Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Water SA
On-line version ISSN 1816-7950
Print version ISSN 0378-4738
Water SA vol.44 n.4 Pretoria Oct. 2018
http://dx.doi.org/10.4314/wsa.v44i4.04
ORIGINAL ARTICLES
Suitability of treated wastewater with respect to pH, electrical conductivity, selected cations and sodium adsorption ratio for irrigation in a semi-arid region
Pholosho M KgopaI, *; Phatu W MashelaI; Alen ManyevereII
IDepartment of Plant Production, Soil Science and Agricultural Engineering, University of Limpopo Private Bag X 1106, Sovenga 0727, South Africa
IIDepartment of Agronomy, University of Fort Hare, Private Bag X1314, King William's Town Rd, Alice 5700, South Africa
ABSTRACT
Increasing incidents of drought spells in most Sub-Saharan African countries call for exploration of innovative alternative sources of water for irrigation. A study was conducted to investigate the cation concentrations for different disposal points of treated wastewater and for borehole water. A 4 × 5 factorial experiment included a borehole as a reference sampling site plus three other sampling sites along the wastewater disposal system over 5 months. Monthly collected water samples were analysed for pH, EC, Ca, Mg, Na and K, with sodium adsorption ratio (SAR) computed and compared with those of water from the borehole, the FAO-desired ranges and the South African (SA) quality standards for irrigation water. Except at two sampling sites during one month, pH values were within the FAO-desired range. Relative to the FAO desired ranges and SA water quality standards, most variables in treated wastewater were much lower, suggesting that the test treated wastewater was suitable for irrigation.
Keywords: cation content, temporal effects, treated wastewater disposal, water scarcity
INTRODUCTION
Worldwide, water scarcity due to consistent droughts and increased water quality degradation are concerns that intensify with the advent of global warming. The resulting increase in water demand impacts negatively on agricultural water supplies, particularly in water-scarce countries like South Africa (Qadir et al., 2007; DWAF, 2013). Consequently, alternative sources for irrigation water are being investigated as a strategy to combat water scarcity challenges in various countries (Angelakis, 2003; AATSE, 2004; Ensink et al., 2004), with the use of treated wastewater being one such strategy. However, irrigation with wastewater could result in unintended consequences such as introducing cations Ca, K, Mg and Na (Angelakis et al., 2003; Rusan et al., 2007), thereby impacting either negatively or positively on the chemical component of soil health (NRCS, 2015).
Mulidzi et al. (2015) observed high Na and K concentrations in treated wastewater, along with low Ca and Mg concentrations. Imbalances in cations could explain the reported high soil pH (Schipper et al., 1996), fluctuations in wastewater pH (Rusan et al., 2007) and changes in soil fertility when agricultural lands have been irrigated with treated wastewater (Mosse et al., 2011). However, high acidic or basic cation concentrations could result in ionic toxicities to crops, with increased incidents of leaching into groundwater resources or introduction into other surface water resources through unmanaged run-offs (Christen et al., 2010).
Generally, the quality of treated wastewater depends to a great extent on the quality of municipal water supply, the efficacy of the treatment plants, the nature of chemical wastes added during use, post-treatment handling and disposal of such water prior to irrigation (Pedrero et al., 2010). Effluents from households, restaurants and hospitals discharge numerous acidic and basic chemical compounds that could be detrimental to agricultural soils (Al Salem, 1987; Amouei et al., 2014). Treatment of wastewater entails physical, chemical and biological operative methods (Kumar and Chopra 2012), which are intended to debulk most of the undesirable entities, with the remaining water being disposed of as treated wastewater. Generally, the cation concentration of water in wastewater resources is dependent on sources of disposed wastes.
Post-treatment, wastewater is disposed through a combination of furrows and canals to storage dams, where the liquid part is further evaporated to leave the solid part that is removed to enhance the repeated use of such disposal dams. Part of treated wastewater on its way to the main disposal dam could be diverted for use in irrigation, as had been the case at the University of Limpopo Experimental Farm (ULEF). After a series of treatments at the Mankweng Wastewater Treatment Plant (MWWTP), the treated wastewater flows in an open furrow from the initial point of discharge to the canal prior reaching the night-dam. An assumption, which had not been subjected to empirical analysis, had been that in the night-dam most cations settle to the bottom soon after disposal through the entry site, thus improving the quality of treated wastewater prior to use for irrigation.
The 'pure' treated wastewater was delivered to the irrigated lands through the exit site, which was on the opposite side of the entry site to the night-dam. Approximately 50 m from the night-dam is a borehole, used for potable water and irrigation of fields for production of a variety of vegetables. The cation concentrations of water from the exit of Pond 16 in MWWTP (which discharges wastewater into an approximately 2.9 km furrow to the canal entry site of the night-dam, followed by the piped exit site of the night-dam), along with that of the nearby borehole, had not been documented. The objective of this study was to quantify and compare the cation concentrations of borehole water and treated wastewater at the entry into the furrow from Pond 16, entry into night-dam at ULEF, and exit site from the night-dam, over a period of 5 months, from July to November 2016.
METHODOLOGY
Study site description
The study was conducted on water from MWWTP (23°50′59″ S; 29°42′27″ E) exit of Pond 16 into the furrow that conveyed water to the night-dam at ULEF (23°49′58″ S; 29°42′27″ E), the entry and exit sites of the night-dam and the adjacent borehole. MWWTP received effluent from a number of industries in Mankweng Township (23°53′12″ S; 29°43′53″ E), namely, University of Limpopo (23°52′51″ S; 29°44′18″ E), Mankweng Hospital (23°52′51″ S; 29°43′33″ E), two local shopping centres, filling stations, various human settlements and runoff water from buildings. The effluent undergoes physical, biological and chlorine treatments prior to disposal into the furrow for conveying treated wastewater to the night-dam at ULEF. After physical treatment, excess water is disposed through a series of 16 dams, referred to as maturation ponds, each being 30 m × 90 m. In Pond 01, the disposed water is subjected to chlorine treatment - primarily for biological treatment. After the disposed water has been temporarily stored in a series of 16 ponds, the disposed water through the furrow is technically referred to as treated wastewater. Water samples were collected at 4 sites, namely, (a) exit of Pond 16 into the furrow, (b) entry into night-dam, (c) exit of night-dam to irrigated fields and (d) exit from borehole to irrigated fields. The samples were collected on the 15th of each month, from July to November 2016.
Water sampling and analysis
Water samples were collected in 1-L sterile bottles and transported in portable ice chests to the University of Limpopo soil science laboratory, for determination of pH and electrical conductivity using pH and conductivity meters, respectively. Total suspended solids and total dissolved solids were determined following American Public Health Association (APHA) standard methods (2005). For cations, prior to sampling, the 1 L polypropylene containers were filled with diluted hydrochloric acid and then rinsed several times with water collected from the sampling site. Containers were kept at less than 4°C prior to analysis. Water samples were pre-treated using ultrapure HNO3 for 16 h to reduce pH to less than 2 and then subjected to ICP-OES analysis for Ca, Mg, Na, K and Al (USEPA, 1996). Sodium adsorption ratio was then calculated using the following formula (Suarez et al., 2006):

where Na, Ca, and Mg concentrations were expressed in mEq·L−1.
Statistical analysis
Data were subjected to factorial analysis of variance (ANOVA) using Stata 12 software (StataCorp, 2011). Interactive effects of sampling site and sampling period were assessed, with matrix tables used to further assess the effects of sampling site and period on pH, EC and cation concentrations relative to samples collected from the borehole site. Treatment means were separated using Duncan multiple range test (P ≤ 0.05) and, unless otherwise stated, results were discussed at the probability level of 5%. The total treatment variation (TTV) for all factors was computed using the formula:

where MSS = mean sum of squares and total = total mean sum of squares
Relative impacts of the different factors were calculated using the formula:

RESULTS
pH and electrical conductivity
The site × time interaction was highly significant for pH and EC of test water, contributing 14% and 12% of total treatment variation (TTV) for the respective variables (Table 1). The interaction results were further subjected to the two-way matrix table, where the magnitude and direction of the effects were shown. Relative to borehole water, pH was significantly reduced during July in night-dam exit and Pond 16 exit by 38% and 71%, respectively, and then remained stable throughout the sampling period (Table 2). Generally, relative to the borehole water, the EC of the treated wastewater was also stable, showing significant increases of the variable as affected by the sampling site and the sampling time period.
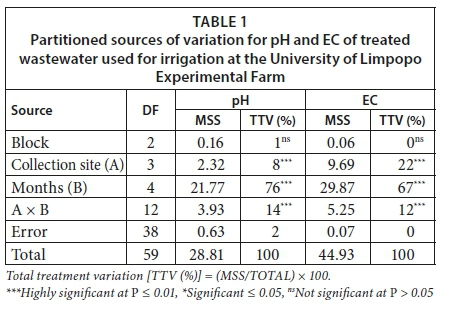
Cations and SAR
The site × time interaction was highly significant for Ca, Mg and SAR, with contributions in TTV for the respective variables being negligible, and therefore not discussed (Table 3). Similar negligible effects were observed for the time factor. In contrast, the collection site had highly significant effects on Ca, Mg, K, Na and SAR, contributing 98%, 100%, 84%, 55% and 70% of total treatment variation for the respective variables.
Relative to the borehole water, Ca was reduced from 11.87 mg·kg−1 to 8.37 mg·kg−1 in Pond 16. Magnesium at the treated wastewater sites was as low as 3.48 mg·kg−1, 3.19 mg·kg−1 and 3.04 mg·kg−1, when compared to 80.93 mg·kg−1 in the borehole water. Sodium, with the exception of the night-dam entry, was reduced from 40.3 mg·kg−1 to 27.10 mg·kg−1 and K reduced from 8.52 mg·kg−1 to 8.27 mg·kg−1 from Pond 16 to night-dam exit, respectively (Table 4). Generally, Ca, Mg, Na and K values in the borehole water were far above the FAO-desired ranges, whereas those of treated wastewater, except for Na that was within the FAO-desired range at the night-dam entry and K that was within (night-dam exit and Pond 16 exit) and slightly above (night-dam entry), were all far below the FAO-desired ranges.
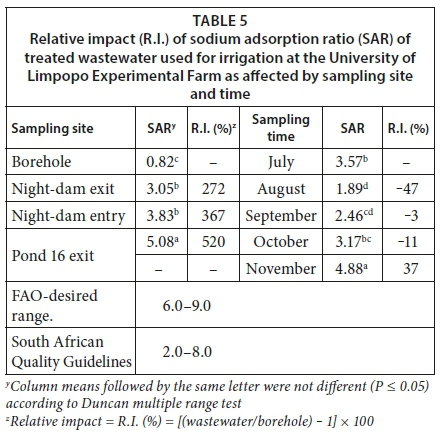
Relative to the borehole water, SAR in Pond 16 exit, night-dam entry and night-dam was increased by 520%, 367% and 272%, respectively (Table 5). However, at all sampling sites, water had SAR values that were below the FAO-desired range. Except in November where SAR of treated wastewater was increased by 37% and in August and September where it was reduced by 47 and 3%, respectively, SAR in October did not differ relative to July as the initial sampling time (Table 5). In all sampling months, SAR values of treated wastewater were below the FAO-desired range.
DISCUSSION
pH and electrical conductivity
Hydrogen ion concentration denoted by pH is an essential variable, on the basis of which water could quickly be assessed for its suitability for irrigation. The observed pH value in both borehole and wastewater sources were well within the FAO limits and SA water quality guidelines for vegetable production, including for onions, indicating that the water might be safe for irrigation. However, in August, the three wastewater sources reported an average pH of 8, which could result in high pH soils if a continued use is observed. The observed high pH in August could have been due to the increase in temperature, which could have promoted higher rates of chemical reactions in water (Barron et al., 2006). In contrast the high values of pH could be beneficial in soils experiencing low pH, as it affects mobility of nutrients (Oliveira et al., 2016). Normally, the safe pH range in water is 6.5 to 8.4 (Jeong et al., 2016), but this could vary according to soil type. The highest observed pH values in the current study were similar to those observed in Isfahan, Iran, by Abedi-Koupai et al. (2006), who reported a pH of 7.8 for wastewater samples. Although pH values fluctuated with the different months, treated wastewater had higher pH values thanborehole water. Irrigation with high pH water could result in high root-zone pH, which can tie up micronutrients, especially in crops with a high demand for iron (Hulme, 2012).
The observed EC values from this study varied with the different months of sampling. The lowest EC of 0.43 dS·m−1 was observed in Pond 16 exit, in August, while the highest value of 7.21 dS·m−1 was in night-dam exit which is the exit point for irrigation. The observed variation could be due to the added salts that move with water in open furrows when transported from Pond 16 to the night-dam. Electrical conductivity is associated with salinity and could cause severe damage to crops if it is above recommended standards (Kiziloglu et al., 2008). The value is above set standards of 3.00 dS·m−1 which are considered severe (Ayers and Westcot, 1995; Jeong et al., 2016). Castro et al. (2011) reported that soils irrigated with high-EC water resulted in high soil EC and salinity, for this study of the effects of wastewater irrigation on soil properties and turf grass growth. Results of the current study were in contrast with the EC values in Isfahan, Iran (Abedi-Koupai et al., 2006), which were within the standards set by FAO (2010).
Cations and sodium adsorption ratio
The observed lower Ca concentrations in wastewater sources relative to that in the borehole water indicated, to an extent, the efficacy of the treatment plant in reducing this critical ion in treated wastewater. Calcium concentrations of treated wastewater sources in various sampling sites and times were below the recommended maximum Ca guideline of lower than 40 mg·L−1 for treated wastewater irrigation (Ayers and Westcot, 1994; Alberta Environment, 2000; Al-Jasser, 2011). Water with low Ca concentrations is generally viewed as soft water, which is suitable for irrigation (Swistock, 2017) and automatically qualifies for use in best agricultural practices since it ameliorates soil hardness. Calcium concentrations in treated wastewater have been reported as being too high, with ranges from 52 to 100 Ca mg·L−1 (Al-Jasser, 2011). However, in other areas the cation concentration ranged from 15 to 84 mg·L−1 (Balkhair and Ashraf, 2016).
The observed Ca concentrations from the borehole in the current study were considerably lower when compared with those in Chitar River Basin, India (Subramani et al., 2005). In other countries, water used for irrigation is moderately hard (40-60 Ca mg·L−1) (Swistock, 2017), whereas in other countries groundwater and surface water used for irrigation is exceedingly hard (> 280 mg·L−1) (Orzepowski and Pulikowski, 2008). In the current study, the high Ca values in borehole water could be associated with groundwater pollution from the storage facilities of treated wastewater, as the night-dam for water discharged from MWWTP was approximately 50 m upslope of the borehole. Generally, salts that leach deeper into the soil have been reported to be the major contaminants of underground water (Tandyrak et al., 2005).
Magnesium concentrations in the treated wastewater sources relative to the borehole water were generally low when compared to those from borehole water in other countries (Orzepowski and Pulikowski, 2008). Both Mg and Ca are associated with soil aggregate stability and friability (Swistock, 2017). These two elements are usually required in high quantities in soil. However, Mg concentrations that are higher than 200 mg·L−1 in irrigation water when in soil solution could result in high pH values, with resultant reduction in the availability of P, Cu and Zn (Khodapanah et al., 2009).
Similar to Ca and Mg, higher K concentrations in borehole water were observed when compared to treated wastewater sources. The lowest concentration which was observed in the night-dam exit could be associated with settling to the bottom of the night-dam prior to release. The K value in borehole water (16.73 K mg·L−1) was slightly above the desirable range (0-10 mg·L−1) of K in irrigation water (Swistock, 2017). Potassium is vital in tolerance of plants to various stress situations such as drought, low temperature and/or salinity (Tisdale et al., 1999).
High Na levels in wastewater would not be suitable for the soils to which it would be applied due to Na ions adsorbing onto the soil cation exchange sites, causing soil aggregates to disperse, sealing the soil pores, and making it impermeable to water flow (Emongor and Ramolemana, 2004). Generally, Na concentrations are considered moderate when they are just above 70 mg·L−1 (Pedrero et al., 2010), which was close to the concentration observed for borehole water in the current study. Thus, Na in the wastewater sampling sites in night-dam exit, night-dam entry and Pond 16 were at 27.10, 50.50 and 40.3 mg·L−1, respectively. For all of the sites sampling suggested that, in terms of Na, the water was suitable for irrigation. However, the values could only be recommended for short-term use, as a build-up in Na in soils could lead to sodicity (Abrol et al., 1988). Also, Na concentrations observed in the treated wastewater sources in the current study were considerably lower when compared to 109.7 mg·L−1 from similar sources (Orzepowski and Pulikowski, 2008) and treated river water streams (123.60 mg·L−1) in other countries (Alobaidy et al., 2010).
Sodium adsorption ratio is often used as a suitability indicator for irrigation water use. Generally, the higher the SAR, the less suitable is the water for irrigation purposes (Abrol et al., 1988). The current study demonstrated that, in terms of SAR, the treated wastewater was in Class S1, which is a low Na hazard and suitable for irrigation. All the observed SAR values were below the FAO and SA water quality desired ranges. Furthermore, the results of the current study demonstrated that borehole water had the lowest SAR when compared to the treated wastewater sampling sites. In terms of SAR, variability was mainly introduced by the sampling period, which is in agreement with observations for effluents from Al-Rustamia Wastewater Treatment Plant in Iraq (Shakir et al., 2017). Water with SAR values above the FAO-desired ranges is not suitable for use in irrigation (Ayers and Westcot, 1985), since it invariably leads to deterioration of the physical structure of soil (Shakir et al., 2017).
CONCLUSION
Comparison of the variables measured in the test treated wastewater and the FAO-desired range standard for irrigation water, suggested that the treated wastewater was suitable for irrigation. The causes of the variability of the treated wastewater in terms of location along the disposal system and temporary storage sites, as well as during the sampling period, were not clear. In the test treated wastewater, some caution should be taken for K, since it hovered within and slightly higher than the FAO-desired range. Also, in the current study, it was clearly shown that the treated wastewater had relatively lower concentrations of cations than the borehole water, with SAR being the highest in Pond 16. However, all SAR values were below the desired range which confirmed the suitability of the test treated wastewater and the borehole water for current irrigation purposes.
ACKNOWLEDGEMENTS
The authors are grateful to the National Research Foundation (NRF) of South Africa for financial support through the Thuthuka grant.
REFERENCES
AATSE (Australian Academy of Technological Sciences and Engineering) (2004) Water Recycling in Australia. AATSE, Victoria, Australia. [ Links ]
ABEDI-KOUPAI JB, MOSTAFAZADEH F, AFUNYI M and BAGHER MR (2006) Effect of treated wastewater on soil chemical and physical properties in an arid region. Plant Soil Environ. 52 (8) 335-344. https://doi.org/10.17221/3450-PSE [ Links ]
ABROL P, YADAV JSP and MASSOUD FI (1988) Salt-affected soils and their management. FAO Soils Bulletin 39. Food and Agriculture Organization of The United Nations, Rome. [ Links ]
ALBERTA ENVIRONMENT (2000) Guidelines for municipal wastewater irrigation. Municipal Program Development Branch, Environmental Sciences Division Edmonton, Alberta. [ Links ]
AL-JASSER AO (2011) Saudi wastewater reuse standards for agricultural irrigation: Riyadh treatment plants effluent compliance. J. King Saud Univ. Eng. Sci. 23 (1) 1-8. https://doi.org/10.1016/j.jksues.2009.06.001 [ Links ]
ALOBAIDY AHMJ, MAULOOD BK and KADHEM AJ (2010) Evaluating raw and treated water quality of Tigris River within Baghdad by Index Analysis. J. Water Resour. Prot. 2 629-635. https://doi.org/10.4236/jwarp.2010.27072 [ Links ]
AL SALEM SS (1987) Evaluation of the Al Samra waste stabilization pond system and its suitability for unrestricted irrigation. Paper prepared for the Land and Water Development Division, FAO, Rome. [ Links ]
AMOUEI A, HOSEINI R and ASGHARNIA H (2014) Investigation of household hazardous wastes production in the Amirkola Township, Iran, in 2012-2013. Iran. J. Health Sci. 2 (3) 8-14. [ Links ]
ANGELAKIS AN, BONTOUX L and LAZAROVA V (2003) Challenges and prospective for water recycling and reuse in EU countries. Water Sci. Technol. 3 (4) 59-68. https://doi.org/10.2166/ws.2003.0046 [ Links ]
APHA (American Public Health Association) (2005) Standard Methods for the Examination of Water and Wastewater (21st edn). American Public Health Association/American Water Works Association/Water Environment Federation, Washington DC. [ Links ]
AYERS RS and WESTCOT DW (1994) Water quality for agriculture. FAO irrigation and drainage paper. 29 Rev. 1. Food and Agriculture Organization of the United Nations, Rome. [ Links ]
BALKHAIR KS and ASHRAF MA (2016) Field accumulation risks of heavy metals in soil and vegetable crop irrigated with sewage water in western region of Saudi Arabia. Saudi J. Biol. Sci. 23 (1) S32-S44. https://doi.org/10.1016/j.sjbs.2015.09.023 [ Links ]
BARRON JJ, ASHTON C AND GEARY L (2006) The effects of temperature on pH measurements. 57th Annual meeting of the International Society of Electrochemistry. URL: www.reagecon.com (Accessed 18 April 2016). [ Links ]
CASTRO E, MAÑAS MP and DE LAS HERAS J (2011) Effects of wastewater irrigation on soil properties and turfgrass growth. Water Sci. Technol. 63 (8) 1678-1688. https://doi.org/10.2166/wst.2011.335 [ Links ]
CHRISTEN EW, QUAYLE WC, MARCOUX MA, ARIENZO M and JAYAWARDANE NS (2010) Winery wastewater treatment using the land filter technique. J. Environ. Manage. 91 1665-1673. https://doi.org/10.1016/j.jenvman.2010.03.006 [ Links ]
DWAF (Department of Water Affairs and Forestry, South Africa), 2013. Overview of the South African water sector. Department of Water Affairs and Forestry, Pretoria, South Africa. [ Links ]
EMONGOR VE and RAMOLEMANA GM (2004) Treated sewage effluent (water) potential to be used for horticultural production in Botswana. Phys. Chem. Earth 29 1101-1108. https://doi.org/10.1016/j.pce.2004.08.003 [ Links ]
ENSINK JHJ, MAHMOOD T, VAN DER HOEK W, RASCHID-SALLY L and AMERASINGHE FP (2004) A nation-wide assessment of wastewater use in Pakistan: an obscure activity or a vitally important one? Water Polic. 36 197-206. https://doi.org/10.2166/wp.2004.0013 [ Links ]
HULME F (2012) Managing highly alkaline irrigation water. Media and Fertilizer: Greenhouse Product News. URL: www.gpnmag.com (Accessed 23 January 2017). [ Links ]
JEONG H, KIM H and JANG T (2016) Irrigation water quality standards for indirect wastewater reuse in agriculture: a contribution towards sustainable wastewater reuse in South Korea. Korea: Institute of Green Bio Science and Technology, Seoul National University, Gangwon. [ Links ]
KHODAPANAH L, SULAIMAN WNA and KHODAPANAH N (2009) Groundwater quality assessment for different purposes in Eshtehard District, Tehran, Iran. Eur. J. Sci. Res. 36 (4) 543-553. [ Links ]
KIZILOGLU F M, TURAN M, SAHIN U, KUSLU Y and DURSUN A (2008) Effects of untreated and treated wastewater irrigation on some chemical properties of cauliflower (Brassica olerecea L. var. botrytis) and red cabbage (Brassica olerecea L. var. rubra) grown on calcareous soil in Turkey. Agric. Water Manage. 95 (6) 716-724. https://doi.org/10.1016/j.agwat.2008.01.008 [ Links ]
KUMAR V and CHOPRA AK (2012) Monitoring of physico-chemical and microbiological characteristics of municipal wastewater at a treatment plant, Haridwar City (Uttarakhand) India. J. Environ. Sci. Technol. 5 109-118. [ Links ]
MOSSE KPM, PATTI AF, CHRISTEN EW and CAVAGNARO TR (2011) Review: Winery wastewater quality and treatment options in Australia. Aus. J. Grape Wine Res. 17 111-122. https://doi.org/10.1111/j.1755-0238.2011.00132.x [ Links ]
MULIDZI AR, CLARKE CE and MYBURGH PA (2015) Effect of irrigation with diluted winery wastewater on cations and pH in four differently textured soils. S. Afr. J. Enol. Vit. 36 (3) 400-410. https://doi.org/10.21548/36-3-973 [ Links ]
NRCS (Natural Resources Conservation Services) (2015) Soil Health-Healthy soil for life. United States Department of Agriculture. URL: www.nrcs.usda.gov (Accessed 12 March 2016). [ Links ]
OLIVEIRA PCP, GLOAGUEN TV, GONÇALVES RAB, SANTOS DL and COUTO CF (2016) Soil chemistry after irrigation with treated wastewater in semiarid climate. Braz. J. Soil Sci. 40 1-13. https://doi.org/10.1590/18069657rbcs20140664 [ Links ]
ORZEPOWSKI O and PULIKOWSKI K (2008) Magnesium, calcium, potassium and sodium content in groundwater and surface water in arable lands in the commune (Gmina) of Katy Wroclawskie. J. Elementol. 13 (4) 605-614. [ Links ]
PEDRERO F, KALAVROUZIOTIS I, ALARCÓN JJ, KOUKOULAKIS P and ASANO T (2010) Use of treated municipal wastewater in irrigated agriculture-review of some practices in Spain and Greece. Agric. Water Manage. 97 1233-1241. https://doi.org/10.1016/j.agwat.2010.03.003 [ Links ]
QADIR M, WICHELNS D, RASCHID-SALLY L, MINHAS PS, DRECHSEL P and BAHRI A (2010) The challenges of wastewater irrigation in developing countries. Agric. Water Manage. 97 561-568. https://doi.org/10.1016/j.agwat.2008.11.004 [ Links ]
RUSAN MJM, HINNAWI S and ROUSAN L (2007) Long term effect of wastewater irrigation of forage crops on soil and plant quality parameters. Desalination 215 143-152. https://doi.org/10.1016/j.desal.2006.10.032 [ Links ]
SCHIPPER LA, WILLIAMSON JC, KETTLES HA and SPIER TW (1996) Impact of land-applied tertiary-treated effluent on soil biochemical properties. J. Environ. Qual. 25 1073-1077. https://doi.org/10.2134/jeq1996.00472425002500050020x [ Links ]
SHAKIR E, ZAHRAW Z, and AL-OBAIDY AHMJ (2017) Environmental and health risks associated with reuse of wastewater for irrigation. Egy. J. Petroleum. 26 (1) 95-102. https://doi.org/10.1016/j.ejpe.2016.01.003 [ Links ]
STATACORP (2011) Stata Statistical Software: Release 12. College Station, TX: StataCorp LP. [ Links ]
SUAREZ DL, WOOD JD and LESCH SM (2006) Effect of SAR on water infiltration under a sequential rain-irrigation management system. Agric. Water Manage. 86 150-164. https://doi.org/10.1016/j.agwat.2006.07.010 [ Links ]
SUBRAMANI T, ELANGO L and DAMODARASAMY SR (2005) Groundwater quality and its suitability for drinking and agricultural use in Chitar River Basin, Tamilnadu, India. Environ. Geol. 47 1099-1110. https://doi.org/10.1007/s00254-005-1243-0 [ Links ]
SWISTOCK B (2017) Interpreting irrigation water tests. Penn State College of Agricultural Sciences Research and Extension, The Pennsylvania State University, USA. [ Links ]
TANDYRAK R, GROCHOWSKA J and DUNALSKA J (2005) Effect of terrain management on hydro-chemical parameters of water and bottom sediments in small reservoirs of Kortowo district, Olsztyn. Zesz. Probl. Post. Nauk Rol. 505 459-465. [ Links ]
TISDALE SL, HAVLIN JL, BEATON JD, NELSON WL (1999) Soil Fertility and Fertilizers. An Introduction to Nutrient Management (6th edn.) Prentice-Hall, Upper Saddle River, New Jersey. [ Links ]
USEPA (United States Environmental Protection Agency) (1996) Inductively Coupled Plasma-Atomic Emission Spectrometry, Method 6010B, Revision 2.0, SW-846 Manual, 3rd edition, Office of Solid Waste and Emergency Response. USEPA, Washington DC. [ Links ]
Received 31 May 2017
Accepted in revised form 20 August 2018
* To whom all correspondence should be addressed. e-mail: Pholosho.kgopa@ul.ac.za














