Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Water SA
On-line version ISSN 1816-7950
Print version ISSN 0378-4738
Water SA vol.44 n.2 Pretoria Apr. 2018
http://dx.doi.org/10.4314/wsa.v44i2.16
ORIGINAL ARTICLES
Investigating the effects of different physical and chemical stress factors on microbial biofilm
Cansu VatanseverI, *; Irfan TuretgenII
IAltinbas University, Faculty of Pharmacy, Department of Pharmaceutical Microbiology, 34145 Bakirkoy, Istanbul, Turkey
IIIstanbul University, Faculty of Science, Department of Biology, 34134 Fatih, Istanbul, Turkey
ABSTRACT
Microorganisms that adhere to surfaces in order to protect themselves from many adverse environmental conditions form a layer called biofilm. Biofilms protect bacteria from changing environmental conditions such as starvation, antibiotics, disinfectants, pH and temperature fluctuation, dryness and UV rays. In this study, biofilms were formed on surfaces of glass coupons in a cooling tower model system over a period of 180 days. The biofilms were treated with various stress factors monthly. These stress factors were: exposure to temperatures of 4°C and 60°C, pH of 3, 5, and 11, 3 M aqueous NaCl and distilled water, as well as, monochloramine at 2, 500, and 1 000 mg/L (ppm). Following the treatment with stress factors, both the numbers of actively respiring bacteria and the total bacteria in the biofilms were determined by CTC-DAPI staining. The aerobic heterotrophic plate counts (HPC) in the biofilms were determined by the conventional culture method of spread plating on R2A agar. The aim of this study was to determine the impact of these stressors on the model cooling-tower biofilms. Of the stressors tested, those that had the greatest impact were a temperature of 60°C, pH of 3, 3 M NaCl, and monochloramine at both 500 and 1 000 mg/L. However, when using a non-culture-based viability assay (CTC-DAPI staining), an extremely high number of live bacteria were detected even after applying the most effective stress factors (with the exception of pH 3) of 60°C, 3 M NaCl, monochloramine at 500 and 1 000 mg/L. Results showed that biofilm protects the bacteria from extreme physical and chemical stress conditions. Additionally, the conventional culture technique cannot detect the presence of bacteria that have entered the viable but non-culturable (VBNC) phase; the use of different techniques, such as microscopy and cytometry (flow and solid-phase), is therefore important to obtain more accurate results.
Keywords: biofilm, stress factors, monochloramine, CTC-DAPI staining
INTRODUCTION
Biofilms are microbial communities attached to either an inert or a living surface within a polymeric matrix. This matrix includes extracellular polymeric substrate (EPS) made up of polysaccharides, protein, lipids, nucleic acids and various heteropolymers that are secreted by the microorganisms into their environment (Costerton et al., 1999; Kim et al., 2012; Allison et al., 2003). Water makes up more than 97% of this biofilm matrix. While 5% of the matrix is composed of cells, 1-2% of the matrix is formed from EPS (Allison et al., 2003).
Biofilms may be present in any niche that supports life, and develop at the interfaces of solid-liquid, solid-air and liquid-air environments (Beveridge et al., 1997; Flemming, 2002). Biofilms can be found in a variety of habitats, including terrestrial and aquatic environments, living tissues (plant, animals, humans) and built environments such as filters, porous materials, water tanks, piping systems, ship hulls, heat exchangers, etc. (Flemming et al., 2000). Biofilms occurring in industrial fields can cause problems such as decrease in heat exchange capacity, loss of efficiency, increase in fluid frictional resistance, corrosion of equipment and decrease of water quality (Tanji et al., 2007; Gilbert et al., 2003; Wood et al., 1996). Public health institutions have documented that biofilms in water distribution systems contain primary and opportunistic bacteria, viruses, protozoa and fungi. The most important of these opportunistic bacteria are Pseudomonas aeruginosa, Legionella pneumophila and Mycobacterium avium complex (Mains, 2008).
Living in the biofilm form provides many advantages to bacteria. The matrix may be considered one of the most important elements of biofilm. In addition to ensuring the adhesion of microorganisms, it can provide resistance against multiple stress conditions such as water or food scarcity, biocides and other antimicrobial agents. This structure enables the adhesive base and the protective barrier to also protect embedded cells from breakage by the flow of water (Tsuneda et al., 2003; Kives et al., 2006). Further, the matrix retards the movement of small molecules through and out of the biofilm, thus creating a perfect environment for metabolic changes (Kierek-Pearson and Karatan, 2005). Another advantage of the matrix is concentrating nutrients, an important part of the overall strategy for microbial survival under oligotrophic conditions (Beveridge et al., 1997; Decho, 1990; Decho, 2000).
Cooling towers are well-known reservoirs of biofilm. The main function of a cooling tower is to transfer heat from circulating water to the atmosphere through evaporation (Liu et al., 2009). These systems have large water reservoirs where the temperature is maintained between 25°C and 35°C and thereby offer a conducive environment for microbial growth and spread (Breiman, 1996; Liu et al., 2009). Immediately after the water enters the system, organic molecules adhere to the surfaces creating a conditioning layer that enables bacterial attachment by neutralizing the surface charge. In the initial phase, pioneer bacteria attach to the surface by electrostatic interactions and physical strength and then daughter cells produce EPS, which leads to the development of biofilm. Finally, a mature biofilm is formed (Tanji et al., 2007). Moreover, the majority of the biomass found in cooling towers is due to the formation of biofilm attached to the cooling tower surfaces (Critcheley and Bentham, 2007). Aerosols, which are produced in cooling towers during the removal of heat, provide transport to microorganisms. Therefore, cooling towers have been responsible for some outbreaks such as legionellosis originating from Legionella (Nygard et al., 2008). For this reason, elimination of the biofilm layer that occurs in the cooling tower system is an important issue for public health. Determining the effective method of biofilm removal will help to prevent health problems that may originate from cooling towers.
Bacteria in industrial processes or in natural environments are exposed to a variety of abiotic stresses. Cells develop mechanisms to protect themselves from potential harm when they detect adverse environmental conditions or stressors (Jan et al., 2000). Planktonic and biofilm bacteria, by inducing stress response genes, become more tolerant phenotypes to environmental stresses such as changes in food quality, cell density, temperature, pH and osmolarity (Novick, 2003). This stress adaptation involves a form of complex regulation of gene expression and studies have shown that stress-activated genes in prokaryotes are well-protected (Segal and Ron, 1998; Jan et al. 2000). Mechanisms developed by bacteria to cope with stress conditions, like biofilm formation, prevent effective elimination. Moreover, assessments based solely on conventional culture methods can give misleading results since they cannot detect bacteria that have entered a VBNC phase.
The aim of this study was to determine the stress factors that most affect bacteria in biofilm form, and thus contribute by identifying treatments that may provide successful results in the elimination of biofilm that develops in cooling tower systems. In addition, we sought to determine the most appropriate technique for the detection of bacterial viability.
MATERIALS AND METHODS
Circulation system setup
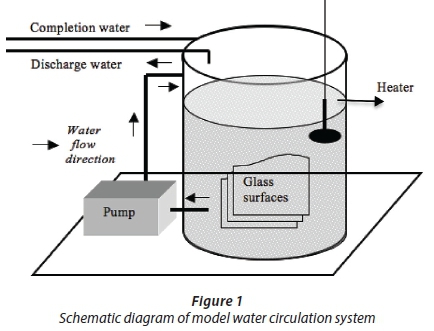
This study was conducted with a system described by Turetgen (2004). Their results showed that this closed-loop water circulation system reflected the actual cooling tower with respect to biofilm formation on surfaces. A water circulation system where the temperature was fixed at 32°C was used in the experiment. For biofilm growth surfaces, 2 mm-thick 18 cm2 (9x2) sterilized microscope slides were used. Prior to installation in the system, the glass coupons were washed with detergent and rinsed with distilled water. The coupons were then dipped in 70% ethanol (Turetgen and Cotuk, 2005) and flame-sterilized. Following sterilization, the glass coupons were placed in the circulation system with coupon carrier equipment made from plexiglass. Plexiglass carrier equipment was sterilized in a biosafety cabinet under 254 nm UV radiation for 24 h (Sanli-Yurudu, 2009). Then the system was filled with 100 L of tap water and circulated under non-turbulent shear stress (Reynolds number: 2000) (Fig. 1). During a 6-month period, sampling was conducted monthly.
Bacteriological analysis and application of stress factors
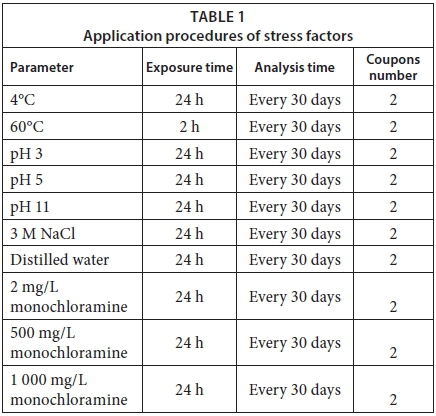
Monthly, 22 coupons were removed from the system, two of which were to serve as control coupons. Duplicates of the remaining 20 coupons were exposed to various stress factors: temperatures of 4°C and 60°C; pH of 3, 5, 11; 3 M aqueous NaCl and distilled water, and monochloramine at 2, 500, and 1 000 mg/L. Numbers of live bacteria, total bacteria and HPC on the coupon surfaces were determined. The amount of carbohydrate on the surfaces was analysed monthly, as were the values of pH, dissolved oxygen and total dissolved solids in the bulk water.
For bacteriological analysis, 2 coupons for each experiment were exposed to the described stress factors for 24 h, except 60°C heat (2 h) (Table 1). The coupons were placed in sterile glass containers having a volume of 90 mL. pH solutions, distilled water, NaCl and monochloramine solutions were prepared as described below and were placed in sterile containers before adding the coupons. These tests were performed at room temperature. For heat (60°C) and cold (4°C) temperature stress experiments, bulk water was transferred from the water circulation system to glass containers. Coupons were added and the containers were placed into a heating bath at 60°C and a refrigerator at 4°C. At the end of the treatment periods, biofilm samples on the coupon surfaces were collected using sterile swabs, placed into polypropylene bags containing 20 mL of sterile phosphate buffer (PBS) and homogenized with a masticator (IUL Instruments 200 strike/min, IUL SA, Barcelona, Spain) for 90 s. The buffer suspensions were serially diluted in PBS (1:100 series). For HPC, 100 μL were then spread plate in triplicate on R2A agar (Oxoid, CM0906) and incubated at 27°C for 10 days.
Preparation of experimental solutions
Bulk water from the water circulation system was used for experiments evaluating temperatures as stressors. Distilled water for pH treatments was adjusted to 3, 5 and 11 using KOH and HCl. Monochloramine stock solution (1 000 mg/L) was prepared with 1 g of ammonium chloride, 0.832 g of sodium carbonate, 8 mL of 6% sodium hypochlorite (commercial bleach) and 200 mL distilled water. Distilled water was used to dilute the stock solution to 2 and 500 mg/L monochloramine solutions. Treated coupons were placed into 0.5% sodium thiosulphate for 20 min after exposure to monochloramine solutions to neutralize residual disinfectant activity (Sanli-Yurudu, 2009).
Epifluorescence microscopy analysis
Epifluorescence microscopy (NIKON 80i, Japon) was used to observe biofilms. The numbers of actively respiring bacteria and total bacteria were determined for each treatment by taking a 900 μL aliquot from 20 mL of biofilm suspension and adding 100 μL CTC (5-cyano-2,3-ditolyl tetrazolium chloride) dye (Sigma-Aldrich, 94498), stirring, then incubating at 28°C for 4 h (Rodriguez et al. 1992). Following the incubation period, 110 μL DAPI (4',6-diamino-2-phenylindole) dye (ThermoFisher Scientific, D1306) was added to the mixture and incubated at 28°C for 1 h. DAPI binds to DNA and allows all bacteria in the suspension to be visible as blue colour when exposed to fluorescent light (Saby et al., 1997). CTC is colourless in oxidized form and only becomes fluorescent after the reduction by actively respiring bacteria through the electron transport system (Rodriguez et al., 1992; Schaule et al., 1993). In this way, the live bacteria can be distinguished red in colour. After incubation, to stop the reaction and to ensure homogenisation of the sample, 3 mL bidistilled sterile water was added to the mixture. The sample was filtered through a black polycarbonate filter of 0.2 μηι diameter (Merck Millipore, Isopore Membrane Filters) (Araya et al., 2002). The air-dried filter was placed on a microscope slide and observed with a 100 χ oil immersion objective (Rodriguez et al., 1992). Images were taken from 10 different areas and the average number of bacteria on the surface was calculated (Turetgen, 2008).

where:
N = number of microorganisms per milliliter
S = filtration area
n = average number of microorganisms in the studied area
C = microscopic area
V = filtered sample volume
D = dilution rate of sample
Carbohydrate analysis
Extracellular polymeric substances in the biofilm were measured colorimetrically according to the phenol-sulphuric acid methods described by Dubois and others (1956). Three sucrose standards of 0, 10 and 100 μg/mL were used with each experiment; the results were given as μg/(carbohydrate·cm2) of biofilms on the coupon surfaces. Data represent the means of three measurements.
Dissolved oxygen measurement
During the experiment, the amount of dissolved oxygen in the water was measured monthly with an oximeter (WTW - Oxi 330; Gemini BV Laboratory, Apeldoorn, NL).
Total dissolved solids measurement
During the experiment, the amount of total dissolved solids (TDS) in the water was measured monthly with a conductometer device (WTW LF 95 Conductivity Meter; Gemini BV Laboratory, Apeldoorn, NL).
Statistical analysis
For each parameter over the 180-day duration of the experiment, the difference between the average number of bacteria in experimental and control groups, in terms of the months and groups, was compared by two-way analysis of variance. A follow-up post-hoc (multiple comparisons) analysis was performed in order to determine which monthly averages were significantly different. Any month which differed significantly was examined using the least significant difference (LSD). The difference was considered significant when p < 0.05. Furthermore, in terms of the parameters, differentiation of the average number of bacteria was analysed by one-way analysis of variance. A post-hoc analysis was performed in order to determine which average of parameter was significantly different. Parameters that differed significantly from each other were examined with LSD. Analyses were performed using SPSS Version 10.0. The above-mentioned analyses were performed separately for results obtained from conventional culture method and epifluorescence microscopy.
RESULTS AND DISCUSSION
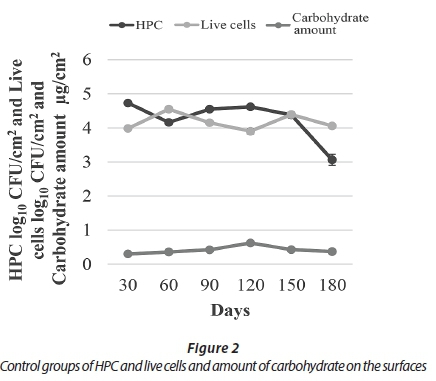
During a study period of 180 days, carbohydrate analysis showed that biofilm thickness increased until Day 120 and decreased thereafter. Consistent with the results of carbohydrate analyses, HPC on R2A plates increased for the first 120 days except Day 60, and subsequently HPC decreased until the last day. The results obtained using live/dead fluorescent microscopy showed consistent results with both the HPC and carbohydrate analyses, in that the number of live bacteria decreased on Day 180 (Fig. 2).
Dissolved oxygen in the biofilm reactor was measured every 30 days. A continuous, controlled feed of water was maintained in the network and no significant changes to TDS or pH were observed throughout the 180-day study period (Table 2).
Heat and cold treatment
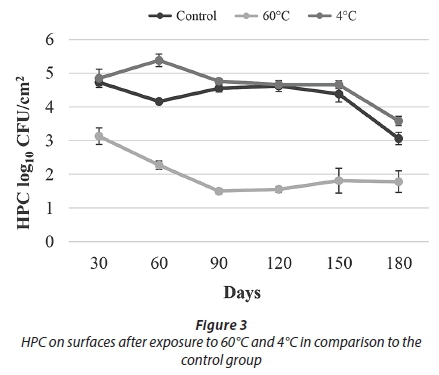
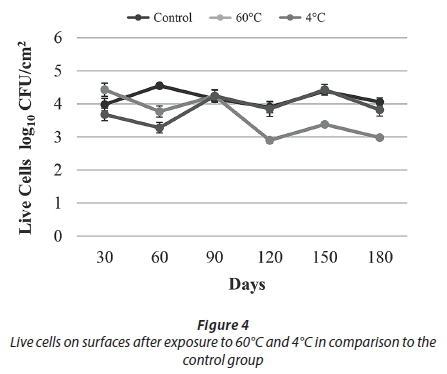
The results of conventional culture indicated that treatment with 60°C resulted in a decrease in the HPC towards Day 90. After Day 90, an increasing trend was observed. At the end of the study, being exposed to 60°C caused an approximately 1 log reduction in HPC and the number of live cells when compared to the control samples (Figs 3-4).
Significant consequences of reprogramming gene expression and protein synthesis of biofilm cells are EPS biosynthesis and matrix production (Landini, 2009). Under normal conditions, cells have their own signal regulation system to control genes involved in cellular defence mechanisms and to respond to environmental stress (Kennelly and Potts, 1996). Under normal circumstances, sigma factor σ70 is responsible for the transcription of diverse gene promotors; in stress conditions, the alternative sigma factors, small proteins bound to the RNA polymerase, are induced. σs(rpoS) is the principal regulator of the general stress response factors in Escherichia coli and other enteric bacteria (Adnan et al., 2010). Studies show that the presence of biofilm as well as biofilm maturity is effective resistance to heat treatment. For example, in a study performed with Panton-Valentine leukocidin-positive community-acquired methicillin-susceptible Staphylococcus aureus, as a result of the temperature treatment with 50°C, 60°C and 70°C for 60 min, increased resistance was observed in parallel with increasing biofilm age. For 50°C and 60°C treatments, 5-day biofilms were more resistant than 2-day biofilms and for 70°C treatment, reproduction was detected in 9-day biofilms for the first time (Al-Azemi et al., 2011).
As a result of the heat treatment in the current study, HPC reduction was observed up to 90 days and increased until the end of the experiment (Fig. 3). It is thought that this result was due to the resistance provided by the presence of biofilm and biofilm maturity.
Protein denaturation and folding are two important types of cell detriment caused by high temperatures. All organisms respond to a rapid increase in temperature by inducing the synthesis of a particular group of polypeptides. These are called heat shock proteins. Heat shock proteins include chaperones such as GroES, GroEL, DnaK, DnaJ, ClpB, ClpA, ClpX, small heat shock proteins (HSPs) and protease. While chaperones provide the correct folding of proteins, which become denatured, proteases degrade the proteins, which are damaged irreversibly (Grover et al. 2011; Munchbach et al. 1999). As a result of increases in temperature, induction of the expression of heat shock proteins has been detected in many bacteria including P. aeruginosa and E. coli (Ali et al., 2009; Rasouly and Ron, 2009). In a study conducted on the heat shock protein of S. aureus, where bacteria were incubated at 42°C for 30 min, heat shock response occurred and the transcription of 98 genes was induced. Using real-time PCR analysis, researchers found that the heat shock response genes clpC and ctsR were upregulated 65-fold and 95-fold, respectively (Anderson et al., 2006). It has been shown that Legionella can be grown rapidly around 40°C, while this value is the temperature at which many bacteria are killed. Living in free-living amoeba allows the growth of Legionella at this high temperature. Increasing the temperature over 60°C does not eradicate it, but efficient bacterial suppression is supplied by the heat flush (Decker and Palmore, 2014).
Over the course of this 180-day experiment, an approximately 1 log reduction was observed in both the number of live bacteria and HPC when compared to the control samples. These results showed that 60°C temperature application effectively reduced cell numbers in the biofilm but did not completely eliminate them (Figs 3-4).
Being exposed to 4°C did not cause a considerable change in the number of live bacteria nor HPC. Under such low temperature conditions, the induction of certain proteins, called cold shock proteins (CSPs), occurs. These proteins are small, highly preserved and structurally related to the nucleic acid binding proteins that play significant roles in the regulation of diverse physiological processes. CSPs bind to DNA or RNA and control replication, transcription and translation processes in the bacterial cell (Ermolenko et al., 2002). These proteins are found in many prokaryotes such as Listeria monocytogenes, Bacillus subtilis and E. coli. In one study about this issue, 4°C cold shock was applied to L. monocytogenes for 2 h. When compared to 37°C, up to 23-fold, 4.5-fold and 7.4-fold increases were detected in the transcription of cspA, cspB and cspD (cold shock genes), respectively (Schmid et al., 2009). In another study, as a result of S. aureus incubation at 10°C for 30 min, it was found that the mRNA titer of 46 genes increased and the transcription of the cold shock gene (cspB) increased 9.3-fold (Anderson et al., 2006). A similar study about E. coli showed that the adhesion genes are important for biofilm formation. When the environmental temperature dropped to 23°C, an increase occurred in the expression of some genes responsible for biofilm development (White-Ziegler et al., 2008).
In our study, after applying a 4°C treatment, no decline in either the HPC or the number of live bacteria was observed when compared to the control samples. It was thought that, as in similar results obtained in the studies mentioned above, this was caused by an increase in the expression of cold shock proteins and presence of EPS. Overall results obtained for temperature treatments showed that heat treatment (60°C) was more effective than cold treatment (4°C) in reducing biofilm bacteria (Figs 3-4).
pH treatment
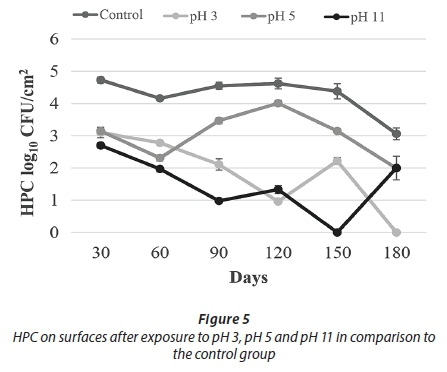
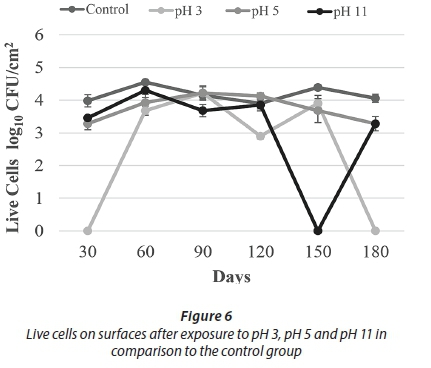
Being exposed to pH 3 resulted in a decrease in HPC towards Day 120. Despite the increase on Day 150, no HPC were detected on Day 180. Similarly, no live cells were observed on Day 180 (Figs 5-6). These results indicated that acid treatment was significantly effective (p < 0.05) against biofilm bacteria.
A study carried out with one of the most important biofilm organisms of the mouth, Streptococcus mutans, showed that the bacteria cope with low pH conditions depending on the activity of F-ATPase (H+-translocating ATPase) that has bound to the cell membrane. This enzyme can export protons outside the cells and produce ATP for growth and persistence under special conditions (Lemos and Burne, 2008). Results of a similar study with S. mutans showed that the expression of molecular chaperones, proteases and DNA repair enzymes were upregulated at low pH (Len et al., 2004). In these ways, bacteria were protected from lethal effects of acid. Another study conducted on lactic acid bacteria that were treated with 8%, 10% and 11% acetic acid for 30 min demonstrated that biofilm bacteria were more resistant than planktonic bacteria. These data can be explained by three resistance mechanisms: The cell membrane becomes more resistant, extracellular polymeric secretions provide protection, and the three-dimensional structure protects the cells that are inside the biofilm. The results showed that cell adhesion and biofilm structure play important roles in stress resistance (Kubota et al., 2008).
A different study examined the effect of different pH values on the quorum sensing autoinducer-2 signalling molecules in Streptococcus intermedius. It was found that low pH did not affect the quorum sensing signal molecules in biofilm formation; the microorganisms continued to communicate and biofilm formation was not blocked. At pH 5.7, biofilm production was approximately 2-3 times higher than at pH 7.5 (Ahmed et al., 2008). Under some acidic conditions, bacteria can undergo an acid tolerance response (ATR). These changes comprise of synthesis of the stress responsive protein, increasing the glycolytic activity and translocation of protons with ATPase that regulates the intracellular pH. A study in which three different strains of S. mutans in 3 h biofilm were incubated in pH 3 for 2 h found that all three strains were more resistant when compared to the planktonic cells (Welin-Neilands and Svensater, 2007). In another study about this issue, it was showed that the change in cellular protein profiles in acid tolerance in mature biofilm cells was 41.5%, while that in newly established biofilm cells was 5.1%. These data indicate that under acidic shock conditions, changes in protein profile continue during the development of biofilms (Welin et al., 2003). During the first 150 days of our study, HPC and live bacteria were detected, but not at the end of the 180-day period. It was thought that this lack of viable bacteria was due to a decrease in bacterial resistance. This decreased resistance occurred due to the increasing age of the biofilm of more than 150 days.
With respect to the results of treatment with pH 11, HPC showed a decreasing trend to Day 150, whereas the number of live cells was comparable to control counts. On Day 150, no cells were recovered by either method. Cell counts were observed, however, on Day 180 (Figs 5-6).
Low pH can cause damage to the pH gradient balance of cell membranes and this leads to the accumulation of volatile fatty acid anions in the cell (Russell and Wilson, 1996). In addition, bacterial DNA is damaged at low pH (Coter and Hill, 2003). When the pH value drops to 3-5, polysaccharide and protein concentrations decrease quickly. At low pH, bacteria are inactivated and thus polysaccharide and protein concentrations decrease. However, polysaccharide and protein concentrations at high pH are more moderate than at low pH. A study conducted on P. aeruginosa biofilm showed that alkaline resistance was higher than acid resistance (Zhou et al., 2014). In a study performed with S. aureus, treatments at pH 9 and 10 were less efficacious against 2-day-old biofilm bacteria than their planktonic counterparts. At least a two-log difference was observed at pH 10 between the numbers of live cells in 2 day-biofilms and the planktonic cells. Additionally, an increase in resistance was found in direct proportion to the increase in biofilm age (Al-Azemi et al., 2011). Sodium proton pumps take 2 H+ ions for each Na+ ion exported. These pumps play an important role in ensuring adaptation of the cell in the presence of alkaline pH (Padan et al., 2001). The results of a proteomics study, where two-dimensional gel electrophoresis was used, demonstrated that high pH promotes the production of metabolic enzymes of amino acid metabolism such as tryptophan deaminase. These enzymes in E. coli convert the surrounding environment from alkaline to acidic by producing acidic products from amino acids (Blankenhorn et al., 1999; Stancik et al., 2002).
In the alkaline pH treatments, in spite of fluctuations during the first 150 days, the number of live cells and HPC increased towards the 180th day. It was thought that this increase in cell number was due to the physical resistance provided by the EPS and a mechanism that can maintain the internal equilibrium of the cell. Overall results of the pH treatments demonstrate that the acid treatment (pH 3) was more effective than the alkaline treatment (pH 11) against biofilm bacteria (Figs 5-6).
For the pH 5 treatment, HPC and the number of live cells obtained were similar to the control experiments. In general, HPC and live cell numbers were higher at this pH than for other pH applications (Figs 5-6).
NaCl solution and distilled water treatment
After exposure to NaCl solution, HPC decreased towards Day 180, as for the distilled water treatment. At the end of the experimental period, an almost 2.5 log reduction was observed in HPC after treatment with NaCl solution when compared to the control sample. Interestingly, the number of live cells did not show a major reduction in either treatments (Figs 7-8).


Many bacteria develop complex mechanisms to survive in hyperosmotic stress. Increasing intracellular K+ concentration by a potassium pump and the accumulation of suitable solutes are two common strategies used to cope with osmotic stress. These suitable solutes are called osmo-protectants and the most effective one is glycine betain (Kempf and Bremer, 1998; Bremer and Kramer, 2000). In a study on S. mutans using real-time PCR, salt stress induced some genes (opuAA) that produced compounds related to transporters of compatible solutes, which are responsible for resistance to osmotic stress (Abranches et al., 2006). An increase in the biofilm structure was observed in parallel with the increase in applied salt concentration, from 0.5% to 7.0% at 22.5°C and 30°C, in a similar study conducted on L. monocytogenes biofilm. The study also showed that after treatment with salt, cells were induced to produce more extracellular matrix substrate (Pan et al., 2010). A different study performed with the halotolerant bacteria Rhodopseudomonas acidophilus showed that the amount of total EPS increased simultaneously with rising salt concentration (Sheng and Yu, 2006). The EPS layer protects bacteria by holding water around the cells and plays a role as an essential component in preventing water loss (Ortega-Morales et al., 2001).
The results of a similar study on P. aeruginosa showed that osmotic shock changed the biofilm structure but did not completely inhibit it. It was estimated that, despite the high salinity, biofilm growth continued slowly. During osmotic shock application, cells from the exponential phase responded with better resistance to high salinity (Bazire et al., 2007). Another study investigated the response of L. pneumophila to salt stress. Survival of L. pneumophila increased with treatments ranging from 0.1-0.5% NaCl. L. pneumophila survived in concentrations above 3% NaCl at temperatures between 4°C and 20°C. Based on the results, it was concluded that sodium plays a role in metabolic transport and as an important cofactor for enzymes (Heller et al., 1998).
In our study, after the application of NaCl, HPC decreased consistently after the 120th day, but the number of live bacteria were similar to the control samples throughout the experiment (Figs 7-8). These results suggest that the biofilm bacteria were resistant against osmotic shock. Another conclusion to be drawn from this data is that studies which are conducted with only conventional culture methods may give false results, because bacteria can enter a VBNC (viable but not culturable) phase when they are exposed to some environmental stresses, such as limitations in available nutrients, temperature, pH, salinity and sunlight (Besnard et al., 2002). Bacteria which enter VBNC phase do not grow on routine culture media but are still alive and their metabolic activities continue at a low level (Oliver, 2005). Studies show that many bacteria can enter a VBNC phase, such as Vibrio cholerae, Mycobacterium tuberculosis, Campylobacter jejuni, Helicobacter pylori, Vibrio vulnificus, E. coli, and especially L. pneumophila and P. aeruginosa in biofilms (Sardeassai, 2005; Türetgen, 2008; Moritz et al., 2010). Sardeassai (2005) showed that 200-5 000 times more bacteria were obtained by microscopic techniques when compared to the culture method. Researchers recommend that a test procedure should be based on culture, photo-analysis and metabolic indicators such as CTC-DAPI (Bredholt et al., 1999).
Monochloramine treatment
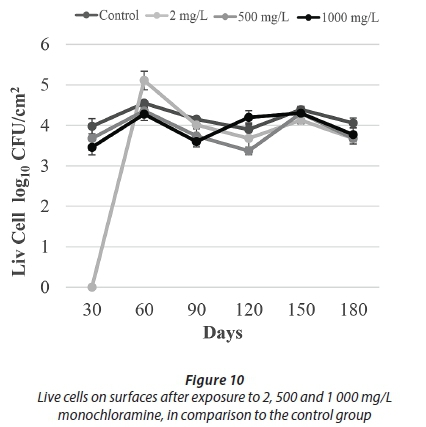
After being exposed to 500 mg/L and 1 000 mg/L monochloramine, no HPC were detected throughout the course of this study. HPC after exposure to 2 mg/L monochloramine was not effective, although no HPC were recovered at the final sampling events. With regards to the results of epifluorescence microscopy, a significant number of live bacteria were detected in all monochloramine treatments (Figs 9-10).

Monochloramine is a disinfectant that has been used in drinking water disinfection since 1916. Monochloramine is more stable than chlorine, which means it can remain in a system long-term and, as a residual disinfectant, can reach long distances in distribution lines. In addition, it enters into the biofilm easier than free chlorine, thereby demonstrating greater efficacy in the fight against biofilm (Koll et al., 1999). It has been shown that many types of bacteria can increase EPS to cope with oxidative stress (Helbling and VanBriesen, 2007). A study with P. aeruginosa in biofilm emerged reporting that the alginate-based EPS blocked the interaction of the reactive sides of cell membranes with monochloramine. The progress of monochloramine into the EPS was also slower. From this point, it was considered that EPS played an effective role in monochloramine disinfection by preventing access to the cell (Xue et al., 2013). A different study about biofilm and disinfection revealed that EPS in biofilm increased the bacterial viability against bactericidal activity provided with nitrogen-doped TiO2 and ZrO2 (Liu et al., 2007).
It was determined that when Legionella is in amoeba-rich biofilm, monochloramine is more effective than free chlorine over long distribution systems (Moore et al., 2006). On the other hand, according to the results of a different study, 99% of Legionella were eliminated with monochloramine application but a very low number of live, resistant bacteria remained after the application and they were able to rapidly recolonize (Jakubek et al., 2013).
In our study, the number of live bacteria was similar to control values after different doses of monochloramine treatment (Fig. 10). It is believed that these similar values were due to the protective effect provided by EPS. By limiting diffusion of monochloramine into the biofilms, any remaining viable bacteria can continue to reproduce. What was also seen after monochloramine applications, as shown with the osmotic shock treatments, was that the number of live bacteria was considerably higher than the HPC. These results indicate that monochloramine treatment causes bacteria to enter a VBNC phase.
CONCLUSION
Microorganisms which are found in water entering systems, such as cooling towers, colonize surfaces and form biofilms, which can decrease equipment efficiency and harbour potential pathogens, among other concerns. These biofilms protect themselves against environmental conditions, making them challenging to remove. This study investigated the efficacy of various parameters against biofilms grown in a model system. The results demonstrated that the most influential parameters on the aerobic heterotrophic bacteria are 60°C, pH 3, 3 M NaCl, 500 and 1 000 mg/L monochloramine, but epifluorescence microscopy results revealed the presence of live microorganisms in biofilm even under these extreme treatments, except at pH 3. Additionally, these results revealed that using only conventional culture methods can give misleading results due to the bacteria entering a VBNC phase. Using different temperature, pH, and osmotic concentration, and different doses of disinfectants to cope with microorganisms was not effective on biofilm bacteria. So, strategies to control the biofilm are still insufficient. Considering the problems in the health and industrial areas that are caused by biofilm, the development of successful control methods, while using the correct techniques to assess their efficacy, plays an important role in the fight against biofilms.
ACKNOWLEDGMENTS
This work was supported by 'Research Fund of the Istanbul University' Project number: 8385.
REFERENCES
ABRANCHES J, LEMOS JA and BURNE RA (2006) Osmotic stress responses of Streptococcus mutans UA159. FEMS Microbiol. Lett. 255 240-246. https://doi.org/10.1111/j.1574-6968.2005.00076.x [ Links ]
ADNAN M, MORTON G, SINGH J and HADI S (2010) Contribution of rpoS and bolA genes in biofilm formation in Escherichia coli K-12 MG1655. Mol. Cell Biochem. 342 207-213. https://doi.org/10.1007/s11010-010-0485-7 [ Links ]
AHMED NAAM, PETERSEN FC and SCHEIE AA (2008) Biofilm formation and autoinducer-2 signaling in Streptococcus intermedius: role of thermal and pH factors. Oral Microbiol. Immunol. 23 492-497. https://doi.org/10.1111/j.1399-302X.2008.00460.x [ Links ]
AL-AZEMI A, FIELDER MD, ABUKNESHA RA and PRICE RG (2011) Effects of chelating agent and environmental stresses on microbial biofilms: relevance to clinical microbiology. J. Appl. Microbiol. 110 1307-1313. https://doi.org/10.1111/j.1365-2672.2011.04983.x [ Links ]
ALI SZ, SANDHYA V, GROVER M, KISHORE N, RAO LV, and VENKATESWARLU B (2009) Pseudomonas sp. strain AKM-P6 enhances tolerance of sorghum seedlings to elevated temperatures. Biol. Fertility Soil46 45-55. https://doi.org/10.1007/s00374-009-0404-9 [ Links ]
ALLISON DG (2003) The biofilm matrix. Biofouling 19 (2) 139-150. https://doi.org/10.1080/0892701031000072190 [ Links ]
ANDERSON KL, ROBERTS C, DISZ T, VONSTEIN V, HWANG K, OVERBEEK R, OLSON PD, PROJAN SJ and DUNMAN PM (2006) Characterization of the Staphylococcus aureus heat shock, cold shock, stringent, and SOS responses and their effects on log-phase mRNA turnover. J. Bacteriol. 188 6739-6756. https://doi.org/10.1128/JB.00609-06 [ Links ]
ARAYA R, TANI K, TAKAGI T, YAMAGUCHI N and NASU M (2002) Bacterial activity and community composition in stream water and biofilm from an urban river determined by fluorescent in situ hybridization and DGGE analysis. FEMS Microbiol. Ecol. 43 111-119. https://doi.org/10.1111/j.1574-6941.2003.tb01050.x [ Links ]
BAZIRE A, DIAB F, JEBBAR M and HARAS D (2007) Influence of high salinity on biofilm formation and benzoate assimilation by Pseudomonas aeruginosa. J. Ind. Microbiol. Biotechnol. 34 5-8. https://doi.org/10.1007/s10295-006-0087-2 [ Links ]
BESNARD V, FEDERIGHI M, DECLERQ E, JUGIAU F and CAPPELIER JM (2002) Environmental and physico-chemical factors induce VBNC state in Listeria monocytogenes. Vet. Res. 33 359-370. https://doi.org/10.1051/vetres:2002022 [ Links ]
BEVERIDGE TJ, MAKIN SA, KADURUGAMUWA JL and LI Z (1997) Interaction between biofilms and the environment. FEMS Microbiol. Rev. 20 291-303. https://doi.org/10.1111/j.1574-6976.1997.tb00315.x [ Links ]
BLANKENHORN D, PHILLIPS J and SLONCZEWSKI JL (1999) Acid-and base- induced proteins during aerobic and anaerobic growth of Escherichia coli revealed by two-dimensional gel electrophoresis. J. Bacteriol. 181 2209-2216. [ Links ]
BREDHOLT S, MAUKONEN J, KUJANPÄÄ K, ALANKO T, OLOFSON U, HUSMARK U, SJOBERG AM and WIRTANEN G (1999) Microbial methods for assessment of cleaning and disinfection of food-processing surfaces cleaned in a low-pressure system. Eur. Food Res. Technol. 209 (2) 145-152. https://doi.org/10.1007/s002170050474 [ Links ]
BREIMAN RF (1996) Impact of technology on the emergence of infectious diseases. Epidemiol. Rev. 18 4-9. https://doi.org/10.1093/oxfordjournals.epirev.a017915 [ Links ]
BREMER E and KRAMER R (2000) Coping with osmotic challenges: osmoregulation through accumulation and release of compatible solutes in bacteria. Bact. Stress Responses 6 79-97. https://doi.org/10.1016/S1095-6433(00)80031-8 [ Links ]
COSTERTON JW, STEWART PS and GREENBERG EP (1999) Bacterial biofilms: a common cause of persistent infections. Science 284 1318-1322. https://doi.org/10.1126/science.284.5418.1318 [ Links ]
COTTER PD and HILL C (2003) Surviving the acid test: responses of gram-positive bacteria to low pH. Microbiol. Mol. Biol. Rev. 67 (3) 429-453. https://doi.org/10.1128/MMBR.67.3.429-453.2003 [ Links ]
CRITCHLEY M and BENTHAM R (2007) Legionella and protozoa in cooling towers: implications for public health and chemical control. Environ. Health 7 36-45. [ Links ]
DECKER BK and PALMORE TN (2014) Hospital water and opportunities for infection prevention. Curr. Infect. Dis. Rep. 16 (432) 1-8. https://doi.org/10.1007/s11908-014-0432-y [ Links ]
DECHO AW (1990) Microbial exopolymer secretions in ocean environments: their role(s) in food webs and marine processes. Oceanogr. Mar. Biol.: Annu. Rev. 28 73-153. [ Links ]
DECHO AW (2000) Microbial biofilms in intertidal systems: an overview. Cont. Shelf Res. 20 1257-1273. https://doi.org/10.1016/S0278-4343(00)00022-4 [ Links ]
DUBOIS M, GILLES KA, HAMILTON JK, REBERS PA and SMITH F (1956) Colorimetric method for determination of sugars and related substances. Anal. Chem. 28 350-356. https://doi.org/10.1021/ac60111a017 [ Links ]
ERMOLENKO DN and MAKHATADZE GI (2002) Bacterial cold- shock proteins. Cell. Mol. Life Sci. 59 1902-1913. https://doi.org/10.1007/PL00012513 [ Links ]
FLEMMING HC, WINGENDER J, MAYER C, KORSTGENS Vand BORCHARD W (2000) Cohesiveness in biofilm matrix Polymers. In: Allison DG, Gilbert P, Lappin-Scott HM and Wilson M (eds.) Community Structure and Co-operation in Biofilms. Cambridge University Press, New York. https://doi.org/10.1017/CBO9780511754814.007 [ Links ]
FLEMMING HC (2002) Biofouling in water systems - cases, causes and countermeasures. Appl. Microbiol. Biotechnol. 59 629-640. https://doi.org/10.1007/s00253-002-1066-9 [ Links ]
GILBERT P, MCBAIN AJ and RICKARD AH (2003) Formation of microbial biofilm in hygienic situations: a problem of control. Int. Biodeterioration Biodegradation 51 245-248. https://doi.org/10.1016/S0964-8305(03)00043-X [ Links ]
GROVER M, ALI SZ, SANDHYA V, RASUL A and VENKATESWARLU B (2011) Role of microorganisms in adaptation of agriculture crops to abiotic stresses. World J. Microbiol. Biotechnol. 27 1231-1240. https://doi.org/10.1007/s11274-010-0572-7 [ Links ]
HELBLING DE and VANBRIESEN JM (2007) Free chlorine demand and cell survival of microbial suspensions. Water Res. 41 44244434. https://doi.org/10.1016/j.watres.2007.06.006 [ Links ]
HELLER R, HOLLER C, SUBMUTH R and GUNDERMANN KO (1998) Effect of salt concentration and temperature on survival of Legionella pneumophila. Lett. Appl. Microbiol. 26 64-68. https://doi.org/10.1046/j.1472-765X.1998.00273.x [ Links ]
JAKUBEK D, GUILLAUME C, BINET M, LEBLON G, DUBOW M and BRUN ML (2013) Susceptibility of Legionella strains to the chlorinated biocide monochloramine. Microbes Environ. 28 (3) 336-345. https://doi.org/10.1264/jsme2.ME12205 [ Links ]
JAN G, ROUAULT A and MAUBOIS JL (2000) Acid stress susceptibility and acid adaptation of Propionibacterium freudenreichii subsp. Shermanii. Lait 80 325-336. https://doi.org/10.1051/lait:2000128 [ Links ]
KEMPF B and BREMER E (1998) Uptake and synthesis of compatible solutes as microbial stress responses to high-osmolality environments. Arch. Microbiol. 170 319-330. https://doi.org/10.1007/s002030050649 [ Links ]
KENNELLY PJ and POTTS M (1996) Fancy meeting you here! A fresh look at "prokaryotic" protein phosphorylation. J. Bacteriol. 178 4759-4764. https://doi.org/10.1128/jb.178.16.4759-4764.1996 [ Links ]
KIEREK-PEARSON K and KARATAN E (2005) Biofilm Development in Bacteria. Adv. Appl. Microbiol. 57 79-111. https://doi.org/10.1016/S0065-2164(05)57003-5 [ Links ]
KIM J, PARK HD and CHUNG S (2012) Microfluidic approaches to bacterial biofilm formation. Molecules 17 9818-9834. https://doi.org/10.3390/molecules17089818 [ Links ]
KIVES J, ORGAZ B and SANJOSE C (2006) Polysaccharide differences between planktonic and biofilm-associated EPS from Pseudomonas fluorescens B52. Colloids Surf. B: Biointerfaces 50 104-108. https://doi.org/10.1016/j.colsurfb.2006.04.018 [ Links ]
KOOL JL, CARPENTER JJ and FIELDS BS (1999) Effect of monochloramine disinfection of municipal drinking water on risk of nosocomial Legionnaires' disease. Lancet 353 272-277. https://doi.org/10.1016/S0140-6736(98)06394-6 [ Links ]
KUBOTA H, SENDA S, NOMURA N, TOKUDA H and UCHIYAMA H (2008) Biofilm formation by lactic acid bacteria and resistance to environmental stress. J. Biosci. Bioeng. 106 (4) 381-386. https://doi.org/10.1263/jbb.106.381 [ Links ]
LANDINI P (2009) Cross-talk mechanisms in biofilm formation and responses to environmental and physiological stress in Escherichia coli. Res. Microbiol. 160 259-266. https://doi.org/10.1016/j.resmic.2009.03.001 [ Links ]
LEMOS JA and BURNA RA (2008) A model of efficiency: stress tolerance by Streptococcus mutans. Microbiology 154 3247-3255. https://doi.org/10.1099/mic.0.2008/023770-0 [ Links ]
LEN AC, HARTY DW and JACQUES NA (2004) Stress-responsive proteins are upregulated in Streptococcus mutans during acid tolerance. Microbiology 150 1339-1351. https://doi.org/10.1099/mic.0.27008-0 [ Links ]
LIU Y, LI J, QIU XF and BURDA C (2007) Bactericidal activity of nitrogen-doped metal oxide nanocatalysts and the influence of bacterial extracellular polymeric substances (EPS). J. Photochem. Photobiol. Chem. 190 94-100. https://doi.org/10.1016/j.jphotochem.2007.03.017 [ Links ]
LIU Y, ZHANG W, SILEIKAA T, WARTAA R, CIANCIOTTOB NP and PACKMANA A (2009) Role of bacterial adhesion in the microbial ecology of biofilms in cooling tower systems. Biofouling 25 (3) 241-253. https://doi.org/10.1080/08927010802713414 [ Links ]
MAINS C (2008) Biofilm control in distribution systems. Natl Environ. Serv. Cent. 8 1-4. [ Links ]
MOORE MR, PRYOR M, FIELDS B, LUCAS C, PHELAN M and BESSER RE (2006) Introduction of monochloramine into a municipal water system: impact on colonization of buildings by Legionella spp. Appl. Environ. Microbiol. 72 (1) 378-383. https://doi.org/10.1128/AEM.72.1.378-383.2006 [ Links ]
MORITZ MM, FLEMMING HC and WINGENDER J (2010) Integration of Pseudomonas aeruginosa and Legionella pneumophila in drinking water biofilms grown on domestic plumbing materials. Int. J. Hyg. Environ. Health 213 190-197. https://doi.org/10.1016/j.ijheh.2010.05.003 [ Links ]
MUNCHBACH M, NOCKER A and NARBERHAUS F (1999) Multiple small heat shock proteins in Rhizobia. J. Bact. 181 83-90. [ Links ]
NOVICK RP (2003) Autoinduction and signal transduction in the regulation of Staphylococcal virulence. Mol. Microbiol. 48 1429-1449. https://doi.org/10.1046/j.1365-2958.2003.03526.x [ Links ]
NYGARD K, WERNER-JOHANSEN O, RONSEN S, CAUGANT DA, SIMONSEN O, KANESTROM A, ASK E, RINGSTAD J, ØDEGARD R, JENSEN T, KROGH T, H0IBY EA, RAGNHILDSTVEIT E, AABERGE IS and AAVITSLAND P (2008) An outbreak of Legionnaires disease caused by long-distance spread from an industrial air scrubber in Sarpsborg, Norway. Clin. Infect. Dis. 46 61-69. https://doi.org/10.1086/524016 [ Links ]
OLIVER JD (2005) The viable but nonculturable state in bacteria. J. Microbiol. 43 93-100. [ Links ]
ORTEGA-MORALES BO, LOPEZ-CORTES A, HERNANDEZ-DUQUE G, CRASSOUS P and GUEZENNEC J (2001) Extracellular polymers of microbial communities colonizing ancient limestone monuments. Meth. Enzymol. 336 331-339. https://doi.org/10.1016/S0076-6879(01)36599-0 [ Links ]
PADAN E, VENTURI M, GERCHMAN Y and DOVER N (2001) Na+/ H+ antiporters. Biochim. Biophys. Acta 1505 144 -157. https://doi.org/10.1016/S0005-2728(00)00284-X [ Links ]
PAN Y, BREIDT F and GORSKI L (2010) Synergistic effects of sodium chloride, glucose, and temperature on biofilm formation by Listeria monocytogenes serotype 1/2a and 4b strains. Appl. Environ. Microbiol. 76 (5) 1433-1441. https://doi.org/10.1128/AEM.02185-09 [ Links ]
RASOULY A and RON EZ (2009) Interplay between the heat shock response and translation in Escherichia coli. Res. Microbiol. 160 288-296. https://doi.org/10.1016/j.resmic.2009.03.007 [ Links ]
RODRIGUEZ GG, PHLPS D, ISHIGURO K and RIDGWAY HF (1992) Use of a fluorescent redox probe for direct visualization of actively respiring bacteria. Appl. Environ. Microbiol. 58 1801-1808. [ Links ]
RUSSELL JB and WILSON DB (1996) Why are ruminal cellulolytic bacteria unable to digest cellulose at low pH? J. Dairy Sci. 79 (8) 1503-1509. http://dx.doi.org/10.3168/jds.S0022-0302(96)76510-4 [ Links ]
SABY S, SIBILLE I, MATHIEU L, PAQUIN JL and BLOCK JC (1997) Influence of water chlorination on the counting of bacteria with DAPI (4,6-diamidino-2-phenylindole). Appl. Environ. Microbiol. 63 (4) 1564-1569. [ Links ]
SANLI-YURUDU NO (2009) Sogutma kulesi su sisteminde biyofilm tabakasina karsi biyosit etkinliginin incelenmesi. PhD thesis, Istanbul University. [ Links ]
SARDEASSAI YN (2005) Viable but non-culturable bacteria: their impact on public health. Curr. Sci. 89 1650. [ Links ]
SCHAULE G, FLEMMING HC and RIDGWAY HF (1993) Use of 5-cyano-2,3-ditolyl tetrazolium chloride for quantifying planktonic and sessile respiring bacteria in drinking water. Appl. Environ. Microbiol. 59 (11) 3850-3857. [ Links ]
SCHMID B, KLUMPP J, RAIMANN E, LOESSNER MJ, STEPHAN R and TASARA T (2009) Role of cold shock proteins in growth of Listeria monocytogenes under cold and osmotic stress conditions. Appl. Environ. Microbiol. 75 (6) 1621-1627. https://doi.org/10.1128/AEM.02154-08 [ Links ]
SEGAL G and RON EZ (1998) Regulation of heat-shock response in bacteria. Ann. New York Acad. Sci. 851 147-151. https://doi.org/10.1111/j.1749-6632.1998.tb08988.x [ Links ]
SHENG GP and YU HQ (2006) Characterization of extracellular polymeric substances of aerobic and anaerobic sludge using three-dimensional excitation and emission matrix fluorescence spectroscopy. Water Res. 40 1233-1239. https://doi.org/10.1016/j.watres.2006.01.023 [ Links ]
STANCIK LM, STANCIK DM, SCHMIDT B, BARNHART DM, YONCHEVA YN and SLONCZEWSKI JL (2002) pH-dependent expression of periplasmic proteins and amino acid catabolism in Escherichia coli. J. Bacteriol. 184 4246-4258. https://doi.org/10.1128/JB.184.15.4246-4258.2002 [ Links ]
TANJI Y, NISHIHARA T and MIYANAGA K (2007) Monitoring of biofilm in cooling water system by measuring lactic acid consumption rate. Biochem. Eng. J. 35 81-86. https://doi.org/10.1016/j.bej.2007.01.001 [ Links ]
TSUNEDA S, AIKAWA H, HAYASHI H, YUASA A and HIRATA A (2003) Extracellular polymeric substances responsible for bacterial adhesion onto solid surface. FEMS Microbiol. Lett. 223 287-292. https://doi.org/10.1016/S0378-1097(03)00399-9 [ Links ]
TURETGEN I (2004) Comparison of free residual chlorine and monochloramine for efficacy against biofilms in model and full scale cooling towers. Biofouling 20 81 -85. https://doi.org/10.1080/08927010410001710027 [ Links ]
TURETGEN I (2008) Induction of viable but nonculturable (VBNC) state and the effect of multiple subculturing on the survival of Legionella pneumophila strains in the presence of monochloramine. Ann. Microbiol. 58 153-156. https://doi.org/10.1007/BF03179460 [ Links ]
TURETGEN I and COTUK A (2005) Formation of bacterial biofilms in model recirculating water system. J. Environ. Micropaleontol. Microbiol. Meiobenthic 2 136-142. [ Links ]
WELIN J, WILKINS JC, BEIGHTON D, WRZESINSKI K, FEY SJ, MOSE-LARSEN P, HAMILTON IR and SVENSTER G (2003) Effect of acid shock on protein expression by biofilm cells of Streptococcus mutans. FEMS Microbiol. Lett. 227 287-293. https://doi.org/10.1016/S0378-1097(03)00693-1 [ Links ]
WELIN-NEILANDS J and SVENSATER G (2007) Acid tolerance of biofilm cells of Streptococcus mutans. Appl. Environ. Microbiol. 73 (17) 5633-5638. https://doi.org/10.1128/AEM.01049-07 [ Links ]
WHITE-ZIEGLER CA, UM S, PEREZ NM, BERNS AL, MALHOWSKI AJ and YOUNG S (2008) Low temperature (23°C) increases expression of biofilm-, cold-shock- and RpoS-dependent genes in Escherichia coli K-12. Microbiology 154 148-166. https://doi.org/10.1099/mic.0.2007/012021-0 [ Links ]
WOOD P, JONES M, BHAKOO M and GILBERT P (1996) A novel strategy for control of microbial biofilms through generation of biocide at the biofilm-surface interface. Appl. Environ. Microbiol. 62 2598-2602. [ Links ]
XUE Z, HESSLER CM, PANMANEE W, HASSETT DJ and SEO Y (2013) Pseudomonas aeruginosa inactivation mechanism is affected by capsular extracellular polymeric substances reactivity with chlorine and monochloramine. FEMS Microbiol. Ecol. 83 101-111. https://doi.org/10.1111/j.1574-6941.2012.01453.x [ Links ]
ZHOU L, XIA S, ZHANG Z, YE B, XU X, GU Z and WANG X (2014) Effects of pH, temperature and salinity on extracellular polymeric substances of Pseudomonas aeruginosa biofilm with N-(3-Oxooxtanoyl)-L-homoserine lactone addition. J. Water Sustainability 4 (2) 91-100. [ Links ]
Received 12 June 2016
Accepted in revised form 9 April 2018
* To whom all correspondence should be addressed. e-mail: cansu.vatansever@altinbas.edu.tr














