilize the BMFS to determine whether the circulating enteroviruses excreted in sewage by Harare residents contain PV (Troy et al., 2013).
WPV was not detected in sewage samples from high-density or low-density suburbs in Harare. SL3 was detected at only four sampling sites using direct ITD. However, five sampling sites were positive via the WHO algorithm for SL3, implying that the vaccine strains were viable, though at low concentrations. This study has enabled us to determine baseline PV presence at the beginning of the OPV-IPV switch. The results suggest there has not been circulating VDPV or reintroduction of WPVs. More importantly, the application of BMFS, which can be done in the field by gravity, in EV and PV surveillance using large volumes is suitable for supplementing AFP surveillance in settings where power shortages are a huge problem. In the context of GPEI, BMFS-assisted EV and PV surveillance could play a critical role during the period between interruption of WPV transmission and certification of polio eradication in resource-limited countries like Zimbabwe. It also would play an important role for continuous monitoring of the sewage for VDPVs, re-emergence of WPVs, or disappearance of all OPV-related strains after OPV cessation (Hovi et al., 2012). EV detection or environmental surveillance may also provide earlier identification of PV circulation in a community before illness occurs and allow intervention before paralytic cases of polio occur, like the silent polio outbreak in Rahat, Israel, in 2013 (Brouwer et al., 2018). The cost of the BMFS kits is available on request from Scientific Methods (https://www.scientificmethods.com/bmfs), which commercially produces the kit. At the time of this study, the BMFS Field Sampling Kits were purchased for 75.79 USD each. When identifying a concentration method, costs to consider include the opportunity cost associated with the ability to analyse for multiple targets from a single sample type, reusable and consumable items, and field and laboratory personnel costs. It has also been observed that the BMFS performs similar to or better than the WHO two-phase separation method (Zhou et al., 2018; Fagnant-Sperati et al., 2020).
The efficient BMFS filtration is supposed to be followed by a sensitive detection method. This study used three detection protocols: direct RT-qPCR, direct ITD, and the WHO algorithm. Direct EV RT-qPCR detected 11.1% more EVs than the WHO algorithm, and 27.8% more EVs than direct EV ITD. Although the isolation results detected less EVs than direct EV RT-qPCR, they have the advantage of differentiating suspected PV from NPEV and have demonstrated that the suspected PV were viable. The fact that all high-density areas were positive for EV via the WHO algorithm and low-density areas reported 33.3% to 66.7% positive detection indicated that some viruses might have lost viability due to better water, sanitation and hygiene (WASH) services in these areas. There was a higher frequency of NPEV in the high-density suburbs, which points to poor hygiene conditions in high-density areas. Therefore, the results of this study also serve as a preliminary demonstration of BMFS-assisted EV environmental surveillance to evaluate WASH services. Depending on the concentration, the chances of finding infectious viruses in the effluent depends on the type of wastewater treatment (Sima et al., 2011; Simmons et al., 2011; Francy et al., 2012). The potential for detecting infectious viruses in source waters in Harare was very high since the pump stations were under repair and the raw wastewater was discharged into the rivers.
We also observed that using the WHO algorithm as the gold standard, direct EV RT-qPCR showed very high sensitivity of 92%, suggesting the BMFS is compatible with both detection methods. ITD results showed that the suspected PV from isolation were SL3. Comparison of detection rates showed that one sampling site, Budiriro, was negative for SL3 when measured via direct PV ITD but was positive using the WHO algorithm. The Budiriro sample was the most turbid and had the longest filtration duration (80 min). There could be some inhibitors in the samples that could have interfered with molecular detection (Ruhanya et al., 2015; Rodríguez-Lázaro et al., 2012). Therefore, it is important to account and adjust for inhibition when reporting RT-qPCR results for viral pathogens in environmental samples, to avoid underestimating target concentration and false negatives (Gibson et al., 2012). The WHO algorithm gave a negative result for New Marlborough but direct ITD gave positive results for both EVs and PVs. It was likely the viability of the viruses was affected by better WASH services in the low-density areas or suburbs. When choosing between detection methods, cost, ease-of-use, and sensitivity should be considered. Direct detection methods are less costly and simpler to conduct than the WHO algorithm as they do not include tissue culture. However, the WHO algorithm has greater poliovirus detection sensitivity, which should be factored in when choosing a method for a specific site and use case. BMFS EVs/PV surveillance could consider either direct ITD or virus isolation methods in order to accurately estimate public health risk while factoring in personnel and cost constraints.
Previous studies have demonstrated a rapid decline in PV isolation around the OPV-IPV transition period, with a disappearance of PV vaccine strains from wastewater within 2 to 3 months after the cessation of OPV administration (Huang et al., 2005). Some studies have reported the shortening of the duration and quantity of PV shedding after the introduction of IPV (Mueller et al., 2009). Our study did not detect SL1 or SL2 but did detect SL3 at the beginning of the OPV2-IPV transitional period. The introduction of IPV might have had an impact on the shedding and duration or persistence of the vaccine serotypes in the sewage. Previous studies reported the disappearance of SL PVs from the environment before OPV immunization had ceased (Nakamura et al., 2015; Fagnant-Sperati et al., 2020).
Our study reported a strong and significant association between relative concentrations of EVs detected in sewage and the absolute numbers of children vaccinated in suburbs seeding sewage to sampled pump stations. These findings imply that the shedding of enteroviruses into sewage is related to the shedding of SL strains and, in the case of vaccines, is an indicator of the number of people vaccinated who eventually shed the vaccine strains in the environment. However, the number of vaccine recipients was higher in high-density suburbs than low-density suburbs, while the relative EV concentrations were higher in low-density suburbs than in high-density suburbs. These results could be explained by inhibition in samples from one type of location but not the other, the overall catchment population contributing to these sampling sites, and/or the wastewater flow at the sites. Unfortunately, this information is not available but should be included in future work. More data is required for association studies between EVs in the sewage and vaccine uptake covering a period of time.
CONCLUSION
The study demonstrated that BMFS can be applicable for surveillance of enteroviruses and is compatible with both the WHO algorithm and direct RT-qPCR. In the context of GPEI, the tool can be used to detect silent PV circulation, assess vaccination efficiency, and the disappearance of OPV strains in the environment in the endgame strategy.
ACKNOWLEDGEMENTS
We thank field, technical and support staff of Harare City Council and the National Virology Lab for their assistance in sampling. This study was supported in part by the UW NIEHS sponsored Biostatistics, Epidemiologic and Bioinformatics Training in Environmental Health (BEBTEH) Training Grant #: NIEHS T32ES015459 and the UW DEOHS Environmental Health Microbiome Initiative.
AUTHOR CONTRIBUTIONS
•Conceptualization and methodology of the study: Vurayai Ruhanya, Nicolette Angela Zhou, John Scott Meschke
•Data collection and fieldwork: Vurayai Ruhanya, Chip Berejena, Paradzai Chibukira, Arnold Mukaratirwa, Simon Takawira Muserere, Kudzai Masunda
•Sample/data analysis: Vurayai Ruhanya, Nicolette Angela Zhou, George Nyandoro
•Interpretation of results: Vurayai Ruhanya, Nicolette Angela Zhou
•Writing of the initial draft: Vurayai Ruhanya, Nicolette Angela Zhou
ORCID
John Scott Meschke
https://orcid.org/0000-0002-8983-9042
REFERENCES
ASGHAR H, DIOP OM, WELDEGEBRIEL G, MALIK F, SHETTY S, EL BASSIONI L, AKANDE AO, AL MAAMOUN E, ZAIDI S, ADENIJI AJ, and co-authors (2014) Environmental surveillance for polioviruses in the global polio eradication initiative. J. Infect. Dis. 210 (suppl_1) S294-S303. https://doi.org/10.1093/infdis/jiu384 [ Links ]
BAICUS A (2012) History of polio vaccination. World J. Virol. 1 (4) 108-114. https://doi.org/10.5501/wjv.v1.i4.108 [ Links ]
BROUWER AF, MASTERS NB and EISENBERG JNS (2018) Quantitative microbial risk assessment and infectious disease transmission modeling of waterborne enteric pathogens. Curr. Environ. Health Rep. 5 (2) 293-304. https://doi.org/10.1007/s40572-018-0196-x [ Links ]
CDC (2003) Progress toward poliomyelitis eradication --- Southern Africa, 2001--March 2003. https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5222a3.htm (Accessed 24 December 2020). [ Links ]
FAGNANT CS, BECK NK, YANG M-F, BARNES KS, BOYLE DS and MESCHKE JS (2014) Development of a novel bag-mediated filtration system for environmental recovery of poliovirus. J. Water Health. 12 (4) 747-754. https://doi.org/10.2166/wh.2014.032 [ Links ]
FAGNANT CS, SÁNCHEZ-GONZALEZ LM, ZHOU NA, FALMAN JC, EISENSTEIN M, GUELIG D, OCKERMAN B, GUAN Y, KOSSIK AL, LINDEN YS and co-authors (2018) Improvement of the bag-mediated filtration system for sampling wastewater and wastewater-impacted waters. Food Environ. Virol. 10 (1) 72-82. https://doi.org/10.1007/s12560-017-9311-7 [ Links ]
FAGNANT CS, TOLES M, ZHOU NA, POWELL J, ADOLPHSEN J, GUAN Y, OCKERMAN B, SHIRAI JH, BOYLE DS, NOVOSSELOV I and co-authors (2017) Development of an elution device for ViroCap virus filters. Environ. Monit. Assess. 189 (11) 574. https://doi.org/10.1007/s10661-017-6258-y [ Links ]
FAGNANT-SPERATI CS, REN Y, ZHOU NA, KOMEN E, MWANGI B, HASSAN J, CHEPKURUI A, NZUNZA R, NYANGAO J, VAN ZYL WB and co-authors (2020) Validation of the bag-mediated filtration system for environmental surveillance of poliovirus in Nairobi, Kenya. J. Appl. Microbiol. https://doi.org/10.1111/jam.14807 [ Links ]
FRANCY DS, STELZER EA, BUSHON RN, BRADY AMG, WILLISTON AG, RIDDELL KR, BORCHARDT MA, SPENCER SK and GELLNER TM (2012) Comparative effectiveness of membrane bioreactors, conventional secondary treatment, and chlorine and UV disinfection to remove microorganisms from municipal wastewaters. Water Res. 46 (13) 4164-4178. https://doi.org/10.1016/j.watres.2012.04.044 [ Links ]
GERLOFF N, SUN H, MANDELBAUM M, MAHER C, NIX WA, ZAIDI S, SHAUKAT S, SEAKAMELA L, NALAVADE UP, SHARMA DK and co-authors (2018) Diagnostic assay development for poliovirus eradication. J. Clin. Microbiol. 56 (2). https://doi.org/10.1128/JCM. 01624-17 [ Links ]
GIBSON KE, SCHWAB KJ, SPENCER SK and BORCHARDT MA (2012) Measuring and mitigating inhibition during quantitative real time PCR analysis of viral nucleic acid extracts from large-volume environmental water samples. Water Res. 46 (13) 4281-4291. https://doi.org/10.1016/j.watres.2012.04.030 [ Links ]
GPEI (2015) GPEI-Global eradication of wild poliovirus type 2 declared. https://polioeradication.org/news-post/global-eradication-of-wild-poliovirus-type-2-declared/ (Accessed 24 December 2020). [ Links ]
GPEI (no date) GPEI-Polio-Free Countries. https://polioeradication.org/where-we-work/polio-free-countries/ (Accessed 24 December 2020). [ Links ]
HOVI T, SHULMAN LM, VAN DER AVOORT H, DESHPANDE J, ROIVAINEN M and DE GOURVILLE EM (2012) Role of environmental poliovirus surveillance in global polio eradication and beyond. Epidemiol. Infect. 140 (1) 1-13. https://doi.org/10.1017/S095026881000316X [ Links ]
HUANG QS, GREENING G, BAKER MG, GRIMWOOD K, HEWITT J, HULSTON D, VAN DUIN L, FITZSIMONS A, GARRETT N, GRAHAM D, and co-authors (2005) Persistence of oral polio vaccine virus after its removal from the immunisation schedule in New Zealand. Lancet (London, England). 366 (9483) 394-396. https://doi.org/10.1016/S0140-6736(05)66386-6 [ Links ]
LAASSRI M, LOTTENBACH K, BELSHE R, WOLFF M, RENNELS M, PLOTKIN S and CHUMAKOV K (2005) Effect of different vaccination schedules on excretion of oral poliovirus vaccine strains. J. Infect. Dis. 192 (12) 2092-2098. https://doi.org/10.1086/498172 [ Links ]
LOPALCO PL (2017) Wild and vaccine-derived poliovirus circulation, and implications for polio eradication. Epidemiol. Infect. 145 (3) 413-419. https://doi.org/10.1017/S0950268816002569 [ Links ]
MAES EF, DIOP OM, JORBA J, CHAVAN S, TANGERMANN RH and WASSILAK SGF (2017) Surveillance systems to track progress toward polio eradication - worldwide, 2015-2016. Morb. Mortal. Wkly Rep. 66. https://doi.org/10.15585/mmwr.mm6613a3 [ Links ]
MOSSER JF, GAGNE-MAYNARD W, RAO PC, OSGOOD-ZIMMERMAN A, FULLMAN N, GRAETZ N, BURSTEIN R, UPDIKE RL, LIU PY, RAY SE, and co-authors (2019) Mapping diphtheria-pertussis-tetanus vaccine coverage in Africa, 2000-2016: a spatial and temporal modelling study. The Lancet. 393 (10183) 1843-1855. https://doi.org/10.1016/S0140-6736(19)30226-0 [ Links ]
MUELLER JE, BESSAUD M, HUANG QS, MARTINEZ LC, BARRIL PA, MOREL V, BALANANT J, BOCACAO J, HEWITT J, GESSNER BD, and co-authors (2009) Environmental poliovirus surveillance during oral poliovirus vaccine and inactivated poliovirus vaccine use in Córdoba Province, Argentina. Appl. Environ. Microbiol. 75 (5) 1395-1401. https://doi.org/10.1128/AEM.02201-08 [ Links ]
NAKAMURA T, HAMASAKI M, YOSHITOMI H, ISHIBASHI T, YOSHIYAMA C, MAEDA E, SERA N and YOSHIDA H (2015) Environmental surveillance of poliovirus in sewage water around the introduction period for inactivated polio vaccine in Japan. Appl. Environ. Microbiol. 81 (5) 1859-1864. https://doi.org/10.1128/AEM.03575-14 [ Links ]
OKEIBUNOR JC, OTA MC, AKANMORI BD, GUMEDE N, SHABA K, KOUADIO KI, POY A, MIHIGO R, SALLA M and MOETI MR (2017) Polio eradication in the African Region on course despite public health emergencies. Vaccine. 35 (9) 1202-1206. https://doi.org/10.1016/j.vaccine.2015.08.024 [ Links ]
RODRÍGUEZ-LÁZARO D, COOK N, RUGGERI FM, SELLWOOD J, NASSER A, NASCIMENTO MSJ, D'AGOSTINO M, SANTOS R, SAIZ JC, RZEŻUTKA A and co-authors (2012) Virus hazards from food, water and other contaminated environments. FEMS Microbiol. Rev. 36 (4) 786-814. https://doi.org/10.1111/j.1574-6976.2011.00306.x [ Links ]
RUHANYA V, DIEZ-VALCARCE M, D'AGOSTINO M, COOK N, HERNÁNDEZ M and RODRÍGUEZ-LÁZARO D (2015) Monitoring of extraction efficiency by a sample process control virus added immediately upon sample receipt. Food and Environmental Virology. 7 (4) 413-416. https://doi.org/10.1007/s12560-015-9214-4 [ Links ]
SCHWAB KJ, DE LEON R and SOBSEY MD (1995) Concentration and purification of beef extract mock eluates from water samples for the detection of enteroviruses, hepatitis A virus, and Norwalk virus by reverse transcription-PCR. Appl. Environ. Microbiol. 61 (2) 531-537. [ Links ]
SIMA LC, SCHAEFFER J, SAUX J-CL, PARNAUDEAU S, ELIMELECH M and GUYADER FSL (2011) Calicivirus removal in a membrane bioreactor wastewater treatment plant. Appl. Environ. Microbiol. 77 (15) 5170-5177. https://doi.org/10.1128/AEM.00583-11 [ Links ]
SIMMONS FJ, KUO DH-W and XAGORARAKI I (2011) Removal of human enteric viruses by a full-scale membrane bioreactor during municipal wastewater processing. Water Res. 45 (9) 2739-2750. https://doi.org/10.1016/j.watres.2011.02.001 [ Links ]
TROY SB, MUSINGWINI G, HALPERN MS, HUANG C, STRANIX-CHIBANDA L, KOUIAVSKAIA D, SHETTY AK, CHUMAKOV K, NATHOO K and MALDONADO YA (2013) Vaccine poliovirus shedding and immune response to oral polio vaccine in HIV-infected and -uninfected Zimbabwean infants. J. Infect. Dis. 208 (4) 672-678. https://doi.org/10.1093/infdis/jit208 [ Links ]
VAN ZYL WB, ZHOU NA, WOLFAARDT M, MATSAPOLA PN, NGWANA FB, SYMONDS EM, FAGNANT-SPERATI CS, SHIRAI JH, KOSSIK AL, BECK NK, and co-authors (2019) Detection of potentially pathogenic enteric viruses in environmental samples from Kenya using the bag-mediated filtration system. Water Supply. 19 (6) 1668-1676. https://doi.org/10.2166/ws.2019.046 [ Links ]
WHO (2020) Global polio eradication initiative applauds WHO African region for wild polio-free certification. https://www.who.int/news/item/25-08-2020-global-polio-eradication-initiative-applauds-who-african-region-for-wild-polio-free-certification (Accessed 14 January 2021). [ Links ]
ZHOU NA, FAGNANT-SPERATI CS, SHIRAI JH, SHARIF S, ZAIDI SZ, REHMAN L, HUSSAIN J, AGHA R, SHAUKAT S, ALAM M and co-authors (2018) Evaluation of the bag-mediated filtration system as a novel tool for poliovirus environmental surveillance: Results from a comparative field study in Pakistan. PloS One. 13 (7) e0200551. https://doi.org/10.1371/journal.pone.0200551 [ Links ]
 Correspondence:
Correspondence:
John Scott Meschke
Email: jmeschke@uw.edu
Received: 26 March 2021
Accepted: 14 June 2022
APPENDIX















