Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
Water SA
versión On-line ISSN 1816-7950
versión impresa ISSN 0378-4738
Water SA vol.45 no.4 Pretoria oct. 2019
http://dx.doi.org/10.17159/wsa/2019.v45.i4.7553
SHORT COMMUNICATION
The rate of release of Cry1Ab protein from Bt maize leaves into water
Amy du Pisanie; Louis du Preez; Johnnie van den Berg; Rialet Pieters*
Unit for Environmental Sciences and Management, North-West University, Potchefstroom, 2531, South Africa
ABSTRACT
Transgenic Bt maize plants are genetically modified to contain genes of Bacillus thuringiensis that encode for δ-endotoxins (Cry1Ab protein) that have insecticidal properties. These endotoxins target certain lepidopteran pests of maize. There are several entry routes by which Cry proteins enter the aquatic ecosystem in which aquatic organisms are exposed to these proteins. The main route is through plant debris such as leaves, stalks and postharvest detritus that are transported by means of run-off, rain and wind. While several studies have been conducted on the fate of Cry1Ab protein in terrestrial ecosystems, little is known of the release rates of Cry1Ab proteins from maize plant tissues that end up in aquatic ecosystems. In this study, leaves of Bt-maize and its isoline were submerged in containers filled with deionised or borehole water for a period of 16 days, and kept at 3 different temperatures (10±1, 21±1 and 30±1°C). Samples were collected at 1, 2, 4, 8, 16, 24, 48, 96, 192 and 384 h post submersion and analysed for Cry protein content using enzyme-linked immunosorbent assay (ELISA). The release of Cry protein from submerged maize leaves was influenced by temperature, and duration of immersion. An increase in Cry protein levels in the water was observed from the first hour onwards in both water types until the end of the experiment. The highest concentration of Cry protein was found at 30°C. This study showed that temperature and time period influence the release rate of Cry proteins from dried leaf matter into the aquatic environment.
Keywords: aquatic ecosystem, arthropods, GM crops, environment, risk assessment
INTRODUCTION
Genetically modified (GM) transgenic Bt maize plants contain genes of the soil bacterium Bacillus thuringiensis that encode for δ-endotoxins (Cry1Ab protein) that have insecticidal properties. These endotoxins target certain lepidopteran pests of maize (Betz et al., 2000; Baumgarte and Tebbe, 2005; Bravo et al., 2007; Chambers et al., 2010). Production of Cry endotoxins by GM plants confers pesticidal properties to these plants, for example, Bt maize, of which various maize hybrids express different Cry proteins such as Cry1Ab, Cry1Ac and Cry1F, that are toxic for specific insect species (Baumgarte and Tebbe, 2005; Bravo et al., 2007; Swan et al., 2009). While GM Bt maize that expresses only Cry1Ab protein has been planted in South Africa from 1998 onwards, Bt maize hybrids that express both Cry1.105 and Cry2Ab2 proteins started to replace these hybrids from 2012 onwards. South Africa is currently the 9th largest producer of Bt maize in the world, with approximately 1.9 million ha planted to Bt crops in 2017 (ISAAA, 2017). The most Bt maize planted per country in 2017 was the 29.4 million ha in the USA and almost 15 million ha in Brazil (second-most). Globally, a combined surface area of 101 million ha is planted to Bt cotton, Bt maize and Bt soybean (ISAAA, 2017).
Between 65 and 75% of the maize planted annually in South Africa is Bt maize (Masehela et al., 2016), which is planted for control of the maize stem borer complex that consists of Busseola fusca (Lepidoptera: Noctuidae), Chilo partellus (Lepidoptera: Crambidae) and Sesamia calamistis (Lepidoptera: Noctuidae) (Van den Berg and Van Wyk, 2007; Van den Berg et al., 2013). The high adoption rates for Bt crops in the world are ascribed to the many benefits they provide to farmers. Reduced use of chemical insecticides, increased target specificity and ease of crop management are widely reported to be the drivers of these high levels of adoption (Raybould and Quemada, 2010; Kruger et al., 2009; Brookes and Barfoot, 2014). However, these crops have largely not been approved for cultivation in Europe and most countries in Africa. The benefits these crops hold are strongly debated, leading to controversies regarding environmental safety and effects on biodiversity (Naranjo, 2009; Lövei and Arpaia, 2005) and especially aspects around aquatic ecosystem health (Venter and Bøhn, 2016). It is especially with regards to aquatic environments where risk assessments are lacking (Carstens et al., 2012). Despite growing recognition that aquatic ecosystems near agricultural fields receive significant amounts of run-off and crop residues that contain these toxins (Böttger et al., 2015; Li et al., 2013), environmental risk assessments tend to neglect aquatic ecosystems as a relevant context for assessing the potential risks associated with GM crops.
A critical evaluation of the fate of Cry proteins is imperative in order to gain an accurate characterization of exposure levels to sensitive species for risk assessments. Arthropods that feed on Bt crop tissue ingest Bt protoxins which are then activated before they can have an effect on susceptible individuals (Broderick et al., 2006). The gut protease of the target pest species cleaves the protoxin producing an active toxin which binds to the mid-gut epithelial cells, creating pores in the cell membrane. This causes immobilization of the gut, lyses of epithelial cells and ultimately death due to both septicaemia and starvation (Broderick et al., 2006).
Direct exposure to Cry proteins takes place when transgenic crop residues are consumed by organisms (Kratz et al., 2010), while indirect exposure may be through leaching of protein from crop plant tissue into the aquatic environment, with possible adverse effects on exposed organisms (Li et al., 2007; Venter and Bøhn, 2016). There are several routes through which Cry proteins enter the aquatic ecosystem in which aquatic organisms are exposed to these proteins through different exposure pathways (Carstens et al., 2012). Rosi-Marshall et al. (2007) indicated the main entry route into aquatic systems to be through plant debris (which includes pollen, leaves, crop dust, stalks and postharvest detritus) that are transported by means of run-off, rain and wind. The leaching of Cry proteins from plant tissue can be influenced by temperature, plant tissue type, sediment composition and the presence of microbes (Li et al., 2007).
The presence and persistence of Cry1Ab protein in the environment has been studied by several authors. Bøhn et al. (2008) showed that Cry1Ab protein is present in maize grain at a concentration of approximately 67 ng/g tissue, and 2 530 ng/g in leaves (Holderbaum et al. 2015), and for stacked events the concentration can be much higher. The Bt toxin load in pollen of 'Smartstax' can be 100 to 200 ng/mg pollen dry mass (Phillips, 2008; Stillwell and Silvanovich, 2008). A study by Tank et al. (2010) indicated that the presence of Cry1Ab protein in natural streams containing maize detritus was above the minimum detection limit of 6 ng/L in 23% of the sites sampled.
In terrestrial ecosystems the concentration of Bt toxins in soil depends on soil type (Palm et al., 1994). Higher clay and organic matter results in stronger toxin binding, making extraction difficult (Palm et al., 1994). For example, 27% and 60% B. thuringiensis var. kurstaki (Btk) toxins were recovered from soil by Palm et al. (1994), with high and low clay-organic matter content, respectively. The degradation rate of Cry1Ab proteins in different soil types (differed in texture but not in silt or pH) showed that carbon dioxide (CO2) production in soil was initially high and then declined over a period of 135 days, indicating that the Cry protein was used by microorganisms as a growth substrate (Valldor et al., 2015). The latter study also reported that the low levels of Cry1Ab in soil indicate that these proteins mineralize faster due to microbial degradation (Valldor et al., 2015). Cry toxin may also remain active in the soil where it binds quickly and tightly to humic acid and clays (Saxena et al., 1999; Carstens et al., 2012). Though bound, the Cry toxin maintains its insecticidal characteristic and, since it is bound to soil particles, is protected from degradation. Depending on the soil type, the toxin can persist for at least 234 days (Saxena et al., 1999).
Using an enzyme-linked immunosorbent assay (ELISA) to determine Cry1Ab levels, Zwahlen et al. (2003) studied how long the toxin remains in the plant tissue when left on field after harvest and over different periods of the year. They reported the degradation rate of Cry protein to be temperature dependent and reduced at lower soil temperatures. Feng et al. (2011) also reported that soil temperature had a significant effect on the degradation of Cry1Ab protein, but that pH had no obvious effect.
Jensen et al. (2010) reported that Cry1Ab protein present inside maize leaves lost its bioactivity after 2 weeks of immersion in water. However, they did not quantify the level of Cry1 proteins either in the plant tissue or aquatic medium, which made it difficult to determine whether the protein in the plant tissue degraded or leached out into the water. Another study reported a decline of Cry3Bb1 content in maize tissue following water immersion (Prihoda and Coats, 2008).
Cry proteins enter aquatic systems by leaching into the soil from plant material (Victorov, 2011). Leaf detritus are also left on fields to provide nutrition and as animal feed, while at the same time Bt proteins can leach into the ground and make its way to nearby streams or water bodies (Swan et al., 2009; Chambers et al., 2010; Carstens et al., 2012). Large amounts of maize debris have been reported to end up in water systems over very short periods of time (<7 days) (Victorov, 2011; Venter and Bøhn, 2016), which may lead to sudden increases in Bt protein concentration in such aquatic systems. The amount of Bt maize debris in water streams has been reported to correlate with the amount of Cry protein found in stream water (Tank et al., 2010). Whiting et al. (2014) reported high concentrations (33 ng/L) of Cry1Ab protein in run-off water sampled in maize fields. Shogren et al. (2019) found that Cry1Ab proteins are removed from the water column in riverine systems either via sorption of the protein to the biofilm or by biological removal thereof.
This study investigated the release rate of the Cry1Ab protein from water-submerged Bt maize leaves, with a specific focus on different periods of immersion, temperature and type of water. These results will provide insight into how the immediate environment influences the extent of Cry protein release into aquatic systems, and will provide useful information for use in the design of laboratory bioassays in which potential non-target effects of Cry proteins are studied in aquatic environments. This study was undertaken as a pilot study in order to glean necessary information to allow planning of more comprehensive studies.
MATERIALS AND METHODS
The experiment consisted of 12 treatments, each replicated 3 times. Dried maize leaf tissue was exposed to different water types and different temperatures. The treatments were as follows: Bt maize leaf tissue in either borehole water or deionised water, maintained at 3 different temperatures. The control treatment consisted of non-Bt maize leaves exposed to similar water and temperature treatments.
Maize leaves were collected from Bt and non-Bt plants grown under the same field conditions. The leaves were removed from the stems just before flowering (7 weeks after seedling emergence) and dried under natural conditions for 5 weeks in a well-ventilated plant growth tunnel. Maize hybrids DKC 7815B (MON810) and CRN 3505 were used as the Bt and non-Bt iso-hybrid, respectively.
The leaves were cut into 9 cm long pieces after which infusions were prepared by submerging 24 g of maize leaves into 1 L of water in glass containers. After putting the leaf tissue into the water, the glass containers were kept at 3 different temperatures (10±1oC, 21±1oC and 30±1oC) for the duration of the experiment. The beakers were covered to limit evaporation, but not sealed airtight. Gas exchange could still take place.
Water samples were taken from each treatment at the following time intervals after submersion of leaf tissue: 1, 2, 4, 8, 16, 24, 48, 96, 192, and 384 h. Each sample consisted of 9 mL infusion, made up by three 3 mL sub-samples taken from each container at the respective time intervals. All samples were immediately frozen at −80°C until assays were performed. Once all the samples were acquired, analyses were done to determine the concentration of Cry1Ab protein content.
ELISA analysis
The ELISA procedure used was similar to that described by Strain et al. (2014), although the high amount of proteins present in our samples precluded the necessity to concentrate the samples. Analyses were done following the manufacturer's instructions and using a commercially available ELISA kit (EnviroLogixQuantiPlate assay Kit for Cry1Ab/Cry1Ac).
The ELISA was carried out as described by the product manufacturer. Briefly, 50 µL enzyme conjugate was added to each well which was followed by 50 µL sample, or positive control or Cry1Ab analytical standard and incubated for 2 h at room temperature. The commercial kit does not include known concentrations of Cry1Ab protein to create a calibration curve. Lyophilised, activated Cry1Ab toxin prepared from Cry1Ab protoxin was acquired from Marianne Pusztai-Carey at the Department of Biochemistry, Case Western University, Cleveland, Ohio. The protoxin from B. thuringiensis subsp. kurstaki HD-1 was expressed as a single gene product in Escherichia coli, cleaved with trypsin and deionised by high-pressure liquid chromatography (HPCL). The lyophilised powder was dissolved in Tris-EDTA (Sigma 93302) at pH = 4. Two 12-point calibration series were created independently with known Cry1A concentrations ranging between 0.03 ng/mL and 3.5 ng/mL and run on each plate. This series was optimised for the capabilities of the commercial ELISA kit and the plate reader used to quantify absorbance. This meant that dilutions were made of selected samples if their initial protein concentration caused the maximum absorbency of the plate reader. After incubation the conjugate was removed and washed with phosphate-buffered saline before adding the substrate. The stop solution was added after 30 min and concentrations of Bt Cry1Ab were determined by subtracting optical density values read at 650 nm (reference wavelength) and 450 nm on a Berthold Tristar LB941 plate reader.
The water samples were allowed to thaw at room temperature. Strain et al. (2014) found that samples stored above freezing point (4 and 23°C) had low recoveries, but that there was no significant difference in the recoveries between the two sub-zero temperatures (−20 and −80oC). Should the samples not be analysed immediately, they suggested that the samples be frozen at either of the latter two temperatures.
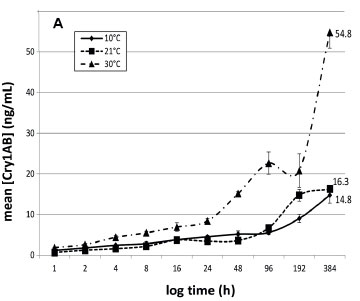
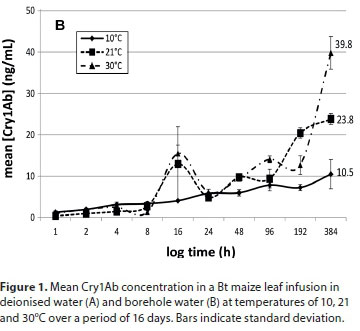
The entire ELISA protocol was repeated 3 times for each sample. Data are graphically presented to indicate the concentration of Cry1Ab protein in water over time (Fig. 1). Dilution factors were taken into consideration and corrections were made for the gradual decrease of water volume due to the consecutive sampling.
A sample was considered a non-detect (ND) if the optical density value was below that of the blank plus 3 times the standard deviation. The limit of detection (LOD) and limit of quantification (LOQ) were determined using regression analysis of the calibration curves where LOD = 3Sb/b and LOQ = 10Sb/b (Sb = slope uncertainty and b = slope). The LOD was 0.13 ± 0.05 ng/mL and the LOQ was 1.06 ± 0.85 ng/mL.
Statistical analysis
The normality of the data distribution was investigated with the Shapiro-Wilk test to decide on the application of one-way analysis of variance (ANOVA) (if the dataset was normally distributed) or Kruskal-Wallis ANOVA if the dataset was non-normally distributed. IBM's SPSS software package was used for the calculations.
RESULTS AND DISCUSSION
There were no detectable levels of Cry proteins in any of the water samples at the start of the experiment before leaf matter was added. The Cry1Ab protein concentration in the control treatments (non-Bt iso-hybrid) was very low with the highest concentration in deionised water and borehole water being 0.13 ng/mL and 0.10 ng/mL, respectively, which were <LOQ (Data not shown). These levels might be ascribed to light absorption in the relevant nanometer range caused by other dissolved compounds that leached out of the plant material or that could also have had an affinity for the antibody on the plates. Nevertheless, these levels were lower than the limit of quantification.
The Cry1Ab concentrations in deionised water and borehole water at the three different temperatures are presented in Figs 1A and 1B, respectively. This study indicated that Cry protein released from Bt maize leaves is more pronounced at higher ambient temperatures. Both infusions at 10°C had lower concentrations of Cry1Ab proteins, varying between 11 and 15 ng/mL up to 16 days after the exposure commenced. Exposure of submerged Bt maize leaves under an ambient temperature of 30oC resulted in the highest Cry concentration throughout the experiment. The infusions exposed to 30°C had the highest Cry1Ab concentrations after 16 days, ranging between 39.8 ng/mL (borehole water) and 54.8 ng/mL (deionised water), with those exposed at room temperature having concentrations between 16.3 and 23.8 ng/mL (Fig. 1A). For the 10°C treatment, the maximum Cry protein concentrations after 16 days of leaf exposure were 14.8 ng/mL in borehole water and 10.5 ng/mL in deionised water.
The general tendency in all treatments was a slow increase in Cry1Ab concentration over time until the end of the 16-day period. No significant difference in Cry protein concentrations between the two types of water was found. Compared to the two lower temperature treatments, a marked increase in Cry1Ab protein concentration in both water treatments over the latter half of the experiment (192 h onwards) was observed in water kept at 30°C. The concentrations of Cry1Ab protein in the two types of water kept at 30°C were approximately 3.4- and 1.7-fold higher at the end of the exposure period, compared to the other temperature treatments. It would be expected that the highest concentration of Cry1Ab in the water would be reached later at lower temperatures when compared to higher temperatures. When comparing the entire infusion period, there was a statistically significant difference between Bt protein released into the water at 10°C and 30°C and between 21°C and 30°C, but not between 10°C and 21°C for both the water types (Kruskal-Wallis; p < 0.05). A study by Tank et al. (2010) indicated that the maximum concentration of Cry1Ab protein recorded in natural streams containing maize detritus was 32 ng/L (Tank et al., 2010), much lower than those recorded in this study. Carstens et al. (2012) reported that aquatic organisms in a pond or ditch with maize detritus could be exposed to a maximum concentration of 22.5-1 125 ng/mL or 0.67-33ng/mL of Bt protein, respectively, in the worst-case scenario assumptions for the risk assessment they did. The range of concentrations of Cry proteins recorded after 16 days in the two water types used in this study (Fig. 1A and 1B) ranged between 23.8 and 54.8ng/mL. Comparing this to an approximate 14.7 x 106 µg/mL that could potentially be present in the initial 24 g leaf material (we did not quantify the Cry1Ab contents in the leaf material in this study, but Andreassen et al. (2015) reported 612.51 ng Cry1Ab in 1 mg Mon810 leaf), what seems to be leaching out in these 16 days is but a tiny fraction of the potentially available Cry1Ab. While these concentrations are in the lower range of that predicted by Carstens et al. (2012) for a pond system, they are much higher than that reported by Tank et al. (2010) in natural streams. The methods described in this study can therefore be used as a guideline in planning of risk assessment studies on aquatic organisms, since this study gives an indication of the exposure scenarios that can be developed using these methods.
Strain and Lydy (2015) found that exposure time played a significant role in the release rate of the Cry1Ab proteins into the surrounding environment. They observed that the Cry proteins leached out of the leaf tissues at a rapid rate, peaking within a day or two (depending on the temperature) and then declining. This observation is not supported by our findings, where there was a gradual increase in Cry concentrations after 48 h. Strain and Lydy (2015) also reported higher concentrations of the Bt protein in colder temperatures than warmer temperatures, which is also opposite to the findings we report here. However, the experimental set-up used by Strain and Lydy (2015) was different to ours. The one difference that might explain the decline of Bt proteins from the water in their experiment is the presence of sediment in their microcosm, whereas sediment was absent from our experimental set-up. It is known that Cry proteins strongly adsorb to surface active particles of clay and organic matter in soils (Stotzky, 2005; Mueting et al., 2014), but in the absence of sediment, the Cry protein concentration increased up until the termination of our experiment at the end of Day 16.
The presence of sediment in the Strain and Lydy (2015) microcosm could possibly also explain the higher concentrations reported in the colder water (4°C). In contrast, we report higher concentrations for Cry protein at the higher temperatures and we did not add sediment. The temperatures in the current study were 10°C, 21°C and 30°C, while Strain and Lydy (2015) evaluated the influence of a similar temperature range (4°C, 23°C and 37°C). They added 10 dry leaf disks of 1.8 cm diameter to 75 mL water on top of 5 g sediment, whereas we added strips of dry leaf to the equivalent of 24 g to 1 L of water. Although we cannot definitively compare the leaf mass used in the two experiments, it is clear from the reported levels that the leaf mass:water ratio resulted in two orders of magnitude lower Cry concentrations in this study compared to that reported by Strain and Lydy (2015). Both Strain and Lydy (2015) and this study made use of unsterilised water (and unsterilised sediment in the case of Strain and Lydy (2015)), and although they reported no significant difference between the Cry levels in the unsterilised and sterilised aquatic environments, we postulate that the soil bacteria in the sediment could have metabolised the Cry proteins (Valldor et al., 2015). Furthermore, at higher temperatures higher metabolic rates may lead to decreased Cry protein levels. Because the present study was conducted without sediment, it is likely that there would have been less bacterial activity in our experimental set-up, explaining the steady increase in Cry levels over time. The plant material would also degrade quicker at higher temperatures, releasing the Cry protein and, in the absence of adsorbing sediment, contributing to the higher Cry levels at higher temperatures in this study.
No studies that can inform risk assessments regarding the effect of Bt maize on aquatic ecosystems have been done in South Africa. It is important that future studies address the possible effects of Cry proteins on non-target species that are closely related to the target pests of Cry proteins. Although the target Lepidoptera species of the Cry1Ab protein are all crop pests, off-target effects on other lepidopteran species may result if susceptible and closely related species ingest such proteins. Although Lepidoptera are characteristically terrestrial, the Pyralidae family includes several species with truly aquatic larvae (Gerber and Gabriel, 2002). The Pyralidae family also includes several maize and sugarcane pests which are susceptible to Cry1Ab protein, for example, Eldana saccharina (Walker) (Keeping et al. 2007) and Chilo partellus (Swinhoe) in South Africa (Van Rensburg, 1999).
CONCLUSIONS
Our data showed that accumulation of Cry1Ab protein released by Bt maize leaves is influenced by temperature and that the concentration of Cry proteins may increase over time. This study also quantified levels of Cry protein present in water that contains Bt maize leaf tissue in the absence of confounding factors such as sediment (and its associated microbial activity). These factors should be considered during risk assessment studies with aquatic organisms. The characterization of exposure of aquatic organisms along with the known specificity of the insecticidal trait, linked to the ecology of non-target species present in that habitat (in particular those closely related to Lepidoptera or other target groups), will contribute to improved risk assessment studies on aquatic environments.
AUTHOR CONTRIBUTIONS
Amy du Pisanie performed all experimental and analytical procedures, and prepared the first draft of the manuscript for submission. LH. du Preez and J van den Berg were supervisors of the study and R Pieters assisted with the ELISA and writing of the manuscript.
SOURCES OF FUNDING
This study was funded by Biosafety South Africa (Project BS08-001) and we accordingly give due acknowledgment.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to declare.
REFERENCES
ANDREASSEN M, BØHN T, WIKMARK O-G, VAN DEN BERG J, LØVIK M, TRAAVIK T and NYGAARD UC (2015) Cry1Ab protein from Bacillus thuringiensis and MON810 cry1Ab-transgenic maize exerts no adjuvant effect after airway exposure. Scand. J. Immunol. 81 192-200 https://doi.org/10.1111/sji.12269 [ Links ]
BAUMGARTE S and TEBBE CC (2005) Field studies on the environmental fate of Cry1Ab Bt-toxin produced by transgenic maize (MON810) and its effect on bacterial communities in the maize rhizosphere. Molec. Ecol. 14 2539-2551. https://doi.org/10.1111/j.1365-294X.2005.02592.x [ Links ]
BETZ FS, HAMMOND BG and FUCHS RL (2000) Safety and advantages of Bacillus thuringiensis - Protected plants to control insect pests. Regul. Toxicol. Pharmacol. 32 56-173. https://doi.org/10.1006/rtph.2000.1426 [ Links ]
BØHN T, PRIMICERIO R, HESSEN DO and TRAAVIK T (2008) Reduced fitness of Daphnia magna fed a Bt-transgenic maize variety. Arch. Environ. Contam. Toxicol. 55 584-592. https://doi.org/10.1007/s00244-008-9150-5 [ Links ]
BÖTTGER R, SCHALLER J, LINTOW S and DUDEL EG (2015) Aquatic degradation of Cry1Ab protein and decomposition dynamics of transgenic corn leaves under controlled conditions. Ecotox. Environ. Saf. 113 454-459. https://doi.org/10.1016/j.ecoenv.2014.12.034 [ Links ]
BRAVO A, GILL SS and SOBERóN M (2007) Mode of action of Bacillus thuringiensis Cry and Cyt toxins and their potential for insect control. Toxicon 49 423-435. https://doi.org/10.1016/j.toxicon.2006.11.022 [ Links ]
BRODERICK NA, RAFFA KF and HANDELSMAN J (2006) Midgut bacteria required for Bacillus thuringiensis insecticidal activity. PNAS 103 15196-15199. https://doi.org/10.1073/pnas.0604865103 [ Links ]
BROOKES G and BARFOOT P (2014) Economic impact of GM crops. GM Crops Food 5 65-75. https://doi.org/10.4161/gmcr.28098 [ Links ]
CARSTENS K, ANDERSON J, BACHMAN P, DE SCHRIJVER A, DIVELY G, FEDERICI B, HAMER M, GIELKENS M, JENSEN P, LAMP W, RAUSCHEN S, RIDLEY G, ROMEIS J. and WAGGONER A (2012) Genetically modified crops and aquatic ecosystems: considerations for environmental risk assessment. Transgenic Res. 21 813-842. https://doi.org/10.1007/s11248-011-9569-8 [ Links ]
CHAMBERS CP, WHILES MR, ROSI-MARSHALL EJ, TANK JL, ROYER TV, GRIFFITHS NA, EVANS-WHITE MA and STOJAK AR (2010) Responses of stream macro invertebrates to Bt maize leaf debris. Ecol. Applic. 20 1949-1960. https://doi.org/10.1890/09-0598.1 [ Links ]
FENG Y, LING L, FAN H, LIU Y, TAN F, SHU Y and WANG J (2011) Effects of temperature, water content and pH on degradation of Cry1Ab protein released from Bt corn straw in soil. Soil Biol. Biochem. 43 1600-1606. https://doi.org/10.1016/j.soilbio.2011.04.011 [ Links ]
GERBER A and GABRIEL MJM (2002) Aquatic Invertebrates of South African Rivers. Field Guide. Institute for Water Quality Studies, Department of Water Affairs and Forestry, Pretoria. [ Links ]
HOLDERBAUM DF, CUHRA M, WICKSON F, ORTH AI, NODARI RO and BØHN T (2015) Chronic responses of Daphnia magna under dietary exposure to leaves of transgenic (event MON810) Bt-maize hybrid and its conventional near isoline. J. Toxicol. Environ. Health A 78 (15) 998-1007. https://doi.org/10.1080/15287394.2015.1037877 [ Links ]
ISAAA (2017) Global Status of Commercialized Biotech/GM Crops: 2017. ISAAA Brief No. 53. ISAAA: Ithaca, New York. [ Links ]
JENSEN PD, DIVELY GP, SWAN CM and LAMP WO (2010) Exposure and non-target effects of transgenic Bt corn debris in streams. Environ. Entomol. 39 707-714. https://doi.org/10.1603/EN09037 [ Links ]
KEEPING MG, RUTHERFORD RS AND CONLONG DE (2007) Bt-maize as a potential trap crop for management of Eldana saccharina Walker (Lep., Pyralidae) in sugarcane. Appl. Entomol. 131 241-250. https://doi.org/10.1111/j.1439-0418.2007.01147.x [ Links ]
KRATZ W, MANTE C, HOFMANN F, SHLECHTRIEMEN U, KUHN U, OBER S. and VÖGELD R (2010) Exposure of maize harvest by-products to aquatic ecosystems and protected nature reserves in Brandenburg, Germany. In: Breckling B and Verhoeven R (eds) Implications of GM-Crop Cultivation at Large Spatial Scales. Peter Lang, Frankfurt, Germany. 21-23. [ Links ]
KRUGER M, VAN RENSBURG JBJ and VAN DEN BERG J (2009) Perspective on the development of stem borer resistance to Bt maize and refuge compliance at the Vaalharts irrigation scheme in South Africa. Crop Protect. 28 684-689. https://doi.org/10.1016/j.cropro.2009.04.001 [ Links ]
LI Y-L, DU J, FANG Z-X and YOU J (2013) Dissipation of insecticidal Cry1Ac protein and its toxicity to nontarget aquatic organisms. J. Agric. Food. Chem. 61 10864-10871. https://doi.org/10.1021/jf403472j [ Links ]
LI Y, WU K, ZHANG Y and YUAN G (2007) Degradation of Cry1Ac protein within transgenic Bacillus thuringiensis rice tissues under field and laboratory conditions. Environ. Entomol. 36 1275-1282. https://doi.org/10.1093/ee/36.5.1275 [ Links ]
LöVEI GL and ARPAIA S (2005). The impact of transgenic plants on natural enemies: a critical review of laboratory studies. Entomol. Exp. Applic. 114 1-14. https://doi.org/10.1111/j.0013-8703.2005.00235.x [ Links ]
MASEHELA TS, TERRAPON H, WINKER H and MAPHISA D (2016) An assessment of land use patterns for genetically modified crops in South Africa 2016: Technical Report Volume 1: GMO Monitoring and Research. Report Number: SANBI/GMO2016/2016/Vol1/A. South African National Biodiversity Institute, Newlands, Cape Town. [ Links ]
MUETING SA, STRAIN KE and LYDY MJ (2014) Validation of an extraction method for Cry1Ab protein from soil. Environ. Toxicol. Chem. 33 18-25. https://doi.org/10.1002/etc.2383 [ Links ]
NARANJO SE (2009) Impacts of Bt crops on non-target invertebrates and insecticide use patterns. CAB Reviews: Perspectives in Agriculture, Veterinary Science, Nutrition and Natural Resources 4, No. 011. [ Links ]
PALM CJ, DONEGAN K, HARRIS D and SEIDLER RJ (1994) Quantification in soil of Bacillus thuringiensis var. kurstaki δ‐endotoxin from transgenic plants. Molec. Ecol. 3 145-151. https://doi.org/10.1111/j.1365-294X.1994.tb00115.x [ Links ]
PHILLIPS AM (2008) Cry34Ab1, Cry35Ab1, Cry1F and PAT protein levels in hybrid maize TC1507, DAS-59122-7, MON 89034 x TC1507 x MON 88017 x DAS-59122-7, and a conventional control from the Monsanto 2006 production plan 06-01-52-04, Dow AgroSciences. LLC Sub-Report ID: 061026.05 https://www.testbiotech.org/sites/default/files/SmartStax_Expression_data_Testbiotech.pdf [ Links ]
PRIHODA KR and COATS JR (2008) Aquatic fate and effects of Bacillus thuringiensis Cry3Bb1 protein: Toward risk assessment. Environ. Toxicol. Chem. 27 793-798. https://doi.org/10.1897/07-300.1 [ Links ]
RAYBOULD A and QUEMADA H (2010) Bt crops and food security in developing countries: realised benefits, sustainable use and lowering barriers to adoption. Food Secur. 2 247-259. https://doi.org/10.1007/s12571-010-0066-3 [ Links ]
ROSI-MARSHALL EJ, TANK JL, ROYER T, WHILES MR, EVANS-WHITE M, CHAMBERS C, GRIFFITHS NA, POKELSEK J and STEPHEN ML (2007) Toxins in transgenic crop by products may affect headwater stream ecosystems. PNAS 104 16204-16208. https://doi.org/10.1073/pnas.0707177104 [ Links ]
SAXENA D, FLORES S and STOTZKY G (1999) Transgenic plants: Insecticidal toxin in root exudates from Bt corn. Nature 402 (6761) 480. https://doi.org/10.1038/44997 [ Links ]
SHOGREN AJ, TANK JL, ROSI EJ, DEE MM, SPEIR SL, BOLSTER D and EGAN SP (2019) Transport and instream removal of the Cry1Ab protein from genetically engineered maize is mediated by biofilms in experimental streams. PLOSONE 14 (5) e0213481. [ Links ]
STILLWELL L and SILVANOVICH A (2008) Assessment of Cry1A.105, Cry2Ab2, Cry3Bb1, and CP4 EPSPS 59122-7 protein levels in the combined trait corn product MON 89034 × TC1507 × MON 88017 × DAS- 59122-7 produced in U.S. field trials during 2006. Monsanto Company Biotechnology Regulatory Sciences, MSL0021070. URL: https://www.testbiotech.org/sites/default/files/SmartStax_Expression_data_Testbiotech.pdf (Accessed 21 May 2017). [ Links ]
STOTZKY G (2005) Persistence and biological activity in soil of the insecticidal proteins form Bacillus thuringiensis, especially from transgenic plants. Plant Soil 266 77-89. https://doi.org/10.1007/s11104-005-5945-6 [ Links ]
STRAIN KE, WHITING SA and LYDY MJ (2014) Laboratory and field validation of a Cry1Ab protein quantitation method for water. Talanta 128 109-116. https://doi.org/10.1016/j.talanta.2014.04.036 [ Links ]
STRAIN KE and LYDY MJ (2015) The fate and transport of the Cry1Ab protein in an agricultural field and laboratory aquatic microcosms. Chemosphere 132 94-100. https://doi.org/10.1016/j.chemosphere.2015.03.005 [ Links ]
SWAN CM, JENSEN PD, DIVELY GP and LAMP WO (2009) Processing of transgenic crop residues in stream ecosystem. J. Appl. Ecol. 46 1304-1313. https://doi.org/10.1111/j.1365-2664.2009.01728.x [ Links ]
TANK JL, ROSI-MARSHALL EJ, ROYERC TV, WHILES MR, GRIFFITHS NA, FRAUENDORF TC and TREERING DJ (2010) Occurrence of maize detritus and a transgenic insecticidal protein (Cry1ab) within the stream network of an agricultural landscape. PNAS 107 17645-17650. https://doi.org/10.1073/pnas.1006925107 [ Links ]
VALLDOR P, MIETHLING-GRAFF R, MARTENS R and TEBBE C (2015) Fate of the insecticidal Cry1Ab protein of GM crops in two agricultural soils as revealed by 14C-tracer studies. Appl. Microbiol. Biotechnol. 99 7333-7341. https://doi.org/10.1007/s00253-015-6655-5 [ Links ]
VAN DEN BERG J, HILBECK H and BØHN T (2013) Pest resistance to Cry1Ab Bt maize: field resistance, contributing factors and lessons from South Africa. Crop Protect. 54 154-160. https://doi.org/10.1016/j.cropro.2013.08.010 [ Links ]
VAN DEN BERG J and VAN WYK A (2007) The effect of Bt maize on Sesamia calamistis in South Africa. Entomol. Exp. Applic. 122 45-51. https://doi.org/10.1111/j.1570-7458.2006.00492.x [ Links ]
VAN RENSBURG JBJ (1999) Evaluation of Bt.-transgenic maize for resistance to the stem borers Busseola fusca (Fuller) and Chilo partellus (Swinhoe) in South Africa. S. Afr. J. Plant Soil. 16 38-43. [ Links ]
VENTER H and BØHN T (2016) Interactions between Bt crops and aquatic ecosystems: a review. Environ. Toxicol. Chem. 35 2891-2902. https://doi.org/10.1002/etc.3583 [ Links ]
VICTOROV AG (2011) Transfer of Bt corn byproducts from terrestrial to stream ecosystems. Russ. J. Plant Phys. 58 543-548. https://doi.org/10.1134/S1021443711040224 [ Links ]
WHITING SA, STRAIN KE, CAMPBEL LA, YOUNG BG and LYDY MJ (2014) A multi-year field study to evaluate the environmental fate and agronomic effects of insecticide mixtures. Sci. Total Environ. 498 543-542. https://doi.org/10.1016/j.scitotenv.2014.07.115 [ Links ]
ZWAHLEN C, HILBECK A, GUGERLI P and NENTWIG W (2003). Degradation of the Cry1Ab protein within transgenic Bacillus thuringiensis corn tissue in the field. Molec. Ecol. 12 765-775. https://doi.org/10.1046/j.1365-294X.2003.01767.x [ Links ]
Received 22 May 2018
Accepted in revised form 20 September 2019
* Corresponding author, email: rialet.pieters@nwu.ac.za