Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Water SA
On-line version ISSN 1816-7950
Print version ISSN 0378-4738
Water SA vol.47 n.4 Pretoria Oct. 2021
http://dx.doi.org/10.17159/wsa/2021.v47.i4.3859
RESEARCH PAPER
Effect of chemical compounds in water on surface properties and adhesion capacity of Pseudomonas aeruginosa and Escherichia coli in turbulent conditions
Mourad Elgoulli; Hafida Zahir; Oubid Aitlahbib; Mostafa Ellouali; El Mostafa Mliji; Hassan Latrache
Bioprocess and Bio-interfaces Laboratory, Faculty of Science and Techniques, Sultan Moulay Slimane University, BP 523 Beni Mellal, Morocco
ABSTRACT
The aim of this study was to evaluate the effect of chemical compounds found in different water types on the physico-chemical properties of bacteria and the adhesion of two strains (Pseudomonas aeruginosa and Escherichia coli) to glass, PVC and stainless steel. P. aeruginosa and E. coli were exposed to two sterile water types (distilled water and tap water) for 3 h. Contact angle measurements were used to assess the surface properties of both strains and coupons of different materials. The hydrophobicity (∆Giwlp.a = 70mJ/m2, ∆GiwlE.c = 49mJ/m2) and the electron donor properties (yPa = 106,81mJ/m2, yEc = 74mJ/m2) of the bacterial strains seems to increase when exposed to sterile tap water compared to distilled water, while the electron acceptor property is largely unchanged (y+p.a = 0,98mJ/m2, y+Ec = 0mJ/m2). The adhesion tests were carried out in a water circuit creating turbulence. The number of adhered cells was determined after their detachment from the coupons using an ultrasonic bath for 2 min. The findings showed that the type of water affects the adhesion of both strains, which is stronger in tap water than distilled water. A correlation test to determine the surface property that governs adhesion in these conditions, suggested that the adhesion is mainly governed by hydrophobicity.
Keywords: type of water, type of materials, bacterial adhesion, physicochemical properties
INTRODUCTION
Drinking water is a complex product, characterized by its diverse and dependent composition. During its transfer to the consumer, water comes into contact with piping materials. Domestic drinking water networks are a major source of contamination in public health, because they are likely colonized by single and multi-species biofilms. Water contamination can occur from the suspended flora; however, immobilized biofilms are the major source of drinking water contamination (Abdel-Nour et al., 2013; Jefferson, 2004). Flemming et al. (2002) reported that 95% of the water flora is attached to the inner surface of water pipes. Therefore, it appears that biofilms are the survival mechanism used by bacteria against all environmental stresses (Aparna and Yadav, 2008). It is well known that biofilms are very complex and difficult structures to eradicate. However, prevention seems to be the most appropriate approach to control bio-adhesion and biofilm formation. This phenomenon is always preceded by an adhesion step. We believe that the events that precede adhesion are the keys to understand this phenomenon, and therefore to prevent it.
Once a surface is exposed to an aqueous medium of varying chemical composition, its surface properties are very often modified by the adsorption of organic and/or inorganic molecules from the surrounding fluid (Chmielewski and Frank, 2003; Somers and Wong, 2004; Sheng et al., 2008; Fard, 2010). The adhesion phenomenon depends on several physicochemical properties, namely, the energetic properties of the bacterial and the substrate surfaces. Yet, the surrounding medium plays a crucial role in the adhesion process. Drinking water is a heterogeneous medium characterized by its diverse and dependent chemical matrix, mainly composed of lime (CaCO3), ions (Al3+, Ca2+, Fe2+, Mg+, Mn2+, K+, Na+, NO3-, PO43+, SO42-...), dissolved gases (O2, CO2), several organic molecules, and microorganisms of different categories (Gao et al., 2009). In an aqueous environment such as drinking water networks, it is very likely that once a bacterium comes into contact with water, its surface is immediately covered by new compounds, especially ions. Thence, this adsorption and attachment of new chemical agents to bacterial surfaces will probably change the surface properties of bacteria (Hamadi et al., 2012) as well as their ability to attach to the inner surfaces of water pipes. Therefore, in order to fully understand and control adhesion in water systems, it is first necessary to focus on the events that precede it.
There have been a multitude of studies which have tested the bio-adhesion of waterborne bacteria to the inner surface of pipes. However, to our knowledge, no work has investigated this adhesion based on the adsorption of the chemical components of water to the surface of the microorganisms. The dynamic adsorption mechanism must be established. In particular, the effect of water ions and molecules that adsorb and bind to bacterial surfaces requires deeper study. Indeed, studying the impact of bacterial physicochemical changes on adhesion under turbulence conditions seems to be of most importance. Thus, the significance of our work lies in that we test two types of water with differences in their chemical compositions. Therefore, the main objectives of this study were to evaluate the bacterial surface properties when suspended in water and the effect on adhesion. Then, it will be possible to have an idea of the contamination rate based on the water chemistry. Pseudomonas aeruginosa and Escherichia coli were tested in this study because they are recognized as two bacterial models of drinking water quality, and because of their pathogenic nature. Stainless steel and PVC were used during this study because they are widely used in the manufacture of domestic drinking water pipes.
MATERIALS AND METHODS
Bacterial strains, growth conditions and preparation of bacterial suspensions
The strains used in this study were Pseudomonas aeruginosa ATCC27853, and Escherichia coli ATCC25922. The preparation of the bacterial suspensions began with incubation of bacteria in solid LB (Luria Bertani) medium for 24 h at 37°C. Then, the cells were scraped and suspended in a 0.1 M (KNO3) solution and washed twice by centrifugation (5 000 g for 15 min) (Hamadi et al., 2014; Elgoulli et al., 2021).
Cleaning of coupons
The materials used during this study were PVC and stainless steel, both widely used for the manufacture of fittings and tubes for water pipelines in Morocco, and glass (glass was used in this study as a reference material because of its simple molecular structure and its clear hydrophilic character). The tested PVC and stainless-steel coupons were obtained from new pipes, while the glass coupons were obtained from microscope slides.
Before cleaning, the supports were cut into coupons with 1 cm x 1 cm surfaces. Then they were soaked for 10 min in 70% (vol/vol) ethanol, and rinsed 3 to 6 times with sterile distilled water and autoclaved at 120°C for 15 min (Hamadi et al., 2014a; Assaidi et al., 2018).
Exposure of bacteria and supports to the water types
After being washed twice by centrifugation, the selected strains were re-suspended in sterile distilled water or sterile tap water (sterile tap water: pH 7.5, conductivity 400.75 μS/cm, 6.15 mg/L of chlorates, 30.55 mg/L of magnesium, 53.40 mg/L of nitrates, 102.80 mg/L of nitrites, 5.10 mg/L of dissolved oxygen, 10.25 mg/L of potassium, 19.50 mg/L of sodium, and 5.66 mg/L of sulfates (Elgoulli et al. 2021)) for 3 h.
Then, the cells of each strain were placed on a cellulose acetate filter membrane (0.45 μm of port diameter) and filtered by means of negative pressure (Busscher et al., 1984; Hamadi et al., 2014). The filters, on which a layer of bacterial cells was formed, were left to dry in the open air for 10 min. Likewise, the supports were put in contact with water for 3 h, then rinsed 2 to 3 times with sterile distilled water and left to dry in the open air.
Surface characterization
The contact angle measurements were performed using a goniometer (GBX instruments, France) by the sessile drop method.
One to three drops of a liquid were placed on each solid support or layer of bacterial cells (as described above). Three to six contact angle measurements were made for all liquids probed, including water, formamide or diiodomethane.
The Lifshitz-Van der Waals (yLW), electron donor (y-) and electron acceptor (y+) components of the bacterial surface, glass, stainless steel and PVC were estimated according to the approach proposed by Van Oss et al. (1988). In this approach the contact angle (0) can be expressed as:
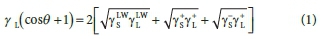
Lewis acid-base components can be identified by:

The approach of Van Oss et al. (1988) was also used during this study to determine the surface hydrophobicity. This approach explains the hydrophobicity as the interaction free energy between two entities of this material when immersed in water; it is noted as ΔGiwi. The surface is considered hydrophobic or hydrophilic if this free energy is negative (ΔGiwi<0) or positive (ΔGiwi>0), respectively. ΔGiwi can be estimated through tensions on the surfaces of interacting entities, according to:
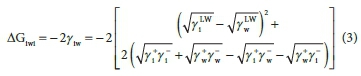
Adhesion experiments
The bacterial suspension of Pseudomonas aeruginosa ATCC27853 and Escherichia coli ATCC25922 - 12 mL containing approximatively 108 CFU/mL - was poured into 2 L of water (sterile distilled water or sterile tap water) circulating in the pilot station (flow: 300 L/h, presser: 160 mbar, temperature: 25°C) for 3 h (Fig. 1). The assays were carried out in turbulent conditions, to be as close as possible to the real conditions that apply when water is flowing through pipes.
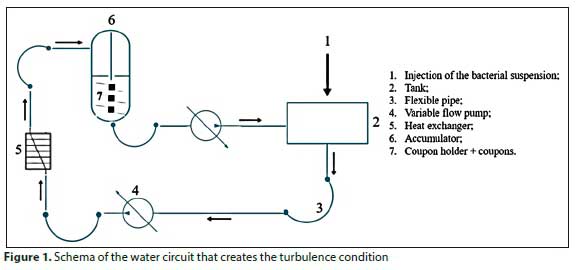
After 3 h of circulation, the coupons were recovered and rinsed gently 3 times with sterile distilled water in order to remove loose bacteria. Each support was immersed in a test tube with 10 mL of sterile physiological water (NaCl: 9 g/L). The adhered cells were detached from each support using a sonication bath for 2 min. The number of adhered cells is determined by a series of dilutions of the mother solutions obtained after sonication. The enumeration is done on solid LB medium after incubation at 37°C for 24 h. The experiments are repeated 3 times.
RESULTS
Physicochemical characterization of the surfaces of supports and bacterial strains
Table 1 presents the physicochemical characterization of the material surfaces used. Table 2 gives the physicochemical characterizations of the bacterial strains' surfaces after being exposed to the two types of sterile waters at 25°C. The surface hydrophobicity can be deduced directly from the contact angle with water (Oliveira et al., 2001). The absolute degree of hydrophobicity of a surface (I) with regard to water (W) can be determined (Van Oss, 1997). According to the approach of Van Oss (1997), the surfaces of P. aeruginosa and E. coli are hydrophilic (ΔGiwlp.a = 41mJ/m2, ΔiwlE.c = 29mJ/m2), strongly electron donors (yPa = 55,5mJ/m2, yEc = 50mJ/m2), and weakly electron acceptors (y+Ra = 0.8mJ/m2, y+Ec= 2.6mJ/m2). Likewise, glass is a hydrophilic (ΔGiwi=15 mJ/m2), strong electron acceptor (y+ = 40.36 mJ/m2) and weak electron donor (y- = 1.54 mJ/m2) surface. On the other hand, stainless steel and PVC are hydrophobic (ΔGiwi = -49.60 mJ/m2, ΔGiwi = -25.50 mJ/m2), moderate electron donor (y- = 6.9 mJ/m2, y- = 11.41 mJ/m2) and weak electron acceptor (y+ = 0.51 mJ/m2, y+= 3.03 mJ/m2) surfaces. The exposure of these supports to water apparently has no effect on their surface physico-chemistry.
Clear changes were revealed in the properties of the bacterial surfaces after the exposure to the two types of water. Changes in surface physicochemical characteristics were typically stronger when the strains were exposed to the sterile tap water. On the contrary, the bacterial surfaces underwent fewer changes in contact with sterile distilled water. Results showed that sterile tap water caused significant increases in hydrophilicity (70.20 mJ/m2 for P. aeruginosa, 49.80 mJ/m2 for E. coli) and electrons donor potential (106.81 mJ/m2 for P. aeruginosa, 74.00 mJ/m2 for E. coli) of surfaces for both strain, while electron acceptor capacity was been affected. Clearly, the degree of modification in surface properties is linked to many parameters, mainly water composition, cellular structure, extracellular polysaccharides produced by the cells, environmental conditions such as pH, ionic forces, temperature, and the exposure period. In this case, these changes are more likely due to the differences in the chemical composition of the two water types. In particular, we suggest that the level of surface modification depends on the differences in the concentrations of lime and dissolved ions in the water. It is possible to establish a relationship between these concentrations and the surface properties of the strains used in this study.
Effect of water on the adhesion of bacterial strains to glass, PVC and stainless steel
Bacterial adhesion is governed by several forces, which are generated bythe surfaces ofbacteria and substrate. The surrounding medium can change these forces when some of its compounds are fixed on the bacterial or substrate surface. In this study we have shown that water (as a surrounding medium) has changed the energetic properties of the bacterial surface and we have given some possible explanations as to how and which compounds could attach to the surface of bacteria and change its surface energy. Consequently, the adhesion capacity will be influenced. Figures 2 and 3 respectively show the adhesion of P. aeruginosa and E. coli. Generally, microbial adhesion is a combination of surface physicochemical properties of both the cells and the types of materials, and the density of the microorganisms. Regardless of the type of water running through the water supply system, both bacterial strains have the ability to adhere to all studied materials. More specifically, the adhesion was stimulated by sterile tap water under turbulence conditions, under which stainless steel (105,61 ufc/cm2, 105,18 ufc/cm2) and PVC (105,96 ufc/cm2, 105,18 ufc/cm2) were the most colonized surfaces.
DISCUSSION
Firstly, our objective was to quantify and explain the physicochemical proprieties of Pseudomonas aeruginosa and Escherichia coli after being exposed to two waters with different chemical composition. Tap water significantly enhances some surface characteristics of both bacterial strains but not to the same degree (Table 2). The observed discrepancies are very likely due to the molecular structure of the bacterial surfaces, which could govern the adsorption and fixation of the appropriate water compound. Numerous studies have shown that hydrophobicity is linked to the presence of nitrogen, proteins or carbon in the shape of hydrocarbons, whereas hydrophilicity is linked to the existence of oxygen and polysaccharides exposed on the surface of bacteria (Cowan et al., 1992; Cuperus et al., 1993; Dufrêne and Rouxhet, 1996; Latrache et al., 2002). The physicochemical differences observed between E. coli and P. aeruginosa could be caused by the chemical composition of the bacterial walls, particularly the external structure of their molecular composition, as reported by some studies (El Ghmari et al., 2002; Latrache et al., 2002; Hamadi et al., 2005). On the other hand, the increase in hydrophilicity of both strains could be the result of adsorption and attachment of some water compounds to these bacterial wall structures.
The relationship between the chemical structure of bacterial surface and its energy has been investigated by various scientists (Mozes et al., 1988; Van der Mei et al., 1989; Mozes et al., 1989; Latrache et al., 1994; Van der Mei and Busscher, 1997; Boonaert and Rouxhet, 2000; Hamadi et al., 2012). For example, a number of research studies (Mozes et al., 1988; Mozes et al., 1989; Latrache et al., 1994; Hamadi et al., 2012) have reported that phosphate groups play an important role in the creation of surface energy. In addition, carboxyl groups are at the same time important for the energy of microbial surfaces (Boonaert and Rouxhet, 2000; Hamadi et al., 2005; Hamadi et al., 2008). The acid-base property is also relevant to the adhesion phenomenon (Henriques et al., 2004; Hamadi et al., 2005; Hamadi et al., 2008).
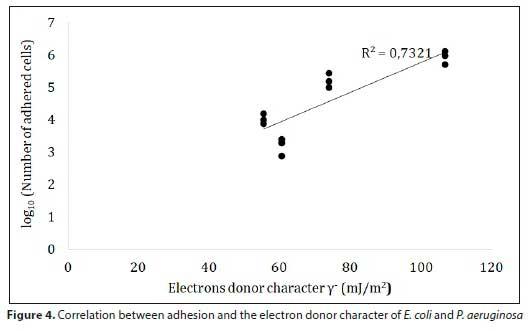
According to these findings, the type of water affects the electron donor character (base) of both strains. Usually, this character is generated by some functional groups exposed on the surface, namely, carboxylic, phosphoric and amino groups (Hong and Brown, 2006). Hamadi et al. (2012) found a strong positive correlation between the electron donor character and the P/C ratio of E. coli. The results showed an increase in the electron donor character with sterile tap water, which could be related to the adsorption of certain groups found in drinking water. On the other hand, nitrogen (N) could play an important role in decreasing the electron acceptor (acid) capacity. Indeed, Hamadi et al. (2012) also found a negative correlation between the electron acceptor character and the N/C ratio for E. coli. There was a depletion of the electron acceptor capacity of E. coli when exposed to sterile tap water (Table 2), which is likely caused by the adsorption of nitrogenous compounds commonly found in drinking water. Similarly, the electron acceptor capacity increases with the amount of polysaccharides concentrated on the bacterial surface (Hamadi et al., 2012). However, this study revealed a reduction in this property (Table 2). The adsorption of negatively charged ions and/ or molecules and the stabilization of acid radicals linked to these forces, in particular O=C structures, and the reduction of the N/C ratio, could be the cause of the reduction in this property.
An investigation of the adhesion ability of P. aeruginosa and E. coli in turbulence conditions on glass, PVC and stainless steel was carried out. To our knowledge, no previous studies have been published on the effect of water type on bacterial adhesion, which have shown that water components can contribute to reducing or increasing bacterial adhesion. The obtained results showed that the bacteria tested in this study could adhere readily on all materials regardless of the type of water. In addition, the exposure of strains to sterile tap water may enhance their adhesive capacity. Several studies have reported that the physicochemical properties of the bacterial wall as well as those of the material, mainly hydrophobicity, surface charge and electron donor/acceptor properties, are the key parameters of microbial adhesion (Hamadi et al., 2005; Hamadi and Latrache, 2008; Henriques et al., 2004; Teixeira et al., 2008; Silva et al., 2008). On the contrary, some other research studies have not found a relationship between adhesion and the physico-chemistry of surfaces (Flint et al., 1997; Oliveira et al., 2006; Teixeira et al., 2007). However, our data exhibit a good positive correlation (R2 = 0.9042) between hydrophobicity and cell adhesion (Fig. 5).
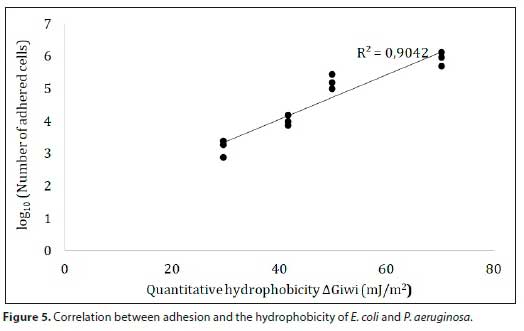
These findings suggest that adhesion could be strongly governed by the electron donor property and the hydrophobicity. In fact, an enhancement of these two physicochemical properties was observed when the bacterial strains were exposed to sterile tap water. Moreover, this enhancement was accompanied by an increase in the adhesive capacity, which is probably due to the adsorption of some water compounds such as ions and molecules, as described above.
CONCLUSION
In order to develop strategies to prevent the contamination of drinking water, it is essential to understand the relationship between the water's chemical composition and its contamination. For this reason, this study was based on the exposure of two bacteria to two different waters. The findings showed that tap water enhances the physicochemical characteristics of the bacterial walls, especially the hydrophilicity and the electron donor property. As a result, the adhesive ability of both strains seems to increase in the presence of tap water. Therefore, it can be assumed that there is a strong relationship between the adhesion of Pseudomonas aeruginosa and Escherichia coli and water type, in turbulent conditions. Water compounds could enhance the bacterial adhesion in the water pipes by changing the bacterial surface characteristics. Therefore, water and its chemical composition have to be taken into consideration as important factors when a strategy of water disinfection is being developed, which will require further in-depth studies.
ACKNOWLEDGMENTS
M Elgoulli would like to thank the Centre National pour la Recherche Scientifique et Technique of Morocco for Scholarship 7USMS2018.
REFERENCES
ABDEL-NOUR M, DUNCAN C, LOW DE and GUYARD C (2013) Biofilms: The stronghold of Legionella pneumophila. Int. J. Molecular Sci. 14 (11) 21660-21675. [ Links ]
APARNA MS and YADAV S (2008) Biofilms: Microbes and disease. Brazilian J. Infect. 12 526-530. [ Links ]
ASSAIDI A, ELLOUALI M, LATRACHE H, MABROUKI M, TIMINOUNI M, ZAHIR H, TANKIOUINE S, BARGUIGUA A and MLIJI EM (2018) Adhesion of Legionella pneumophila on glass and plumbing materials commonly used in domestic water systems. Int. J. Environ. Health Res. 8 (2) 125-133. https://doi.org/10.1080/09603123.2018.1429580 [ Links ]
BOONAERT CJP and ROUXHET PG (2000) Surface of lactic acid bacteria: Relationships between chemical composition and physicochemical properties. Appl. Environ. Microbiol. 66 (6) 25482554. https://doi.org/10.1128/AEM.66.6.2548-2554.2000 [ Links ]
BUSSCHER HJ, WEERKAMP AH, VAN DER MEI HC, VAN PELT AW, DE JONG HP and ARENDS J (1984) Measurement of the surface free energy of bacterial cell surfaces and its relevance for adhesion. Appl. Environ. Microbiol. 48 (5) 980-983 [ Links ]
CHMIELEWSKI RAN and FRANK JF (2003) Biofilm formation and control in food processing facilities. Compr. Rev. Food Sci. Food Saf. 2 (1) 22-32. https://doi.org/10.1111/j.1541-4337.2003.tb00012.x [ Links ]
COWAN MM, VAN DER MEI HC, ROUXHET PG and BUSSCHER HJ (1992) Physico-chemical and structural properties of the surfaces of Peptostreptococcus micros and Streptococcus mitis as compared to those of mutans streptococci, Streptococcus sanguis and Streptococcus salivarius. J. Gen. Microbiol. 138 (12) 2707-2714. https://doi.org/10.1099/00221287-138-12-2707 [ Links ]
CUPERUS PL, VAN DER MEI HC, REID G, BRUCE AW, KHOURY AH, ROUXHET PG and BUSSCHER HJ (1993) Physicochemical surface characteristics of urogenital and poultry lactobacilli. J. Colloid Interface Sci. 156 (2) 319-324. https://doi.org/10.1006/jcis.1993.1118 [ Links ]
DUFRÊNE YF and ROUXHET PG (1996) Surface composition, surface properties, and adhesiveness of Azospirillum brasilense - Variation during growth. Can. J. Microbiol. 42 (6) 548-556. https://doi.org/10.1139/m96-074 [ Links ]
EL GHMARI A, LATRACHE H, HAMADI F, EL LOUALI M, EL BOUADILI A, HAKKOU A and BOURLIOUX P (2002) Influence of surface cell structures on physicochemical properties of Escherichia coli. New Microbiol. 25 (2) 173-178. [ Links ]
ELGOULLI M, AITLAHBIB O, TANKIOUINE S, ASSAIDI A, LOUALI M EL, ZAHIR H and LATRACHE H (2021) The theoretical adhesion of Pseudomonas aeruginosa and Escherichia coli on some plumbing materials in presence of distilled water or tap water. Folia Microbiol. (Praha) 17 1-7. https://doi.org/10.1007/s12223-021-00868-y [ Links ]
FARD PS (2010) Production and purification of biosurfactants and study of their influence on surface properties of stainless steel and Teflon. PhD thesis, University of Lille. [ Links ]
FLEMMING HC, PERCIVAL SL and WALKER JT (2002) Contamination potential of biofilms in water distribution systems. Water Sci. Technol. Water Suppl. 2 (1) 271-280. [ Links ]
FLINT SH, BREMER PJ and BROOKS JD (1997) Biofilms in dairy manufacturing plant - description, current concerns and methods of control. Biofouling. 11 (1) 81-97. https://doi.org/10.1080/08927019709378321 [ Links ]
GAO J, YOUN S, HOVSEPYAN A, LLANEZA VL, WANG Y, BITTON G and BONZONGO J-CJ (2009) Dispersion and toxicity of selected manufactured nanomaterials in natural river water samples: effects of water chemical composition. Environ. Sci. Technol. 43 (9) 33223328 . https://doi.org/10.1021/ES803315V [ Links ]
HAMADI F, ASSERNE F, ELABED S, BENSOUDA S, MABROUKI M and LATRACHE H (2014a) Adhesion of Staphylococcus aureus on stainless steel treated with three types of milk. Food Control. 38 104-108. https://doi.org/10.1016/j.foodcont.2013.10.006 [ Links ]
HAMADI F and LATRACHE H (2008) Comparison of contact angle measurement and microbial adhesion to solvents for assaying electron donor-electron acceptor (acid-base) properties of bacterial surface. Colloids Surf. B Biointerfaces. 65 (1) 134-139 . https://doi.org/10.1016/j.colsurfb.2008.03.010 [ Links ]
HAMADI F, LATRACHE H, MABRROUKI M, ELGHMARI A, OUTZOURHIT A, ELLOUALI M and CHTAINI A (2005) Effect of pH on distribution and adhesion of Staphylococcus aureus to glass. J. Adhes. Sci. Technol. 19 (1) 73-85 . https://doi.org/10.1163/1568561053066891 [ Links ]
HAMADI F, LATRACHE H, MALLOUKI B, MLIJI E, EL GHMARI A, MABROUKI M, BENGOURRAM J and ELLOUALI M (2008) Adhesion of Escherichia coli to glass under different pH. J. Pure Appl. Microbiol. 2 295-302. [ Links ]
HAMADI F, LATRACHE H, ZAHIR H, EL ABED S, ELLOUALI M and SAAD IK (2012) The relation between the surface chemical composition of Escherichia coli and their electron donor/electron acceptor (acid-base) properties. Res. J. Microbiol. 7 (1) 32. https://doi.org/10.3923/jm.2012.32.40 [ Links ]
HENRIQUES M, AZEREDO J and OLIVEIRA R (2004) Adhesion of Candida albicans and Candida dubliniensis to acrylic and hydroxyapatite. Colloids Surf. B Biointerfaces. 33 (3-4) 235-241. https://doi.org/10.1016/jxolsurfb.2003.10.012 [ Links ]
HONG Y and BROWN DG (2006) Cell surface acid-base properties of Escherichia coli and Bacillus brevis and variation as a function of growth phase, nitrogen source and C:N ratio. Colloids Surf. B Biointerf. 50 (2) 112-119. https://doi.org/10.1016/jxolsurfb.2006.05.001 [ Links ]
JEFFERSON KK (2004) What drives bacteria to produce a biofilm? FEMS Microbiol. 236 (2) 163-173. [ Links ]
LATRACHE H, EL GHMARI A, KARROUA M, HAKKOU A, AIT MOUSSE H, EL BOUADILI A and BOURLIOUX P (2002) Relations between hydrophobicity tested by three methods and surface chemical composition of Escherichia coli. New Microbiol. 25 (1) 75. [ Links ]
LATRACHE H, MOZES N, PELLETIER C and BOURLIOUX P (1994) Chemical and physicochemical properties of Escherichia coli: variations among three strains and influence of culture conditions. Colloids Surf. B Biointerf. 2 (1-3) 47-56. https://doi.org/10.1016/0927-7765(94)80017-0 [ Links ]
MOZES N, AMORY DE, LEONARD AJ and ROUXHET PG (1989) Surface properties of microbial cells and their role in adhesion and flocculation. Colloids Surf. 42 (2) 313-329. https://doi.org/10.1016/0166-6622(89)80348-8 [ Links ]
MOZES N, LEONARD AJ and ROUXHET PG (1988) On the relations between the elemental surface composition of yeasts and bacteria and their charge and hydrophobicity. BBA - Biomembr. 945 (2) 324-334. https://doi.org/10.1016/0005-2736(88)90495-6 [ Links ]
OLIVEIRA K, OLIVEIRA T, TEIXEIRA P, AZEREDO J, HENRIQUES M and OLIVEIRA R (2006) Comparison of the adhesion ability of different Salmonella enteritidis serotypes to materials used in kitchens. J. Food Prot. 69 (10) 2352-2356. https://doi.org/10.4315/0362-028X-69.10.2352 [ Links ]
OLIVEIRA R, AZEREDO J, TEIXEIRA P and FONSECA A (2001) The role of hydrophobicity in bacterial adhesion. In: Gilbert P, Allison D, Brading M, Verran J and Walker J (eds) Biofilm Community Interactions: Chance or Necessity? Bioline, Cardiff. 2001. 11-22. [ Links ]
SHENG XX, TING YP and PEHKONEN SO (2008) The influence of ionic strength, nutrients and pH on bacterial adhesion to metals. J. Colloid Interf. Sci. 321 (2) 256-264. https://doi.org/10.1016/j.jcis.2008.02.038 [ Links ]
SILVA S, TEIXEIRA P, OLIVEIRA R and AZEREDO J (2008) Adhesion to and viability of Listeria monocytogenes on food contact surfaces. J. Food Prot. 71 (7) 1379-1385. https://doi.org/10.4315/0362-028X-71.7.1379 [ Links ]
SOMERS EB and WONG ACL (2004) Efficacy of two cleaning and sanitizing combinations on Listeria monocytogenes biofilms formed at low temperature on a variety of materials in the presence of ready-to-eat meat residue. J. Food Prot. 67 (10) 2218-2229. [ Links ]
TEIXEIRA P, LIMA J, AZEREDO J and OLIVEIRA R (2008) Adhesion of Listeria monocytogenes to materials commonly found in domestic kitchens. Int. J. Food Sci. Technol. 43 (7) 1239-1244. https://doi.org/10.1111/j.1365-2621.2007.01598.x [ Links ]
TEIXEIRA P, SILVA S, ARAÚJO F, AZEREDO J and OLIVEIRA R (2007) Bacterial adhesion to food contacting surfaces. In: Méndez-Vilas A (ed.) Communicating Current Research and Educational Topics and Trends in Applied Microbiology. Formatex 2007. [ Links ]
VAN DER MEI HC and BUSSCHER HJ (1997) The use of X-ray photoelectron spectroscopy for the study of oral streptococcal cell surfaces. Adv. Dent. Res. 11 (4) 388-394. https://doi.org/10.1177/08959374970110040301 [ Links ]
VAN DER MEI HC, GENET MJ, WEERKAMP AH, ROUXHET P G and BUSSCHER HJ (1989) A comparison between the elemental surface compositions and electrokinetic properties of oral streptococci with and without adsorbed salivary constituents. Arch. Oral Biol. 34 (11) 889-894 . https://doi.org/10.1016/0003-9969(89)90146-5 [ Links ]
VAN OSS CJ (1997) Hydrophobicity and hydrophilicity of biosurfaces. Curr. Opin. Colloid Interface Sci. 2 (5) 503-512. https://doi.org/10.1016/S1359-0294(97)80099-4 [ Links ]
VAN OSS CJ, CHAUDHURY MK and GOOD RJ (1988) Interfacial Lifshitz-van der Waals and polar interactions in macroscopic systems. Chem. Rev. 88 (6) 927-941. https://doi.org/10.1021/cr00088a006 [ Links ]
 Correspondence:
Correspondence:
Hassan Latrache
Email: latracheh@yahoo.fr
Received: 21 October 2020
Accepted: 18 October 2021














