Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
Water SA
versão On-line ISSN 1816-7950
versão impressa ISSN 0378-4738
Water SA vol.45 no.4 Pretoria Out. 2019
http://dx.doi.org/10.17159/wsa/2019.v45.i4.7543
RESEARCH PAPERS
Finding optimal algal/bacterial inoculation ratio to improve algal biomass growth with wastewater as nutrient source
Le Anh PhamI, II; Julien LaurentI, *; Paul BoisI; Adrien WankoI
IICube, UMR 7357, ENGEES, CNRS, Université de Strasbourg, 2 rue Boussingault, 67000 Strasbourg, France
IIDepartment of Water-Environment-Oceanography, University of Science and Technology of Hanoi, Vietnam Academy of Science and Technology, 18 Hoang Quoc Viet, Hanoi, Vietnam
ABSTRACT
Algal growth, nutrient removal and settling efficiency were quantified while inoculating sequencing batch reactors with a mixture of microalgae and bacteria (activated sludge). Three algae/bacteria inoculation ratios (5:1, 1:1 and 1:5) as well as pure algal biomass (control) were assessed. Algal biomass production increased with the addition of activated sludge. However, the addition of too much activated sludge can cause disturbance to the Al-Bac biomass growth and algal bacterial processes. All reactors including the control with only algae showed similar settling and nutrient removal efficiencies. Good settling was observed in all reactors with only 5% of total biomass found in supernatant after 1 h of settling. Removal efficiencies of COD, TN and PO4-P in all reactors were 79-82%, 61-65% and 15-37%, respectively, with the lowest phosphorus removal efficiency belonging to 1:5 algae/activated sludge ratio. These results may be due to both long hydraulic (7 days) and solids retention times (up to 30 days). Finally, Al-Bac biomass with 1:1 inoculation ratio showed the best enhancement in terms of biomass growth and algal activities.
Keywords: microalgae, activated sludge, nutrients, wastewater, sequencing batch reactor
INTRODUCTION
The role of microalgae in wastewater treatment for photosynthetic aeration has been recognized for a long time (Oswald and Gotaas, 1957). Microalgae provide O2 to heterotrophic aerobic bacteria that oxidize organic pollution for their growth and energy requirements, using in turn the CO2 released from bacterial respiration for algal photosynthesis. This process is naturally driven, requiring only natural light for algal photosynthesis and hence significantly reducing operation costs as well as the carbon footprint of the wastewater treatment system, especially in comparison with conventional activated sludge technology (Van Den Hende et al., 2014). Moreover, during their growth, algal cells might accumulate high amount of lipids and carbohydrates. Hence algal biomass can be used as raw material for anaerobic digestion to produce biomethane or chemical extraction for biofuel production (Sirajunnisa and Surendhiran, 2016; Voloshin et al., 2016). Over the past few years, the use of microalgae for biomass production and wastewater treatment has received considerable attention and has been extensively studied (Park et al., 2011; Sutherland et al., 2015).
However, small cell size and low concentration in culture solution hampers efficient harvesting of algal biomass from water. An efficient harvesting process may account for up to 20 to 30% of total production cost (Mata et al., 2010; Pragya et al., 2013). Algae harvesting remains, therefore, one of the biggest challenges when operating these type of systems (Uduman et al., 2010; Christenson and Sims, 2011; Craggs et al., 2015). One possible solution might be to enhance algal biomass settleability by bio-flocculation (Salim et al., 2010; Vandamme et al., 2013). Indeed, inoculating activated sludge with algae in wastewater has been shown to improve biomass settling while maintaining good treatment efficiency (Gutzeit et al., 2005; Van Den Hende et al., 2011). Studies on algal-bacterial biomass indicated high gravitational settling efficiencies by flocculation between algae and bacteria (Gutzeit et al., 2005; Medina and Neis, 2007; Van Den Hende et al., 2014). Van Den Hende et al. (2014) recovered nearly 100% of algal-bacterial biomass from a pilot scale study via two simple harvesting steps, including gravity settling and dewatering by filter press, requiring no chemical addition or electricity. Most of these promising results, however, were achieved on pilot or semi-industrial scale. Although the potential of this technology is recognized, research on improving algal production while maintaining treatment efficiency is still needed before the system can be applied on an industrial scale (Park et al., 2011).
When co-culturing algae and bacteria, one important factor impacting algal growth is the inoculation ratio. Su et al. (2012b) reported that algae/activated sludge ratio of 5:1 was optimal to achieve good wastewater treatment and biomass settling. Roudsari et al. (2014) also compared several ratios between algae and activated sludge for processing anaerobic effluent from municipal wastewater. They suggested that biomass with a higher proportion of algal than bacterial biomass should be used. However, Van Den Hende et al. (2014, 2016) successfully developed an algal-bacterial biomass process with a higher proportion of activated sludge (1:1.8 to 1:3.8 for aquaculture and food-industrial wastewater treatment, respectively). It is important to note that these studies mainly focused on wastewater treatment efficiency and biomass harvesting. Data showing how the inoculation ratio of algae to activated sludge impacts algal growth, as well as the dynamics between the two biomasses, are still lacking.
Besides inoculation ratio, algal production is also limited by various environmental (light and temperature) as well as operational (pH, oxygen and mixing) factors (Park et al., 2011). Hence fundamental and pilot-scale studies should be conducted prior to application of the system at full scale for comprehensive understanding of these impacts on algal growth as well as performance of the system. An appropriate up-scaling approach involves: (i) studying the impact of inoculation ratio between algae and activated sludge on algal growth, settling and treatment efficiency, so that an optimal biomass ratio can be selected, (ii) applying the obtained optimal biomass ratio to a pilot-scale system for wastewater treatment and biomass production, in order to assess the modification of hydrodynamics and gas transfer due to biochemical processes, (iii) employing the knowledge from pilot studies in designing and operating the system at full scale, and (iv) using data collected from these studies to validate a mathematical model supporting system knowledge, management and optimization.
This study deals with the first part of the approach outlined above. Different algae/activated sludge inoculation ratios were compared in terms of algal growth, treatment efficiency and biomass settling. Lab-scale sequencing batch reactors were inoculated with different ratios and fed with synthetic wastewater. Biomass production, harvesting efficiency and treated effluent quality were monitored.
MATERIAL AND METHODS
Algae and activated sludge inoculations
Traditionally, in algae-based wastewater treatment systems, the term 'algae' usually refers to a consortium of local algal species grown in the wastewater which is allowed to develop in the system at the beginning of the process (Mara and Pearson, 1998). Although specific algal strain selection has been suggested to improve biomass growth and treatment efficiency, maintaining algal monoculture in a wastewater treatment system is difficult (Sutherland et al., 2015). An important advantage of using a local algal consortium is that it ensures compatibility between the algae and bacteria as well as between the microorganisms and wastewater used (Muñoz and Guieysse, 2006; Mata et al., 2010). Therefore, the experiments in this study used a local algal consortium as inoculation source.
Algal inoculation source was a green algal mixture collected by brushing the biomass attached to the wall of a secondary sedimentation tank of a full-scale wastewater treatment plant (WWTP - Rosheim, 67, France). The biomass was then stored in a plastic bottle and transported to the laboratory within 2 h of collection. At the laboratory, biomass was allowed to settle for 1 hour. After this, only settled biomass was collected and served as algal or bacterial inoculum. No purification process was carried out for algal biomass, hence bacterial contamination was unavoidable. Activated sludge was collected from the aeration tank in the same WWTP right before the experiment and processed similarly to algal biomass.
Microscopic observation (light microscope Olympus BH−2) showed that the mixture predominantly contained algae from the following microalgae genera: Chlorella sp., Ulothrix sp. or Klebsormidium sp., Desmodesmus sp., and Pseudanabaena sp.
This mixture was cultivated for 4 weeks with synthetic wastewater in a batch reactor as described under 'Experimental operation' below.
The inoculation ratio was based on final total suspended solids content (TSS) of algae and activated sludge in culture solution. The amount of algae inoculated was the same for all reactors in order to compare the growth of algae with different inoculation ratios. Four reactors were employed in which algal biomass concentration was 0.2 g∙L−1 while activated sludge concentrations inoculated were 0.04, 0.2, 1 and 0 g∙L−1, giving algal/sludge inoculation ratios of 5:1, 1:1, 1:5 and 1:0, respectively. The reactor with only algae (1:0) was used as a control. The algal-bacterial biomass developed in this study was referred as Al-Bac biomass.
Synthetic wastewater
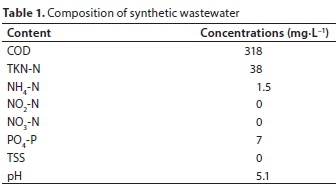
Synthetic wastewater was the only nutrient source used to cultivate the biomass. It was prepared and adapted following the international standard of the Organization for Economic Cooperation and Development (OECD, 2001; O'Flaherty and Gray, 2013). The ingredients included meat extract Viandox (1 mL∙L−1), peptone (160 mg∙L−1), urea (30 mg∙L−1), K2HPO4 (28 mg∙L−1), NaCl (7 mg∙L−1), CaCl2∙2H2O (4 mg∙L−1), and Mg2SO4∙7H2O (2 mg∙L−1). The resulting wastewater parameters, immediately analysed after preparation, are shown in Table 1.
Experimental operation
Each biomass was cultured at room temperature (20.9 ± 0.6°C) in a 5 L (working volume) transparent glass bottle (18 cm diameter) with a cap (Fig. 1). Mixing was ensured by a magnetic stirrer at 300 r∙min−1. Each reactor was operated as a sequencing batch reactor (SBR) without mechanical aeration. The SBR cycle consisted of a feeding phase (pump of 2.5 L of influent wastewater), a reaction phase, and a settling phase. A flexible plastic tube was used for extracting supernatant at the end of the settling phase and feeding the new synthetic wastewater at the beginning of the feeding phase. The volume exchange ratio was 50%. Feeding, reaction and settling phase durations were 1 h, 3.5 days and 1 h, respectively. The mean hydraulic residence time (HRT) was 7 days.

All reactors received the same illumination from 6 cool white light LEDs positioned 10 cm away from the reactors in vertical direction. Light intensity measured at the wall of reactor was 66 µEs−1∙m−2. Photoperiod was set up to 12 h light:12 h dark. Total culturing period was 1 month.
Analytical procedures
Dissolved oxygen concentration (DO) (WTW Inolab Oxi Level II Dissolved Oxygen Meter), pH and temperature (WTW pocket pH meter kits pH330) were measured daily at the central point of each reactor 5 h after illumination started and always before the settling phase. Due to this daily measurement frequency, it should be noticed that pH monitoring was performed once right before the feeding and then 1 day after.
Sampling for biomass analysis was performed twice per week at the end of each reaction phase. 100 mL of the well-mixed solution was sampled, right before the settling phase. Then the first 50 mL of this volume were filtered using 1.2 µm glass fibre filter (FILTRES RS) and used for TSS content determination (AFNOR NF T 90−105, 1997) The remaining 50 mL were filtered using 0.45 µm cellulose nitrate filter paper (Merck Millipore Ltd.) in dark conditions. The filter paper with suspension was then covered by aluminium paper, labelled and frozen before being analysed for total Chlorophyll a (Chl-a) content (AFNOR NF T 90−117, 1999).
The growth curves of TSS and Chl-a were fitted using linear regression in order to compare the global growth rates between the experiments. Standard error was used to evaluate the variances of the fitted values and observed values of the biomass or Chl-a growth rates (Crawley, 2012).
Sampling for nutrient content was performed daily including the same days as biomass sampling. Nutrient content was assessed in both input synthetic wastewater and supernatant effluent. At the beginning of each feeding phase, 300 mL of suspension was collected and filtered through sterile membrane (0.45 µm, filtraTECH) and frozen until analysis (within 1 month) of phosphorus (PO4-P) (ISO 6878:2004, 2004), nitrite nitrogen (NO2-N) (ISO 6777:1984, 1984), nitrate nitrogen (NO3-N) (ISO 7890-3:1988, 1988) and ammonium nitrogen (NH4-N) ((ISO 5664:1984, 1984)). Another unfiltered 100 mL sample was collected and used to analyse total Kjeldahl nitrogen (TKN-N) (ISO 5663:1984, 1984) and chemical oxygen demand (COD) (ISO 15705:2002, 2002).
Data analysis
Data collected were analysed by one-way analysis of variance (one-way ANOVA) with 95% confidence interval to assess if there was a statistical difference between the results for these systems. If a significant difference was detected, Holm tests were used to determine which pair of systems had a statistical difference at a 95% confidence interval. In addition, Welch test with 95% confidence interval was used to compare data representing different growing phases of each reactor. Data analysis was performed using R software (version 3.3.1 (2016-06−21)). Standard error was used to indicate the deviation from the mean with small sample size (n < 30).
RESULTS AND DISCUSSION
Dynamics of dissolved oxygen and pH
The dynamics of algal-bacterial processes can be evaluated by assessing dissolved oxygen (DO) and pH variations. DO concentration is mainly governed by photosynthetic (oxygen production) and oxidative (oxygen uptake) activities of algae and bacteria, respectively (Muñoz and Guieysse, 2006). Via photosynthesis, algae consume inorganic carbon (HCO3-, CO2) leading to an increase of pH in solution (Richmond, 2004; Park et al., 2010; Sutherland et al., 2015) while nitrification releases protons leading to pH decrease. These parameters were measured daily in each reactor to evaluate algal-bacterial processes during feeding and reaction phases (Figs 2 and 3).
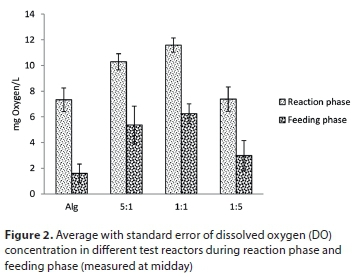
As expected, the feeding phase resulted in higher bacterial activity (heterotrophic growth and nitrification) because a high amount of dissolved organic matter and nutrients was available as substrate. For each reactor, this led to faster DO consumption and a decrease in its concentration: DO measured in the reaction phase was always higher than that in the feeding phase (p < 0.05). Then, during the reaction phase, bacterial activity slowed down and O2 release by algae led to O2 increase in the medium.
The control reactor (algae only) and reactor with 1:5 algae/activated sludge inoculation ratio had similar DO content (p > 0.05). This was also the case between reactors with 1:1 and 5:1 algae/activated sludge ratios (p > 0.05). However, DO contents recorded in reactors with 1:1 and 5:1 algae/activated sludge ratios were higher than the control and 1:5 reactors (p < 0.05). These results are in agreement with Chl-a and TSS data (Figs 4 and 5) that showed that addition of activated sludge enhanced algal growth but that adding too much activated sludge leads to disturbances in algal growth.
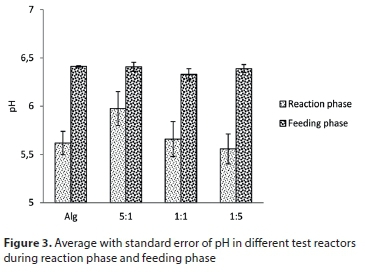
The pH level in an algal bacterial reactor is the consequence of algal productivity, algal/bacterial respiration, the alkalinity and ionic equilibriums, and autotrophic and heterotrophic microbial activities such as nitrification (Park et al., 2010).
Surprisingly, pH measured in the reaction phase was always lower than the pH measured in the feeding phase (Fig. 3), which was statistically proved by the Welch test with 95% confidence interval (p < 0.05). However, one-way ANOVA with 95% confidence interval indicated that there is no significant difference between pH measured during the reaction phase of the four studied systems (p > 0.05). The same conclusion was reached for pH in the feeding phase of all reactors (p > 0.05).
Concerning pH, the observed values are globally acidic. Furthermore, photosynthesis during the reaction is supposed to make pH increase. However, the opposite trend was observed. Several causes can explain these unexpected trends:
•The prepared synthetic wastewater had a low pH (Table 1); this can explain the generally low pH level obtained during the entire experiment.
•The high pH increase (around one pH unit) between reaction and feeding phase indicated intensive photosynthetic activity following a feeding event (pH measurement was performed one day after).
•The decrease observed in the remaining days is mainly due to the nitrification process which acidifies the medium. This is consistent with Su et al. (2011) who observed a slight decrease in pH level over the first 5 days of each batch.
The fact that the nitrification process impact only appears after a few days is linked to the slower kinetics associated with this process: the maximum growth rates have been reported to be 1.3 d−1 and 0.63 d−1 at 20°C for algae and ammonia-oxidizing bacteria, respectively (Solimeno et al., 2017). Also, according to the stoichiometry of these biochemical reactions (Solimeno et al., 2017), the observed oxidation of around 25% of the nitrogen contained in the synthetic wastewater (see below) leads to the release of 1.35 meq H+∙L−1 of protons (2 meq per mmol of N-NH4+) while the maximum observed growth of algae (20 mg∙L−1∙d−1) leads to the release of 0.6 meq alkalinity∙L−1. It should also be mentioned that the synthetic wastewater used had very low alkalinity, making it very sensitive to proton release. These low pH values could exert toxicity and/or inhibition effects on the biomass. However, the use of synthetic wastewater in this study could have played a role in avoiding these types of effects.
These results indicate that nitrification plays a significant role in TKN removal in these reactors.
Biomass growth
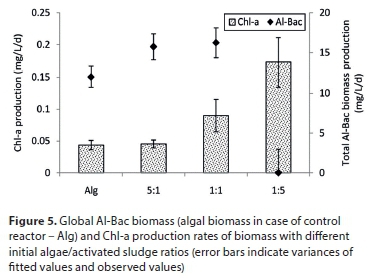
The growth of total Al-Bac biomass during the experimental period was estimated by TSS measurements. Since dissolved organic matter and nutrients were the only supplement provided for each reactor, any increase in total suspended solids inside the reactor was considered as a gain in biomass. Besides the total Al-Bac biomass, the global production of Chl-a in each reactor during the experimental period, which is related to the increase of algae inside Al-Bac biomass (Park and Craggs, 2010), was also monitored (Fig. 4). TSS and Chl-a concentrations increased almost linearly. The slopes of TSS and Chl-a concentrations vs. time were used to derive the production rates displayed in Fig. 5.
After 1 month of experiments, all reactors showed a gain in biomass except the reactor with inoculation ratio of 1:5. The biomass growth rate in the reactor with only algae was lower than the ones inoculated with both algae and activated sludge (5:1 and 1:1). However, there was nearly no differences between growth rate of Al-Bac biomass 5:1 and 1:1. This result suggests that inoculation with both algae and activated sludge increases the production of Al-Bac biomass in comparison with only algae, but that an excessive amount of activated sludge added could decrease the growth of the biomass. A similar result was reported by Su et al. (2012b): with too much activated sludge added, the total algal-bacterial biomass increase at the end of the test was not as high as for other biomasses with lower activated sludge added. Disturbances in Al-Bac biomass growth could originate from the complex interactions between algae and bacteria in activated sludge (Cole, 1982; Kouzuma and Watanabe, 2015). Besides synergistic interactions resulting in fostering the growth of both algae and bacteria, there are antagonistic interactions between these organisms. These interactions, however, are numerous and depend on the species of algae and bacteria, growing states, and environmental conditions (Grossart and Simon, 2007).
In addition, the production rates of Chl-a in reactors with 1:1 and 1:5 ratios were higher than the control reactor with only algae, indicating an acceleration of algal growth with the addition of activated sludge. However, there was no difference between Chl-a production between Al-Bac biomass with 5:1 ratio and the control. This result is in good agreement with the conclusion reached by Roudsari et al. (2014), who observed that addition of activated sludge to up to 40% of the total biomass speeded up algal growth.
In comparison with literature, biomass volumetric production achieved in this study (below 20 mg∙L−1∙d−1) could be considered to be rather low (Mata et al., 2010; Park et al., 2010). Su et al. (2011) cultivated an algae/activated sludge biomass in a batch reactor and reported a volumetric productivity of 38.8 mg∙L−1∙d−1. Van Den Hende et al. (2011) observed a mean value of 181 mg∙L−1∙d−1of algae/activated sludge biomass production in a reactor with flue gas supplement. In addition, Park and Craggs (2010) reported an algal-bacterial biomass volumetric production of 100 mg∙L−1∙d−1 obtained in an outdoor pilot high-rate algal pond wastewater treatment system with CO2 addition. This may be explained by the low light intensity of 66 µEs−1m−2 applied in the present experiment. Indeed, algal growth and activity is enhanced under light intensity ranging from 200 to 400 µE s−1m−2 (Muñoz and Guieysse, 2006; Singh and Singh, 2015).
The increase of Al-Bac biomass and Chl-a with 1:5 inoculation ratio suggests a significant replacement of the activated sludge biomass by algal biomass inside the Al-Bac biomass during the experiment. This illustrates the different dynamics of algal and bacterial growth in the system.
Biomass growth and settleability
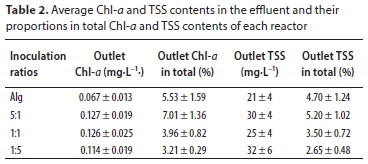
Settling efficiency was evaluated by measuring supernatant TSS and Chl-a concentrations after 1 h of gravitational settling. This reflects both wastewater treatment efficiency in terms of TSS and the possibility of efficiently harvesting the biomass.
All reactors provided good Chl-a and Al-Bac biomass settling efficiencies (Table 2). This indicates good bio-flocculation between algae and activated sludge, as was observed in other studies (Gutzeit et al., 2005; Su et al., 2012b). Surprisingly, the control reactor with only algae also showed similar settling efficiency. This result differs from other studies which reported lower biomass settling efficiency of algae alone (Su et al., 2012b). The settling velocity of microalgal suspensions can differ greatly depending on culturing conditions (Gutiérrez et al., 2016). Several factors are involved, including long SRT (30 days) (Valigore et al., 2012), the dynamics of algae species (Su et al., 2012a) or pH (Vandamme et al., 2014). However, pH-induced flocculation is unlikely in the present study as pH was quite low (Fig. 3). In fact, the most probable factor explaining this observation is granulation as this was recently observed in SBRs (Liu et al., 2017; Cai et al., 2019). Indeed, the experimental conditions applied in this study may have selected for fast-settling biomass.
Nutrient removal efficiency
Similar effluent concentrations were recorded for all reactors (Table 3). The average COD removal efficiencies were 82±2, 79±2, 81±2 and 79±2% for the reactors with only algae, 5:1, 1:1 and 1:5 algae/activated sludge inoculation ratios, respectively. As there was significant COD removal in the control reactor with only algae, it does mean that bacteria growth occurred to some extent even without activated sludge inoculation. In comparison with other algal-bacterial biomass studies, COD removal efficiencies obtained in this study were at a good level (Gutzeit et al., 2005; Medina and Neis, 2007; Su et al., 2012b; Roudsari et al., 2014). However, phosphorus removal was not as high, with removal efficiencies of 30±5, 37±5, 33±3 and 15±11% for reactors with only algae, 5:1, 1:1 and 1:5 ratios, respectively.
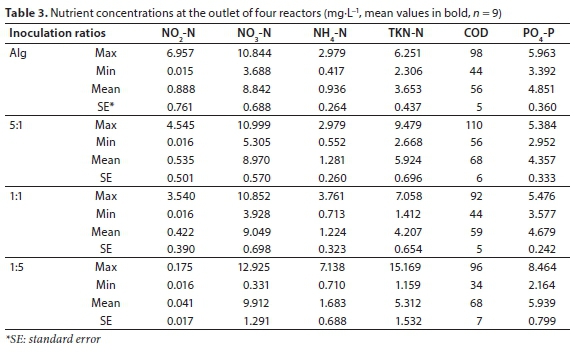
TKN-N removal ranged from 86 to 90%. Moreover, low NH4-N and NO2-N concentrations were measured in the effluent. Nitrification was therefore occurring to a large extent in all reactors, which is not in agreement with other algal-bacterial biomass studies (Gutzeit et al., 2005; Van Den Hende et al., 2011; Su et al., 2012b). Considering the NO3-N concentrations, the total nitrogen removal efficiencies were 65±1, 61±2, 64±2, 61±3% for the reactors with only algae, 5:1, 1:1 and 1:5 algae/activated sludge inoculation ratios, respectively.
Nutrient removal efficiencies were similar between all tested reactors, which is not in agreement with other reports where different inoculation ratios induced varying efficiencies (Su et al., 2012b; Roudsari et al., 2014). Roudsari et al. (2014) conducted a 6-day batch experiment and concluded that a higher proportion of activated sludge improved COD removal while a higher proportion of algae improved ammonium nitrogen removal. The reason for these differences may derive from the long hydraulic and solids retention times (HRT = 7 days, SRT = 30 days) applied in the current experiment (García et al., 2000, 2002; Matamoros et al., 2015; Sutherland et al., 2015).
It is also important to note that the algal biomass inoculated in the control reactor was not pure culture, and thus bacteria, even in small amounts, were expected. Therefore, synthetic wastewater fed to the control reactor may stimulate bacterial growth. Thus, in this study, long HRT and SRT, as well as readily degradable organic matter, provide conditions that can promote the growth of this small amount of bacteria, even in the control reactor (Su et al., 2012b). Consequently, nitrification and heterotrophic growth of bacteria were also observed in this reactor.
The only exception was noted for phosphorus removal efficiency of Al-Bac biomass 1:5 reactor, where the removal efficiency varied widely (15±11%). This instability may originate from the high amount of activated sludge inoculated.
Final choice of optimal inoculation ratio
Results of this study showed an improvement in DO concentration in solution when an appropriate amount of activated sludge is added (1:1 and 5:1 algae/sludge ratios). In comparison, Al-Bac 5:1 had good total biomass growth, and good algal activity and nutrient removal efficiency. Nevertheless, it displayed a low algal growth rate similar the control reactor with only algal inoculum. Finally, Al-Bac 1:1 showed the best improvement in terms of total biomass, algal biomass growth and algal activity. A long-term study with a larger scale system is required to understand more about the dynamics between algae and bacteria. With these considerations, Al-Bac biomass with 1:1 inoculation ratio should be chosen for applying in a pilot culturing system for wastewater treatment and biomass production.
CONCLUSIONS
In this study, sequencing batch reactors were used to cultivate Al-Bac biomass with different algae/sludge inoculation ratios. In order to compare algal growth, initial algal biomass was similar in every test. DO concentration and Chl-a content in all reactors were used to evaluate algal activities, with high levels of DO and Chl-a growth rate indicating good algal activities in the reactor. Local algal biomass showed good incorporation with bacterial biomass (activated sludge): better algal growth occurred with Al-Bac biomass than with only algae.
Several conclusions were drawn as follows:
•Adding activated sludge accelerated the growth of Al-Bac biomass although the addition of too much activated sludge may cause disturbance to the total biomass growth. Algal growth also increased with addition of activated sludge but a significant amount of sludge was required to observe a significant change.
•Biomass settling and nutrient removal efficiencies were similar in every test including the control with only algae. Possible reasons include long hydraulic and solids retention times and occurrence of granulation.
•Among the three inoculation ratios evaluated, Al-Bac biomass with 1:1 inoculation ratio showed the best enhancement in total biomass, algal biomass growth, and algal activities.
REFERENCES
AFNOR NF T 90−105 (1997) Qualité de l'eau - Dosage des matières en suspension - Méthode par centrifugation. 1997. [ Links ]
AFNOR NF T 90−117 (1999) Qualité de l'eau - Dosage de la chlorophylle a et d'un indice phéopigments - Méthode par spectrométrie d'absorption moléculaire. 1999. [ Links ]
CAI W, ZHAO Z, LI D, LEI Z, ZHANG Z and LEE D-J (2019) Algae granulation for nutrients uptake and algae harvesting during wastewater treatment. Chemosphere 214 55-59. https://doi.org/10.1016/j.chemosphere.2018.09.107 [ Links ]
CHRISTENSON L and SIMS R (2011) Production and harvesting of microalgae for wastewater treatment biofuels and bioproducts. Biotechnol. Adv. 29 (6) 686-702. https://doi.org/10.1016/j.biotechadv.2011.05.015 [ Links ]
COLE JJ (1982) Interactions between bacteria and algae in aquatic ecosystems. Annu. Rev. Ecol. Syst. 13 (1) 291-314. https://doi.org/10.1146/annurev.es.13.110182.001451 [ Links ]
CRAGGS R, PARK J, SUTHERLAND D and HEUBECK S (2015) Economic construction and operation of hectare-scale wastewater treatment enhanced pond systems. J. Appl. Phycol. 27 (5) 1913-1922. https://doi.org/10.1007/s10811-015-0658-6 [ Links ]
CRAWLEY MJ (2012) The R Book (2nd edn). Wiley-Blackwell, Chichester. 1076 pp. [ Links ]
GARCÍA J., MUJERIEGO R and HERNANDEZ-MARINE M (2000) High rate algal pond operating strategies for urban wastewater nitrogen removal. J. Appl. Phycol. 12 (3-5) 331-339. https://doi.org/10.1023/A:1008146421368 [ Links ]
GARCÍA J, HERNÁNDEZ-MARINÉ M and MUJERIEGO R (2002) Analysis of key variables controlling phosphorus removal in high rate oxidation ponds provided with clarifiers. Water SA 28 (1) p-55. https://doi.org/10.4314/wsa.v28i1.4868 [ Links ]
GROSSART H-P and SIMON M (2007) Interactions of planktonic algae and bacteria: effects on algal growth and organic matter dynamics. Aquat. Microb. Ecol. 47 (2) 163. https://doi.org/10.3354/ame047163 [ Links ]
GUTIÉRREZ R, FERRER I, UGGETTI E, ARNABAT C, SALVADÓ H and GARCÍA J (2016) Settling velocity distribution of microalgal biomass from urban wastewater treatment high rate algal ponds. Algal Res. 16 409-417. https://doi.org/10.1016/j.algal.2016.03.037 [ Links ]
GUTZEIT G, LORCH D, WEBER A, ENGELS M and NEIS U (2005) Bioflocculent algal-bacterial biomass improves low-cost wastewater treatment. Water Sci. Technol. 52 (12) 9-18. https://doi.org/10.2166/wst.2005.0415 [ Links ]
ISO 5663:1984 (1984) Water quality - Determination of Kjeldahl nitrogen - Method after mineralization with selenium. 1984. ISO, Geneva. [ Links ]
ISO 5664:1984 (1984) Water quality - Determination of ammonium - Distillation and titration method. 1984. ISO, Geneva. [ Links ]
ISO 6777:1984 (1984) Water quality - Determination of nitrite - Molecular absorption spectrometric method. 1984. ISO, Geneva. [ Links ]
ISO 6878:2004 (2004) Water quality - Determination of phosphorus - Ammonium molybdate spectrometric method. 2004. ISO, Geneva. [ Links ]
ISO 7890-3:1988 (1988) Water quality - Determination of nitrate - Part 3: Spectrometric method using sulfosalicylic acid. 1988. ISO, Geneva. [ Links ]
ISO 15705:2002 (2002) Water quality - Determination of the chemical oxygen demand index (ST-COD) - Small-scale sealed-tube method. 2002. ISO, Geneva. [ Links ]
KOUZUMA A and WATANABE K (2015) Exploring the potential of algae bacteria interactions. Curr. Opin. Biotechnol. 33 125-129. https://doi.org/10.1016/j.copbio.2015.02.007 [ Links ]
LIU L, FAN H, LIU Y, LIU C and HUANG X (2017) Development of algae-bacteria granular consortia in photo-sequencing batch reactor. Bioresour. Technol. 232 64-71. https://doi.org/10.1016/j.biortech.2017.02.025 [ Links ]
MARA DD and PEARSON HW (1998) Design Manual for Waste Stabilization Ponds in Mediterranean Countries. Lagoon Technology International, Leeds, UK. 112 pp. [ Links ]
MATA TM, MARTINS AA and CAETANO N (2010) Microalgae for biodiesel production and other applications: A review. Renew. Sustain. Energ. Rev. 14 (1) 217-232. https://doi.org/10.1016/j.rser.2009.07.020 [ Links ]
MATAMOROS V, GUTIÉRREZ R, FERRER I, GARCÍA J and BAYONA JM (2015) Capability of microalgae-based wastewater treatment systems to remove emerging organic contaminants: a pilot-scale study. J. Hazardous Mater. https://doi.org/10.1016/j.jhazmat.2015.02.002 [ Links ]
MEDINA M and NEIS U (2007) Symbiotic algal bacterial wastewater treatment: Effect of food to microorganism ratio and hydraulic retention time on the process performance. Water Sci. Technol. 55 (11) 165-71. https://doi.org/10.2166/wst.2007.351 [ Links ]
MUNOZ R and GUIEYSSE B (2006) Algal bacterial processes for the treatment of hazardous contaminants A review. Water Res. 40 (15) 2799-2815. https://doi.org/10.1016/j.watres.2006.06.011 [ Links ]
OECD (2001) OECD GUIDELINE FOR THE TESTING OF CHEMICALS. Simulation Test - Aerobic Sewage Treatment: 303 A: Activated Sludge Units - 303 B: Biofilms. 22. January 2001. OECD Publishing. https://doi.org/10.1787/9789264070424-en [ Links ]
O'FLAHERTY E and GRAY NF (2013) A comparative analysis of the characteristics of a range of real and synthetic wastewaters. Environ. Sci. Pollut. Res. 20 (12) 8813-8830. https://doi.org/10.1007/s11356-013−1863-y [ Links ]
OSWALD WJ and GOTAAS HB (1957) Photosynthesis in sewage treatment. Trans. Am. Soc. Civ. Eng. 122 (1) 73-97. [ Links ]
PARK JBK and CRAGGS RJ (2010) Wastewater treatment and algal production in high rate algal ponds with carbon dioxide addition. Water Sci. Technol. 61 (3) 633. https://doi.org/10.2166/wst.2010.951 [ Links ]
PARK JBK, CRAGGS RJ and SHILTON AN (2010) Wastewater treatment high rate algal ponds for biofuel production. Bioresour. Technol. 102 (1) 35-42. https://doi.org/10.1016/j.biortech.2010.06.158 [ Links ]
PARK JBK, CRAGGS RJ and SHILTON AN (2011) Wastewater treatment high rate algal ponds for biofuel production. Bioresour. Technol. 102 (1) 35-42. https://doi.org/10.1016/j.biortech.2010.06.158 [ Links ]
PRAGYA N, PANDEY KK and SAHOO PK (2013) A review on harvesting, oil extraction and biofuels production technologies from microalgae. Renew. Sustain. Energ. Rev. 24 159-171. https://doi.org/10.1016/j.rser.2013.03.034 [ Links ]
RICHMOND A (2004) Handbook of Microalgal Culture: Biotechnology and Applied Phycology. Blackwell Publishing, Oxford, UK. 587 pp. [ Links ]
ROUDSARI FP, MEHRNIA MR, ASADI A, MOAYEDI Z and RANJBAR R (2014) Effect of microalgae/activated sludge ratio on cooperative treatment of anaerobic effluent of municipal wastewater. Applied Biochemistry and Biotechnology 172 (1) 131-140. https://doi.org/10.1007/s12010-013-0480-z [ Links ]
SALIM S, BOSMA R, VERMUË MH and WIJFFELS RH (2010) Harvesting of microalgae by bio-flocculation. J. Appl. Phycol. 23 (5) 849-855. https://doi.org/10.1007/s10811-010-9591-x [ Links ]
SINGH SP and SINGH P (2015) Effect of temperature and light on the growth of algae species: A review. Renew. Sustain. Energ. Rev. 50 431-444. https://doi.org/10.1016/j.rser.2015.05.024 [ Links ]
SIRAJUNNISA AR and SURENDHIRAN D (2016) Algae - a quintessential and positive resource of bioethanol production: A comprehensive review. Renew. Sustain. Energ. Rev. 66 248-267. https://doi.org/10.1016/j.rser.2016.07.024 [ Links ]
SOLIMENO A, PARKER L, LUNDQUIST T and GARCÍA J (2017) Integral microalgae-bacteria model (BIO_ALGAE): Application to wastewater high rate algal ponds. Sci. Total Environ. 601-602 646-657. https://doi.org/10.1016/j.scitotenv.2017.05.215 [ Links ]
SU Y, MENNERICH A and URBAN B (2011) Municipal wastewater treatment and biomass accumulation with a wastewater-born and settleable algal-bacterial culture. Water Res. 45 (11) 3351-3358. https://doi.org/10.1016/j.watres.2011.03.046 [ Links ]
SU Y, MENNERICH A and URBAN B (2012a) Comparison of nutrient removal capacity and biomass settleability of four high-potential microalgal species. Bioresour. Technol. 124 157-162. https://doi.org/10.1016/j.biortech.2012.08.037 [ Links ]
SU Y, MENNERICH A and URBAN B (2012b) Synergistic cooperation between wastewater-born algae and activated sludge for wastewater treatment: Influence of algae and sludge inoculation ratios. Bioresour. Technol. 105 67-73. https://doi.org/10.1016/j.biortech.2011.11.113 [ Links ]
SUTHERLAND DL, HOWARD WILLIAMS C, TURNBULL MH, BROADY PA and CRAGGS RJ (2015) Enhancing microalgal photosynthesis and productivity in wastewater treatment high rate algal ponds for biofuel production. Bioresour. Technol. 184 (Advances in biofuels and chemicals from algae) 222-229. https://doi.org/10.1016/j.biortech.2014.10.074 [ Links ]
UDUMAN N, QI Y, DANQUAH MK, FORDE GM and HOADLEY A (2010) Dewatering of microalgal cultures: a major bottleneck to algae-based fuels. J. Renew. Sustain. Energ. 2 (1) 012701. https://doi.org/10.1063/1.3294480 [ Links ]
VALIGORE JM, GOSTOMSKI PA, WAREHAM DG and O'SULLIVAN AD (2012) Effects of hydraulic and solids retention times on productivity and settleability of microbial (microalgal-bacterial) biomass grown on primary treated wastewater as a biofuel feedstock. Water Res. 46 (9) 2957-2964. https://doi.org/10.1016/j.watres.2012.03.023 [ Links ]
VAN DEN HENDE S, BEELEN V, BORE G, BOON N and VERVAEREN H (2014) Upscaling aquaculture wastewater treatment by microalgal bacterial flocs From lab reactors to an outdoor raceway pond. Bioresour. Technol. 159 342-354. https://doi.org/10.1016/j.biortech.2014.02.113 [ Links ]
VAN DEN HENDE S, BEELEN V, JULIEN L, LEFOULON A, VANHOUCKE T, COOLSAET C, SONNENHOLZNER S, VERVAEREN H and ROUSSEAU DPL (2016) Technical potential of microalgal bacterial floc raceway ponds treating food-industry effluents while producing microalgal bacterial biomass: An outdoor pilot-scale study. Bioresour. Technol. 218 969-979. https://doi.org/10.1016/j.biortech.2016.07.065 [ Links ]
VAN DEN HENDE S, VERVAEREN H, DESMET S and BOON N (2011) Bioflocculation of microalgae and bacteria combined with flue gas to improve sewage treatment. New Biotechnol. 29 (1) 23-31. https://doi.org/10.1016/j.nbt.2011.04.009 [ Links ]
VANDAMME D, FOUBERT I and MUYLAERT K (2013) Flocculation as a low-cost method for harvesting microalgae for bulk biomass production. Trends Biotechnol. 31 (4) 233-239. https://doi.org/10.1016/j.tibtech.2012.12.005 [ Links ]
VANDAMME D, MUYLAERT K, FRAEYE I and FOUBERT I (2014) Floc characteristics of Chlorella vulgaris: Influence of flocculation mode and presence of organic matter. Bioresour. Technol. 151 383-387. https://doi.org/10.1016/j.biortech.2013.09.112 [ Links ]
VOLOSHIN RA, RODIONOVA MV, ZHARMUKHAMEDOV SK, NEJAT VEZIROGLU T and ALLAKHVERDIEV SI (2016) Review: Biofuel production from plant and algal biomass. Int. J. Hydrog. Energ. 41 (39) 17257-17273. https://doi.org/10.1016/j.ijhydene.2016.07.084 [ Links ]
Received 26 January 2018
Accepted in revised form 26 September 2019
* Corresponding author, email: julien.laurent@engees.unistra.fr














