Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Water SA
On-line version ISSN 1816-7950
Print version ISSN 0378-4738
Water SA vol.43 n.4 Pretoria Oct. 2017
http://dx.doi.org/10.4314/wsa.v43i4.17
REVIEW
Biosensors for the detection of Escherichia coli
MB MaasI; WJ PeroldI, *; LMT DicksII
IDepartment of Electrical and Electronic Engineering, Stellenbosch University, Stellenbosch 7600, South Africa
IIDepartment of Microbiology, Stellenbosch University, Stellenbosch 7600, South Africa
ABSTRACT
The supply of safe potable water, free from pathogens and chemicals, requires routine analyses and the application of several diagnostic techniques. Apart from being expensive, many of the detection methods require trained personnel and are often time-consuming. With drastic climate changes, severe droughts, increases in population and pollution of natural water systems, the need to develop ultrasensitive, low-cost and hand-held, point-of-use detection kits to monitor water quality is critical. Although Escherichia coli is still considered the best indicator of water quality, cell numbers may be below detection limits, or the cells may be non-culturable and thus only detected by DNA amplification. A number of different biosensors have been developed to detect viable, dead or non-culturable microbial cells and chemicals in water. This review discusses the differences in these biosensors and evaluates the application of microfluidics in the design of ultra-sensitive nano-biosensors.
Keywords: Biosensors, microfluidics, nano-biosensors, E. coli detection
INTRODUCTION
The increase in population numbers, industrial pollution and changes in climate are the main factors leading to water scarcity and a decrease in the quality of potable water (Water Supply and Sanitation Technology Platform, 2006). Polluted water accounts for millions of deaths per annum, especially amongst children under the age of five (WHO, 2003; WHO and UNICEF, 2006). Most of these communities live in drought-stricken countries, often in rural and less developed areas (WHO and UNICEF, 2006). Ten years ago, the World Health Organisation (WHO) and UNICEF estimated that 1.7 billion people in rural areas will not have access to clean, potable water and sanitation (WHO and UNICEF, 2006). The current situation is alarming. Communities in undeveloped rural areas usually have no water purification plants and are prone to develop life-threatening diseases. In many cases, they have to rely on rivers, open reservoirs, springs and open wells for drinking water (WHO and UNICEF, 2006; WHO and United Nations Children's Fund, 2000; Ministry of Health and Social Welfare and WHO, 2002; Gwimbi, 2011). The lack of proper sanitation facilities exacerbates the problem and wells with drinking water are often located close to dug-out latrines, bathing areas and animal camps (WHO and UNICEF, 2006; Ministry of Health and Social Welfare and WHO, 2002; Gwimbi, 2011). Due to the lack in finances and infrastructure, water supplies in informal living areas are seldom tested and are thus not declared safe for human consumption.
Waterborne diseases are not only caused by pathogenic protozoa, viruses and bacteria (WHO and United Nations Children's Fund, 2000), but may also be contracted by the intake of pesticides, hormones, phenols, surfactants, toxins, metals and nitrates (Rodriguez-Mozaz et al., 2006). Testing of water quality relies on testing for the presence of Escherichia coli, which is still considered the best indicator of faecal contamination (WHO, 1996; WHO and UNICEF, 2008; Plate et al., 2004; Ramadan and Gijs, 2012; WHO, 2001). According to WHO guidelines, water is considered of intermediate risk when viable cell numbers of E. coli range between 10 and 100 CFU (colony forming units, thus viable cells) per mL. Water is declared of high risk for consumption when E. coli cells between 100 and 1 000 per mL are recorded (WHO, 1997). In South Africa, one of the drought-stricken countries, the South African National Standards (SANS) for drinking water defines water safe if no viable cells of E. coli are detected (SABS, 2011). The preferred methods for testing microbiological quality of water in South Africa, as specified by SANS, is membrane filtration and colony counts. Confirmation of E. coli is usually done by immunoassay (ELISA) and polymerase chain reaction (PCR) with species-specific DNA primers (Ramadan and Gijs, 2012).
In 2006 the Technical Research Centre (TRC) in Finland identified the need for decentralised monitoring and control of water supplies and advocated an investigation into the development and application of nano-sensors, wireless sensors, rapid detection systems and microbiological sensors to monitor water quality (Könnölä, 2006). Apart from being extremely sensitive (Tokas et al., n.d.), biosensors can be incorporated into portable sensing systems (Rodriguez-Mozaz et al., 2006). Another advantage of portable biosensors is that they can be used to determine spatio-temporal variations in water quality by deploying the sensors in water sources or installing them at the point-of-source (Rodriguez-Mozaz et al., 2006).
The limit of detection (LOD) of a biosensor is defined as the smallest concentration of a compound detectable in a specific volume. Ultra-sensitive biosensors with a low LOD detect a single microbial cell. Although this is the ultimate level of sensitivity required to access water quality, an uneven spread of microbial cells in large volumes of water such as a lake, river or well may not provide accurate cell numbers. This is an important criterion that has to be taken into account when designing a biosensor to assess water quality. From a practical point of view, a point-of-use biosensor needs to be a small, hand-held device and easy to operate. With this in mind, the review focuses on portable biosensors used in the detection of E. coli and summarises the advantages and disadvantages of different technologies used in these sensors.
A number of portable water quality detection kits have been developed. The Nalfleet kit (Figure 1), developed to detect E. coli and Legionella spp. below 100 CFU, records the presence of chemicals (including chlorine), pH and changes in water colour (Wilhelmsen Ships Service). The Potaflex kit (Figure 2) detects changes in microbial growth and was originally designed for laboratory use only (Wagtech WTD, n.d.). These testing kits are useful when analysing water samples, but are difficult to transport and are expensive to use. Rijal and co-workers (2005) developed an E. coli biosensor with antibodies immobilised onto tapered fibres. Apart from being relatively sensitive (the authors recorded LOD values of 70 CFU/mL), the fibres are re-usable after washing with a pH buffer (Rijal et al., 2005). The magnitude of the change was inversely proportional to the concentration of the pathogen. The biosensor differentiated between E. coli O157:H7 and a non-pathogenic variant of E. coli (strain JM101), indicating that it is highly selective for specific antigens. You et al. (2011) developed a handheld lab-on-a-chip device that detected 10 CFU/mL of E. coli K12 and O157:H7 within 6 min. The sensor (Figs 3 and 4) uses Mie light scatter patterns with latex particle immune-agglutination, and detects changes in light intensity at a pre-selected wavelength.



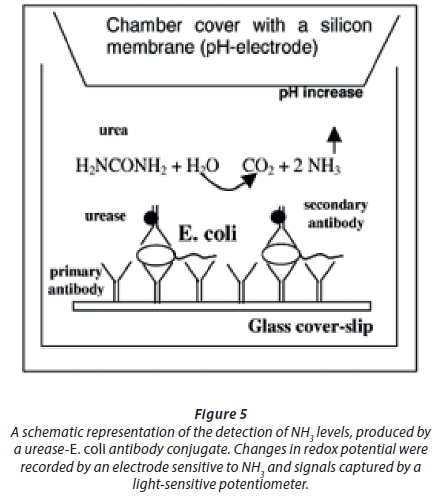
A carbon nanotube (CNT) chemi-resistive biosensor coated with antibodies detected variations in cell numbers of E. coli O157:H7, but was less sensitive with a LOD of 100 000 CFU/mL (García-Aljaro et al., 2010). Teng and co-workers (Teng et al., 2011) used ferrocene-functionalised ZnO nanorods to detect E. coli. The amount of antibodies and ferrocene in the bioconjugates was recorded using the copper reduction/bicinchoninic acid reaction (BCA protein assay) and inductive coupled plasma-atomic emission spectroscopy (ICP-AES), respectively. Changes in current corresponded to changes in E. coli cell numbers and as few as 50 CFU/mL could be detected (Teng et al., 2011). A pre-enrichment step in growth medium allowed the detection of 5 cfu/10 mL E. coli in hospital sewage water (Teng et al., 2011). Ercole et al. (2002) improved the device by recording the interaction between E. coli and a urease-E. coli antibody-conjugate (Figure 5). Changes in redox potential were recorded by an electrode sensitive to NH3 and signals were sent to a light-sensitive potentiometer. As few as 10 CFU E. coli per mL could be recorded over 1.5 h (Ercole et al., 2002). A much simpler method for screening of E. coli in water was described by Mura et al. (2012). The authors used mesoporous thin-film titanium treated with (3-amino-propyl)triethoxysilane (APTES), glutaraldehyde (GA) and antibodies (Mura et al., 2012). Readings were recorded using FTIR (Fourier transform infrared) spectroscopy. The LOD recorded was 100 CFU E. coli per mL (Mura et al., 2012). This biosensor allowed the authors to detect enterohaemorrhagic E. coli O157:H7 in water samples and proved to be an effective screening method. Another approach was to make use of a ferrocene-antimicrobial peptide to develop a biosensor for the detection of E. coli O157:H7 (Li et al., 2014). With the aid of electrochemical impedance spectroscopy (EIS) a LOD of 1 000 CFU E. coli per mL was recorded. The authors differentiated pathogenic E. coli O157:H7 from non-pathogenic E. coli K12, Staphylococcus epidermidis and Bacillus subtilis (Li et al., 2014). Ohk and Bhunia (2013) developed a multiplex fibre optic biosensor for the detection of various microorganisms, including E. coli O157:H7, in meat samples. By immobilising antibodies on optical fibres, the intensity of fluorescence was measured, which correlated with changes in cell numbers of the pathogens (Ohk and Bhunia, 2013). The authors differentiated E. coli O157:H7 from Listeria monocytogenes and Salmonella enterica, the most common pathogens in foodborne outbreaks. The contaminated samples studied were ready-to-eat beef, chicken and turkey meat with a bacterial cell count of approximately 100 CFU/25 g. The limit of detection for the sensor was approximately 1 000 CFU/mL for all three pathogens. The disadvantage of the method was that it took 24 h to get a reading.
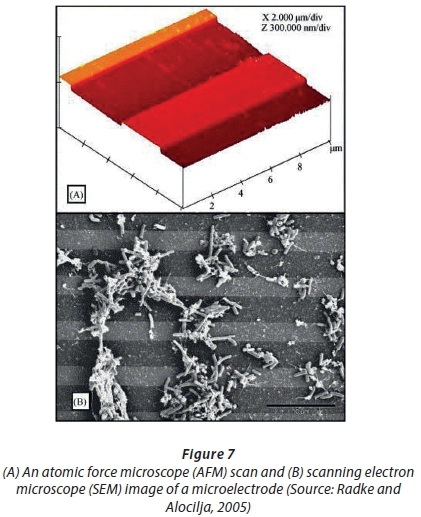
Muhammad-Tahir and Alocilja ( 2003) developed a conductometric biosensor to detect E. coli O157:H7 (Figure 6). The LOD was 79 CFU/mL after 10 min. In this device, a polyaniline-antibody reacted with the antigen and electrons were transferred between two electrodes. Changes in resistance correlated with concentrations of the antigen (Muhammad-Tahir and Alocilja, 2003). The E. coli detection system developed by Radke and Alocilja (2005) relied on impedance. A reduction in impedance was created between two electrodes (Fig. 7). The impedance change was related to the concentration of the pathogen, resulting in a detection limit of 10 000 CFU/mL. The authors could distinguish between cellular concentrations of 104 and 107 CFU/mL E. coli O157:H7 in pure culture and contaminated food samples.
The lowest cell numbers of E. coli (5 CFU/mL) were detected by reporting the activity of two key enzymes (β-glucuronidase and β-galactosidase) characteristic of the species (Hossain et al., 2012). A paper strip was printed with sol-gel-derived silica ink, containing either 5-bromo-4-chloro-3-indolyl-β-D:-glucuronide sodium salt or chlorophenol red β-galactopyranoside (or both) and FeCl3 (Fig. 8).
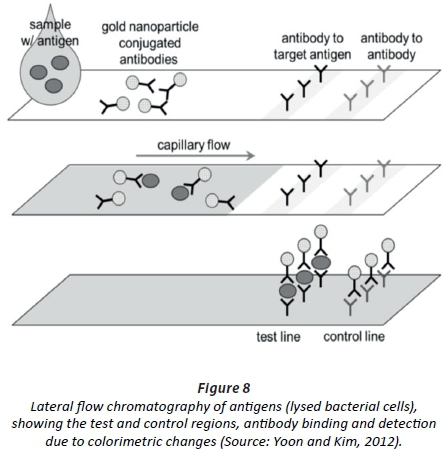
The cells were lysed and the cellular content allowed to migrate, with capillary forces, to the substrate(s) on the opposite end of the paper strip. β-glucuronidase converted the colourless 5-bromo-4-chloro-3-indolyl-β-D:-glucuronide sodium salt to blue and β-galactosidase the yellow chlorophenol red β-galactopyranoside to red. Antibodies were conjugated to immunomagnetic nanoparticles (Hossain et al., 2012). An increase in colour intensity was directly related to the number of E. coli cells (Hossain et al., 2012). The paper strips are stable for weeks without losing effectiveness, and are able to be mass produced at a very low cost (Hossain et al., 2012).
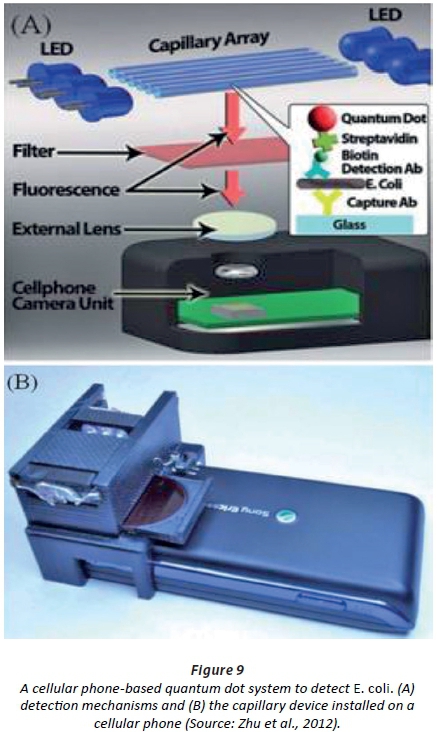
Zhu and co-workers (Zhu et al., 2012) developed a capillary tube system that used quantum dots to detect E. coli. The detection device is linked to a cellphone (Fig. 9). LOD readings of 5 CFU/mL were recorded (Zhu et al., 2012). Each capillary tube acts as a waveguide for UV light emitted by the LEDs, causing the excitation of the quantum dots conjugated on the E. coli cells (Zhu et al., 2012). Fluorescent imaging is then used to relate the light intensity to cell numbers (Zhu et al., 2012). The biosensor could differentiate between E. coli and a number of bacterial species, including Salmonella, and proved effective in the detection of as few as 5 to 10 CFU/mL bacteria in milk. According to the authors, the biosensor may also be used in the screening of other food samples and contaminated water.
BIOSENSORS
A biosensor is defined as 'a self-contained, integrated device capable of providing specific quantitative or semi-quantitative analytical information'. A biosensor model is shown in Figure 10.
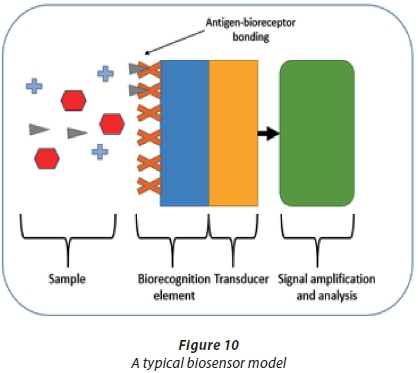
A biosensor typically consists of an analyte in a sample, a bioreceptor (biorecognition element), a transducer and a signal amplification and analysis circuit (Koyun et al., 2012). The analyte is dispersed in the sample with other molecules. The bioreceptor is immobilised with a biomaterial selective to the specific analyte (Koyun et al., 2012). The transducer converts the signal from the analyte, reacts with the biorecognition element and is interpreted by the signal analysis circuit (Sassolas et al., 2012). The concentration of the analyte in the sample is interpreted as the signal increases or decreases, depending on which parameter is tested (Sassolas et al., 2012). The biorecognition element utilises the unique selectivity of biological systems, whilst the transducer amplifies the binding event and the transfer of energy (Yun et al., 2009). The transducer is also used for signal conditioning, sampling time, amplification and electromagnetic interference shielding (Yun et al., 2009). The biorecognition element and transducer surfaces can both be regarded as nanomaterials (Yun et al., 2009).
Biosensors have been applied in various fields, including clinical-, bacterial- and viral diagnostics, medical applications, process control, in bioreactors, quality control, agriculture, veterinary medicine, pharmaceutical production, water treatment, mining, military defence, and environmental monitoring and control (Sadana, 2006; Liu and Lin, 2005). Biosensors are usually highly specific due to the use of selected biorecognition elements and are integrated with existing technology to produce highly robust, low-cost, portable devices (Koyun et al., 2012). Biosensors are thus ideally suited to monitor the microbiological quality of water and the efficiency of filters or membranes in water treatment plants.
Even though there are many advantages, certain problems may occur. These include that heat sterilisation is not possible due to denaturisation of the biomaterial, biomaterial stability is dependent on the natural properties of the material and environmental conditions, and the cells in the biosensor can become contaminated by other unwanted molecules (Koyun et al., 2012).
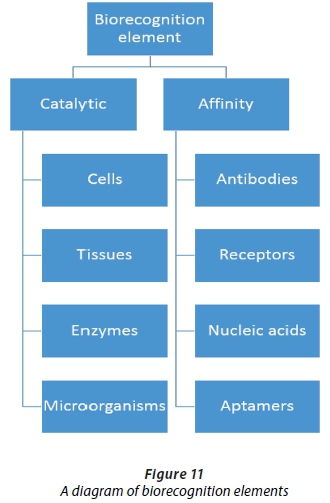
The main biorecognition elements are listed in Figure 11 and a diagram of the transducer types, methods and signals are shown in Figure 12. The elements and their interaction are critical in the design of biosensors.
Transducers and signal analysis
A biosensor is classified according to the transduction mechanism it uses. A transducer is defined as a device that converts physical or chemical changes into electronic signals. In the case of biosensors, the amplification and transfer of the signal may also be facilitated by the transduction element(s). Transduction types are classified as mechanical, magnetic, thermal, piezoelectric, optical or electrochemical (Thévenot et al., 2001). Of these, the electrochemical and optical transduction types are most often used, mainly due to low manufacturing cost, simple design, high sensitivity, robust sensing mechanisms and simple signal analyses (Mairhofer et al., 2009).
Electrochemical transduction methods
Electrochemical transducers either detect the changes occurring between chemical reactions (chemical energy) and transduce these changes into readable electronic signals, or detect electrical changes occurring in mediums due to surface modification by biological elements. Electrochemical biosensors are categorised according to the signal measured, i.e., potentiometric, amperometric, conductometric or capacitive (Thévenot et al., 2001). Potentiometric sensors detect voltage signal changes, amperometric sensors detect current or charge transfer changes (either in a redox reaction or by applying a voltage across a chemiresistor), conductometric sensors measure a change in resistance across electrodes or a surface, and capacitive sensors detect a change in the dielectric constant or electric double layer formed between the material and sample being analysed.
Certain important characteristics of electrochemical biosensors include bioelectroanalysis, the selection of a bioreceptor that is specific to the target analyte, the correct immobilisation method and transducer selection (Koyun et al., 2012). A bioelectrochemical reaction occurs between the bioreceptor and analyte, which may cause a change in current, potential or resistivity between electrodes (Koyun et al., 2012), depending on the geometry and design of the sensor. The performance of the sensor is dependent on the electrode material (electrode and transducer), the surface modification of the electrode and the geometrical dimensions, all of which influence detection ability (Sadana, 2006). Sensor arrays can be integrated with integrated circuits to form microsystems that are able to detect multiple analytes on the same chip (Kim et al., 2003). This enhances the compatibility of the sensors, makes it more functional and can lead to increases in sensitivity (Koyun et al., 2012).
Electrical techniques also include the use of dielectric transducers (capacitive systems) (Spichiger-Keller, 1998). Binding of an antigen to an antibody on an immobilised dielectric insulator causes a change in the electrical double layer (Heineman and Kissinger, 1996) between layered structures containing the sample. When this interface is modified by the binding event, a change in capacitance is observed (Berggren et al., 1999) due to charge transfer from solution to electrode, or vice versa, and by the electrochemical change that may occur in the solution due to redox reactions.
Field effect transistor (FET) biosensors refer to the modification of the gate surface of a field effect transistor. This field is 'tuned' by the interaction of biomolecules and can then be seen by the semiconductor circuit. The I-V characteristic curve of the FET changes according to the concentration of the target antigen. The signal analysis of this electrochemical circuit is simple and FETs can be produced on a large scale using mature electronic technology.
Optical biosensors
Optical biosensors are categorised by either the mode of light used to detect the analyte, or the scattering of light caused by the samples. Simple optical sensors use light emission and detect a change in either light intensity or spectrum shift. This may occur due to the presence of an analyte, or due to the specific antibody-antigen binding of a light source.
Optical sensors can be categorised as absorbent sensors. They use various optical mechanisms for sensing, including transmission in UV-vis (ultra violet visible) light, infrared, evanescent field, surface plasmon resonance (SPR), luminescence and photo emissions (Queirós et al.2012). Absorbency sensors use simple, low-cost, light sources and detectors, are less complex and offer good sensitivities. These sensors are attractive due to their feasibility for use in low-cost, portable sensors that can be mass-produced. Simple optical sensors, with relatively simple detectors can be fabricated from optical fibre cables, or light-emitting diodes (LEDs). LEDs and optical fibres can be immobilised with antibodies, to create highly specific optical absorbency sensors. These light sources and their changes can be detected by a photodetector, light dependent resistor (LDR) or photodiode (O'Toole and Diamond, 2008).
As the concentration of the analyte present in the sample changes, the light intensity will drop or there will be a shift in the spectrum, i.e., a colour change that can be observed. This can be related to the concentration of the analyte.
A unique method using a light source, optical fibre cable and a spectrophotometer is used to detect analytes (Kuswandi et al., 2007). Embedded optical fibres can achieve much lower detection limits, where one fibre delivers light, and another device receives light (Yoon and Kim, 2012). This method is only possible in clean systems, where sample volumes are sufficient (approximately 100 µl sample for a 10 CFU/mL LOD) (Yoon and Kim, 2012). It is possible to immobilise antibodies on the optical fibre tip. When antigen-antibody binding occurs, a photodiode detects a change in the light intensity. This change can then be related to the concentration of antigen in the target.
Another interesting optical sensing mechanism is the use of surface plasmon resonance (SPR), as can be seen in Figure 13.
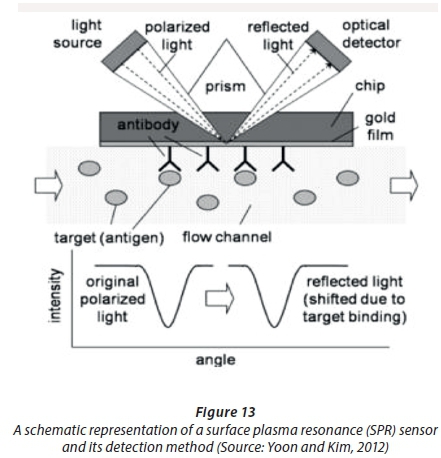
The angle of reflected light shifts due to target binding. This can be detected with an optical detector.
Biorecognition elements
The adhesion of biorecognition elements such as antibodies is important in creating highly specific biosensors. The use of biorecognition elements is critical to biosensor performance, and the understanding of their mechanics is crucial to biosensor development.
Biorecognition elements are biological substances immobilised (attached) to surfaces or transducers. Biorecognition elements use the specificity of biological conjugates to create sensors that only recognise the desired analyte. An appropriate biorecognition element must be selected that only reacts with the specific pathogen or analyte (in this case E. coli), and binds to the surface of the transducer.
There are a variety of biorecognition elements available that can provide a diverse range of applications (Koyun et al., 2012). They can be used create sensitive and specific results due to the fact that they only bind/interact with certain specific target analytes (Koyun et al., 2012). Biorecognition elements should always be specific to the target, should have a high affinity for the target and should form a relatively stable complex with the target (Hunt and Armani, 2010). Biocompatibility is defined as the ability of a material to perform with an appropriate host response in a specific application, and the quality of not having toxic or injurious effects on biological systems (Xiao and Li, 2008). Biorecognition elements are engineered for their size, specificity, affinity, stability, and charge characteristics (Yun et al., 2009).
Biorecognition elements can be used in labelled and label-free biosensors. Labelled biosensors employ external methods of tagging the analyte with secondary or fluorescently marked antibodies, or antibody-nanomaterial conjugates. This is usually done in a pre-processing step. This may complicate the system, making it more expensive and time-consuming (Luo and Davis, 2013). Non-specific signalling issues may also occur (Luo and Davis, 2013). Therefore, the use of label-free sensors is of particular interest. The use of label-free sensors is generally studied, as they do not require auxiliary labelling of pathogens through other mechanisms. Labelled sensors can be used in, for example, colorimetric sensors and lateral flow assays, that indicate colour changes proportionate to analyte concentrations.
Catalytic biosensors refer to electrodes that are immobilised with enzymes and are chemically catalytic, whereas affinity biosensors refer to the binding of a target to immobilised recognition elements on transducer surfaces (Luo and Davis, 2013). Protein-based electrochemical sensors are suited to measure analyte concentrations and to provide continuous and accurate measurements (Kim et al., 2003). Proteins are in the nanometer dimension, and can thus allow the use of smaller electronics (Kim et al., 2003). Recently integrated protein-based biosensor arrays have been developed that can detect multiple analytes (Kim et al., 2003). Proteins used typically include enzymes, antibodies, aptamers, membrane pores and channels, ionophores and receptors (Vo-Dinh and Cullum, 2000; Trojanowicz, 2001).
Affinity biosensors
Affinity biosensors utilise antibodies, nucleic acids, and polymer antibodies (Yun et al., 2009) as biorecognition elements in biosensors. Deoxyribonucleic acid (DNA) and ribonucleic acid (RNA) based biosensors are chemically more stable than antibody based sensors (Luo and Davis, 2013), but may be more complicated, due to DNA amplification required in a pre-conditioning step.
Antibodies are glycoproteins produced by mammals as part of their defence system against foreign matter (WHO, 2001). They possess highly specific binding and recognition domains that can be targeted to specific surface structures of a pathogen (antigen) (WHO, 2001). Two types of antibodies exist, namely, monoclonal and polyclonal antibodies. A comparison of the two is given in Table 1.
The region of the bacterium, antigen or specific protein that needs to be detected and the processing of the sample must both be considered before choosing an appropriate antibody (Abcam, 2016). It is recommended by the WHO (2001) that monoclonal antibodies be used in biosensing devices, but polyclonal antibodies may provide better results depending on the specifications for the device. Antibody immobilised sensors have the ability to be stored and transported at room temperature, but are very sensitive to temperature and humidity (Mairal et al., 2008) during immobilisation. The re-usability of antibody-based biosensors can be increased by washing with certain chemicals after detection, but this may complicate the system required. All these factors need to be considered during biosensor device design.
Recent developments include an E. coli sensor utilising antibodies and ZnO nanorods (Teng et al., 2011; Arya et al., 2012). Saerens et al. (2008) also developed antibody probes for use in biosensors. Conductometric biosensors exploit the insulative properties of cell membranes, so that when cells adhere to the electrode surface, it is expected to increase impedance (Lagarde and Jaffrezic-Renault, 2011).
Aptamers are oligonucleic acids or peptide molecules that selectively bind to low-molecular weight organic and inorganic substrates (Jayasena, 1999) and target molecules. Aptamers can form selective and re-useable sensors, and can form efficient immobilisations and high-density monolayers that are critical to miniaturised systems (Bang et al., 2005; Wu et al., 2007). Aptamers are suitable replacements for antibodies in biosensing systems and can address shortcomings in terms of heat stability.
Catalytic biosensors
Catalytic biosensors typically employ the use of biorecognition elements such as enzymes, cells, tissues and microorganisms (Yun et al., 2009). Enzymes are mostly used in biosensing systems due to their efficient catalytic properties. An enzyme is defined as a biosubstance that acts as a catalyst to bring about a specific biochemical reaction. The enzymatic reaction produces/consumes electrons (for example, enzymes consume dissolved oxygen and produce hydrogen peroxide (De Corcuera and Cavalieri, 2010) in a glucose sensor), which causes electron transfer, and contributes to the double layer potential (Prasad et al., 2012). Biosensors using enzymes can achieve high sensitivities and allow for a lower detection limit due to the catalytic activity that enzymes provide in the biosensor (Vo-Dinh and Cullum, 2000).
Enzyme immobilisation on electrodes has to form an efficient electrical communication, and the electrode surface must retain or improve the biocatalytical effect of the enzyme (Zang et al., 2007). Carbon nanotubes (CNTs) and semiconductive materials such as zinc oxide (ZnO) have been immobilised with enzymes, mostly for use in glucose sensors (Zang et al., 2007). The immobilisation of biorecognition elements to transducer surfaces must be well understood to develop functional and effective biosensors.
Immobilisation techniques
Immobilisation refers to the attachment kinetics of biorecognition elements to transducer surfaces. Inter and intra-cellular signal transduction describes the biochemical mechanism through which cells respond to environmental stimuli (Hunt and Armani, 2010). Correct and effective immobilisation is important in creating high specificity and sensitivity in sensors (Hunt and Armani, 2010). The collection efficiency and the ability of bioreceptors to detect bio-elements in its vicinity is important in creating highly sensitive and specific biosensors (Hunt and Armani, 2010).
The most typical bioreceptors consist of enzymes, antibodies, nucleic acids, cofactors, structured polymers, cells and micro-organisms (Koyun et al., 2012; Queirós et al., 2012). The methods of immobilisation include adsorption, microencapsulation, entrapment, covalent attachment and cross linking (Koyun et al., 2012).
Adsorption can be divided into physical and chemical adsorption techniques. Chemical adsorption is a simpler method but may cause weak bonding of the bioreceptor to the surface of the working electrode or transducer (Koyun et al., 2012). Microencapsulation comprises of an inert membrane that traps the bioreceptor onto the electrode (Koyun et al., 2012). These membranes typically consist of cellulose acetate, collagen, gluten aldehyde, chitosan, nafion or polyurethanes (Koyun et al., 2012). The bilayer lipid membrane (BLM) is the primary electrochemical interface in nature (Kim et al., 2003). Biomimetic membranes act as artificial BLMs and can help to mimic the optimal natural environment in a biosensor (Kim et al., 2003).
The simplest form of immobilisation is physical (electrostatic or hydrophobic) interactions (Luo and Davis, 2013). Better performance can be achieved by immobilisation within an adsorbed matrix, such as a nanostructured transducer (Luo and Davis, 2013).
Antibodies can be immobilised on the surface of transducers by covalent attachment by conjugation of amino, carboxyl, aldehyde, or sulfhydryl groups (De Corcuera and Cavalieri, 2010). Immobilisation techniques are dependent on the physical and chemical characteristics of the transducer, and the environment in which one seeks to operate the biosensor (Luo and Davis, 2013). An example of materials that are functional transducers for biosensors are semiconductive oxides such as ZnO. Oxide surfaces must be functionalised with organosilanes for effective immobilisation of biorecognition elements (Lagarde and Jaffrezic-Renault, 2011). 3-mercaptopropyl trimethoxysilane (MPTS) has been used for antibody immobilisation on ZnO surfaces (Corso et al., 2008). Nanostructures that act as 'smart' materials can facilitate biosensor development, and improve results.
Nanostructures as smart materials in biosensors
There is a major interest in nanostructured materials due to their finite small size, high specific surface area, high porosity and unique physical and chemical properties (Xia et al., 2003). There is also an increased interest in studying the effect nanomaterials have on established structures, such as biosensors. Nanoparticles, nanowires and nanotubes, amongst others, play a significant role in medicine, biomedical engineering, environmental applications and surface science (Tan and Desai, 2005; Bauer et al., 2004). Composite materials, mixing organic and inorganic phases, have flourished and possess unique hybrid qualities (Xiao and Li, 2008). It is for these reasons that the use of nanomaterials in a biosensor would be advantageous to study.
There are various kinds of nanostructures. The structures focussed on in this paper are rods, tubes and wires, which possess similar geometrical properties. Figure 14 shows ZnO nanowires grown on polyethylene fibres and paper sheets.
Nanowires can grow on many substrates. The growth parameters and material properties will vary with different substrates. It is important to consider the growth parameters of nanostructures, while considering the various structural and electrical properties that need to be obtained for the specific application. These parameters will determine the type of material, deposition process and modifications that need to be made to use nanomaterials effectively.
Nanostructures with high surface areas are a suitable platform for adsorption (Corma, 1997) of bioreceptors and sensing (Ramanathan et al., 2005) applications. Nanostructured metal oxide-based composites with large specific surface area and uniform size distribution have been impregnated with biomolecules for use in biosensing applications (Bao et al., 2007a; Bao et al., 2007b; Zhang et al., 2004). The tailored nanostructures could shorten the diffusion distance for the substrate to access the redox centres of immobilised proteins, thus promoting direct electron transfer between the redox protein and electrode (Xiao and Li, 2008). Immobilised enzymes could retain their bioactivity and conduct direct electron transfer between enzyme active sites and electrodes (Zhang et al., 2004). This makes the use of nanostructures advantageous for biosensing applications. It is also possible to produce nanostructures on most substrates, at a low-temperature and using relatively simple deposition processes, which makes devices mass producible at a major cost reduction.
Nanocrystalline metal oxides could play an important role in the adsorption of biomolecules due to the high specific surface area, good biological compatibility and the stability of the materials (Zang et al., 2007). The method developed by Zang and co-authors (2007) was based on flow injection analysis using a bismuth nano-film modified glassy carbon electrode (BiNFE). The marker used was β-d-glucuronidase, an enzyme present in all strains of E. coli. The presence of β-d-Glucuronidase was detected by hydrolysing the cells with polymyxin B and lysozyme and adding 4-nitrophenyl β-d-glucuronide (PNPG) to the cell suspension. The 4-nitrophenol produced from the reaction is electroactive and was easily detected. Levels of 4-nitrophenol produced were directly proportional to the number of E. coli cells within the range of 1.5 × 102 to 1.0 × 106 CFU/ml. The detection limit was 100 CFU/ml and the complete assay was performed within 3 h.
Many types of electrical biosensors are based on the use of nanowires and nanorods including silicon (Si), indium oxide (In2O), zinc oxide (ZnO) and tin oxide (SnO2). They have been used as transduction elements in impedance and capacitive biosensors (Hunt and Armani, 2010). Other nanowire materials that can be used include titanium oxide (TiO2), nickel (Ni), silver (Ag), CNTs, platinum (Pt) and gold (Au) (Luo and Davis, 2013; Prasad et al., 2012).
ZnO and TiO2 are biocompatible, stable and environmentally friendly (Xiao and Li, 2008). ZnO nanostructures can easily be fabricated on any substrate (Qin et al., 2008) with the use of hydrothermal deposition methods at low temperatures (Kenanakis et al., 2009), which is a well-established method.
Zinc oxide (ZnO) based biosensors
ZnO is a n-type, direct wide and-gap II-VI semiconductor with a band gap of 3.37 eV and a large excitonic binding energy of 60 meV at 20°C (Krishnamoorthya and Iliadisa, 2008; Park et al., 2009; Yang et al., 2010). ZnO can be deposited by the wet chemical route or by physical deposition (Yang et al., 2010). The hydrothermal/wet growth methods use amine compounds to direct growth in the c-direction, and a seed layer of ZnO is used (Arya et al., 2012) to facilitate and establish nanowire growth.
An increase in the seed layer thickness improves crystallinity and also increases nanowire diameter (Kenanakis, et al., 2009). Nanostructured ZnO based composites for glucose sensing have been extensively studied, and the synthesis of different ZnO nanostructures for various properties have been established (Xiao and Li, 2008). Biomolecules can be immobilised due to high specific surface area, electrochemical activity, good biocompatibility and chemical stability (Xiao and Li, 2008). High performing sensors can thus be fabricated due to the high specific surface area, and the electron mediating effect by the redox reaction of ZnO (Xiao and Li, 2008). ZnO also has a high isoelectric point value (IEP) of 9.5, which makes it a good matrix to immobilise acidic proteins by electrostatic interactions with high binding stability and insignificant protein denaturalisation (Topoglidis et al., 2005).
Yakimova et al. (2012) reviewed different preparation techniques of ZnO nanocrystals and material issues like wettability, biocompatibility and toxicity which have an important relevance to biosensor functionality. Figures 15 and 16 show the oriented growth of ZnO nanowires in multiple directions and uniformly arranged, respectively.

ZnO nanowires are bio-safe and biocompatible (Zhou et al., 2006). Fe was implanted on ZnO biosensors, which resulted in higher sensitivities (Saha et al., 2010). Song et al. (2007) reported that the performance of biosensors improves by growing highly oriented ZnO nanowires with identical dimensions. Sol-gel, vapour phase and hydrothermal growth methods have been used to fabricate ZnO nanowires (Neveling et al., 2014).
Carbon nanotube (CNT) based biosensors
There are two types of carbon nanotubes (CNTs), multi-walled (2-10 nm internal diameter, 2-100 nm external diameter) and single walled (0.2-2 nm diameter) (Yoon and Kim, 2012). CNTs have a high surface to weight ratio (approximately 300 m2/g), and most of the area is available for electrochemistry and immobilisation of biomolecules. CNTs also have superior conductive properties, low driving voltages, and high energy densities (Yoon and Kim, 2012). CNT immunoassays can allow for rapid electrode kinetics, and higher sensitivities (Yoon and Kim, 2012). The selection of nanostructure for use in a biosensor will depend on the specifications of the device.
Zhang et al. (2009) reviewed recent advances in nanotechnology for use in biosensors. Recent advances include the use of Au nanoparticles as biosensors, carbon nanotubes, magnetic nanoparticles and quantum dots (Zhang et al., 2009; Zhao et al., 2007; Cheng et al., 2008). Cheng et al. (2008) developed a TiO2-based biosensor for the detection of lactate dehydrogenase, Lu et al. (2008) noted that ZnO nanospheres offer a way for enzymes to retain their enzymatic stability, and Zhang et al. (2007) developed an E. coli biosensor using bismuth nanofilm modified gold electrodes.
Other recent developments in nanostructured sensing include using Au nanoparticles/conducting polymer composite for an immunosensor, Pt/nafion composites for the detection of neurotransmitters, CNT-based nanocomposites for use in glucose sensors, and CNT/conducting polymers for use in microbial fuel cells (Xiao and Li, 2008).
LOW-COST MICROFLUIDIC PLATFORMS FOR USE IN BIOSENSING
Microfluidics is defined as the science of manipulating micro-sized droplets on a planar surface or in a micro-channel. Microfluidics can be seen as an enabling technology, allowing the sensing of decreasing sample volumes (Liu et al., 2010). The scaling down of dimensions allow for reduced reagent consumption, higher throughput, enhanced analytical performance, less waste, lower unit cost, and reduced energy consumption, all of which make it an appropriate technology for portable sensing devices (Squires and Quake, 2005).
The basic fluidic operations include droplet moving, mixing, valving and dispensing (Zengerle and Ducrée, 2004). There are two main categories of microfluidics, namely droplet based and continuous flow. Droplet-based microfluidics can be divided into electrowetting, acoustic pumping and two-phase liquid-flow microfluidics (Zengerle and Ducrée, 2004). Continuous-flow microfluidics deals with the mechanisms regarding flow of fluids in micro-sized channels. Continuous-flow microfluidics is less suitable for applications requiring a high degree of flexibility, and complicated fluid manipulations (Liu et al., 2010).
Newman et al. (2004) analysed market trends and developed the roadmap for microfluidics in the life sciences. There is a market in ecology, and specifically water, which includes water quality testing and field tests (Newman et al., 2004). The technological barriers for the development of these technologies include the large volumes of water that need to be analysed, the low concentration of the analyte and the microbiological diversity present in water samples (Newman et al., 2004). All these challenges must be addressed in designing an appropriate biosensor.
Microfluidic-based pathogen sensing can comprise of protein-protein sensing, protein-carbohydrate sensing, and protein-DNA sensing (Lazcka et al., 2007). Another method is the antigen-antibody binding on electrodes (Lazcka et al., 2007), as discussed earlier in this document. Future microfluidic applications and their market trends were evaluated by Zengerle et al. (2004). They identified that microfluidic platforms must be easy to operate. There must be freedom to combine basic microfluidic modules and to build application-specific microfluidic systems (Zengerle and Ducrée, 2004). An important specification is to develop low-cost technologies such as printed circuit boards (PCBs) for use in microfluidics.
Microfluidic analysis can offer a low-cost solution for water quality monitoring, due to the benefits of portability, minimal energy consumption, and cost saving due to their potential for mass-production. The investigation into low-cost platforms such as PCB substrates for use as microfluidic platforms is essential to low-cost sensor development.
Gong and Kim (2005) demonstrated control of droplet volumes on multilayer printed circuit boards (PCBs) with through-substrate electrical contacts to eliminate side connecting lines. They also developed a microfluidic system on a PCB (Gong and Kim, 2008). A cross-section of the device can be seen in Figure 17 (Gong and Kim, 2005).
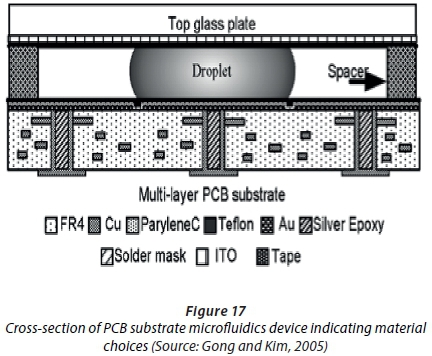
This novel method offers a simple and mature manufacturing technique used in electronics to be used as the base for electrowetting-on-dielectric (EWOD) microfluidic chips. Discrete fluid packets were manipulated on a two-dimensional surface (Gong and Kim, 2008). Two key parameters for microfluidics are volume accuracy and the repeatability of droplet creation (Gong and Kim, 2008), both of which could be achieved with a PCB device. A much lower device cost is made possible by the mass production of PCBs for use in microfluidics.
Electrowetting-on-dielectric (EWOD) microfluidics
The principle of EWOD is defined as the change of free energy on the surface of a dielectric material due to electric charge accumulation when a voltage is applied (Pollack et al., 2000). This changes the wettability of the surface and thus the droplet contact angle (Pollack et al., 2000). EWOD can also be defined as moving discrete droplets by changing the wettability of a surface by an electrical field. When a voltage is applied, the droplet 'sticks' to the surface. This is known as hydrophilic behaviour, meaning an 'affinity for water'. Charge accumulates at the solid-liquid interface, leading to a change in contact angle from hydrophobic to hydrophilic (Saeki et al., 2001), as can be seen in Figure 18.
When there is no applied electrical field, the droplet contact angle changes, and the surface acts as hydrophobic, meaning the 'fear of water'. This causes movement of droplets, by applying a field on an electrode adjacent to the one the droplet sits on. Surfaces acquire a net charge during actuation, but droplets remain electro-neutral (Nelson and Kim, 2012). This can be done using a sandwich device consisting of an electrode, dielectric layer, and hydrophobic coating, as shown in Figure 19. Activating the electrode next to the one on which the droplet sits, deforms the droplet asymmetrically (Gong and Kim, 2008). This exerts a force on the droplet, causing movement.
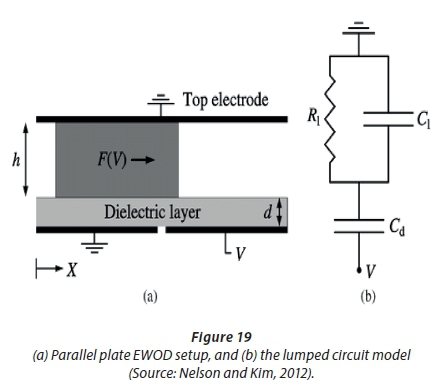
Dielectric insulators, as indicated in Figure 19, guard working fluids from electrodes (Nelson and Kim, 2012). The hydrophobic layer allows simple liquid movement (Nelson and Kim, 2012), and increases the hydrophobicity of the surface in contact with the droplet. The actuation voltage plays a major role in portable devices, because it determines the use of small power sources (batteries) that is critical for device development. The dielectric constant of a material relates to the permittivity of that material (Ahmad, 2012). Permittivity expresses the ability of insulating material to polarise in response to an applied electric field (Ahmad, 2012). If greater polarisation in a given field is achieved, this results in a higher dielectric constant for the material (Ahmad, 2012). One manipulation is the splitting of droplets from a reservoir. To split a droplet, the gap between plates should be smaller than the critical value determined by the material and device parameters (Cho et al., 2003). It must be noted that surface tension is an inherently dominant force in the micro-scale (Saeki et al., 2001).
Zeng and Korsmeyer (2004) provide a comparison between EWOD and dielectrophoresis (DEP) used in microfluidics. The use of thinner dielectric films with higher dielectric constants, and higher dielectric breakdown strengths, can lead to much lower actuation voltages for droplet manipulation, where actuation voltages of 6 V have been achieved (Saeki et al., 2001). A recent development in low-voltage microfluidic actuation was developed (Mita et al., 2009), where a tantalum oxide (Ta2O5) dielectric layer was used in a EWOD system, which allowed droplet actuation under 15 V. A low voltage EWOD device was fabricated (Gao et al., 2011), using silicon nitride (Si3N4) as the dielectric layer, achieving actuation voltages of less than 15 V. Juncker et al. (2002) reported on an autonomous microfluidic capillary system, of which the principles can be used for autonomous inlets and outlets on a portable system.
Producing an EWOD device offers simple device configuration and fabrication, enables the generation of large forces on the micro-scale and consumes very little energy, making it an appropriate platform for portable microfluidics (Gong and Kim, 2008). The dielectric thin-film used greatly influences the device configuration and actuation voltages that can be achieved.
Thin-films for use as dielectric layers
A range of dielectric materials are available for evaluation including silicon oxide (SiO2), silicon nitride (Si3N4), aluminium oxide (Al2O3), yttrium oxide (Y2O3), zirconium oxide (ZrO2), tantalum oxide (Ta2O5) and various liquid polymers.
Lomer (1950) investigated Al2O3 thin films, and noted that the dielectric strength of the material rises as the film thickness decreases. It is also dependent on the temperature of the film (Lomer, 1950). Al2O3 is a wide band gap dielectric material, and methods of depositing Al2O3 thin-films include atomic layer deposition (ALD), plasma-enhanced chemical vapour deposition (PECVD), sol-gel methods, sputtering, pulsed laser deposition (PLD) and physical vapour deposition (PVD) (Kessels et al.). The thickness and stoichiometry of ALD deposited Al2O3 thin-films depend on the underlying surface chemistry during film growth (Elam and George, 2003). The thickness of layers can be determined by using ex-situ stylus profilometry and ellipsometry (Elam and George, 2003), or atomic force microscopy (AFM) step-edge methods.
Radio frequency (RF) sputter coating of Al2O3 results in low deposition rates, while pulsed direct current (DC) reactive sputtering can result in stoichiometric Al2O3 at high deposition rates (Li et al., 2000). A key parameter to notice is the temperature of deposition, which can greatly influence the stoichiometry of the thin-film, as well as limit the type of substrate on which can be deposited (Li et al., 2000).
Pei and Wu (2011) reported on a light-actuated digital microfluidic (LADM) device that uses Al2O3 as the dielectric layer, achieving low voltage actuation (16 VP-P). Advances in ALD have led to the deposition of high quality, conformal, pin-hole free layers of dielectric films, and a superior quality to PECVD techniques (Raj et al., 2009).
Polymers are suitable materials for use as dielectrics due to simpler manufacturing processes, flexibility of the material, and better resistance to chemical attack (Ahmad, 2012). The disadvantages are that they are not temperature resistant, they have large coefficients of thermal expansion and they are susceptible to atmospheric and hydrolytic degradation (Ahmad, 2012). SU-8 and Teflon have been investigated as dielectric and hydrophobic coating layers in EWOD devices (Kumar and Sharma, 2012). Other polymers can also be investigated as dielectric layers in EWOD devices. There is thus a possibility to fabricate thin-film dielectric layers with good material properties, at a low-cost and high throughput.
CONCLUSION
The first E. coli biosensors relied on the detection of colour changes during cell growth (the Potaflex biosensor). The second generation of biosensors made use of antibodies detecting antigens of E. coli or the cells. An improvement on this was the developing of biosensors with higher conductivity, e.g., antibodies adhered to titanium thin films (Mura et al., 2012). The fourth generation of biosensors were made more sensitive by using capillary tubes (Zhu et al., 2012) and fibre-optics (Oak and Bhunia, 2013). The current focus is on developing cost-effective, simple to use biosensors and increasing sensitivity levels. One of the latest developments is the portable fibre-optic biosensor developed in our group (Maas et al., 2017). Polyclonal antibodies against E. coli and fluorescent secondary antibodies were immobilised on borosilicate glass fibres pre-treated with 3-glycidyloxypropyl trimethoxysilane (GPS). A diode placed at one end of the fibres emitted light at an average wavelength of 627 nm. Changes in fluorescence, caused by binding of E. coli to the antibodies, altered the net refractive index of the glass fibres. Photon energy was captured by an ultrasensitive photodiode (Maas et al., 2017). The biggest challenge is to increase the sensitivity of biosensors to levels that would detect less than 5 CFU/mL.
Biosensor development has also been greatly influenced by the advancement of nanotechnology. Biorecognition elements, transducers and analysis techniques developed for nanotechnology have been employed, used and studied in relation to biosensors. These methods have enabled the development of highly sensitive sensors, for the detection of highly specific antigens. The manufacturing methods have also been developed to be scalable for mass-production.
Microfluidics can be used to manipulate, split and move droplets at a low cost. The combination of these methods, including the installation of a wide array of sensors on the same chip, can allow for highly specific sensing. These sensor arrays can detect the same analyte, or be expanded to include different sensors for different antigens.
Biosensors have the ability to be incorporated into highly sensitive, specific, low-cost devices that can detect E. coli at a fraction of the cost and time used for traditional laboratory based methods. Advances in microfluidics, such as electro-wetting on dielectric thin-film layers, and development in nanotechnology and conductive transducers will play a pivotal role in next-gen biosensors.
REFERENCES
ABCAM (2016) Polyclonal and monoclonal: A comparison. URL: http://www.abcam.com/protocols/a-comparison-between-polyclonal-and-monoclonal (Accessed 8 December 2016). [ Links ]
AHMAD Z (2012) Polymer dielectric materials. In: Silaghi M (ed.) Dielectric material. InTech. https://doi.org/10.5772/50638 [ Links ]
ARYA S, SAHA S, RAMIREZ-VICK J, GIUPTA V, BHANSALI S and SINGH S (2012 Recent advances in ZnO nanostructures and thin films for biosensor applications: Review. Anal. Chim. Acta 737 1-21. https://doi.org/10.1016/j.aca.2012.05.048 [ Links ]
BANG G, CHO S and KIM B-G (2005) A novel electrochemical detection method for aptamer biosensors Biosens. Bioelectron. 21 (6) 863-870. https://doi.org/10.1016/j.bios.2005.02.002 [ Links ]
BAO S-J, BAO Q-L, LI C-M, CHEN TP, SUN C-Q, DONG Z-L, GAN Y and ZHANG J (2007a) Synthesis and electrical transport of novel channel-structured beta-AgVO3. Small 3 (7) 1174-1177. https://doi.org/10.1002/smll.200700032 [ Links ]
BAO S-J, LI C-M, LI H-L and LUONG J (2007b) Morphology and electrochemistry of LiMn2O4 optimized by using different Mn-sources. J. Power Sources 164 (2) 885-889. https://doi.org/10.1016/j.jpowsour.2006.11.015 [ Links ]
BARUAH S, PAL S and DUTTA J (2012) Nanostructured zinc oxide for water treatment. Nanosci. Nanotechnol. Asia 14 (1) 613-626. https://doi.org/10.2174/2210681211202020090 [ Links ]
BAUER L, BIRENBAUM N and MEYER G (2004) Biological applications of high aspect ratio nanoparticles. J. Mater. Chem. 14 517-526. https://doi.org/10.1039/b312655b [ Links ]
BERGGREN C, STÅLHANDSKE P, BRUNDELL J and JOHANSSON G (1999) A feasibility study of a capacitive biosensor for direct detection of DNA hybridization. Electroanalysis 11 (3) 156-160. https://doi.org/10.1002/(SICI)1521-4109(199903)11:3<156::AID-ELAN156>3.0.CO;2-O [ Links ]
CHENG J, DI J, HONG J, YAO K, SUN Y, ZHUANG J, XU Q, ZHENG H and BI S (2008) The promotion effect of titania nanoparticles on the direct electrochemistry of lactate dehydrogenase sol-gel modified gold electrode. Talanta 76 (5) 1065-1069. https://doi.org/10.1016/j.talanta.2008.05.006 [ Links ]
CHO S, MOON H and KIM C-J (2003) Creating, transporting, cutting, and merging liquid droplets by electrowetting-based actuation for digital microfluidic circuits. J. Microelectromech. S. 12 (1) 70-80. https://doi.org/10.1109/JMEMS.2002.807467 [ Links ]
COMA A (1997) From microporous to mesoporous molecular sieve materials and their use in catalysis. Chem. Rev. 97 (6) 2373-2420. https://doi.org/10.1021/cr960406n [ Links ]
CORSO C, DICKHERBER A and HUNT W (2008) An investigations of antibody immobilization methods employing organosilanes on planar ZnO surfaces for biosensor applications. Biosens. Bioelectron. 24 (4) 805-811. https://doi.org/10.1016/j.bios.2008.07.011 [ Links ]
DE CORCUERA J and CAVALIERI R (2010) Biosensors. In: Heldman D and Moraru C (eds.) Encyclopedia of Agricultural, Food, and Biological Engineering (2nd edn). CRC Press, Florida, USA. https://doi.org/10.1081/E-EAFE2-120051918 [ Links ]
DUCRÉE J, ZENGERLE R, NEWMAN JD, ERNST H., RIEDMÜLLER K, MESSNER S, ANDRIEUX G, PROVENCE M and ELOY J-C (2004). Flowmap - Microfluidics Roadmap for the Life Sciences. Books on Demand GmbH, Norderstedt, Germany. ISBN 3- 8334-0744-1. 197 pp. [ Links ]
ELAM J and GEORGE S (2003) Growth of ZnO/Al2O3 alloy films using atomic layer deposition techniques. Chem. Mater. 15 (4) 1020-1028. https://doi.org/10.1021/cm020607+ [ Links ]
ERCOLE C, DEL GALLO M, PANTALONE M, SANTUCCI S, MOSIELLO L, LACONI C and LEPIDI A (2002) A biosensor for Escherichia coli based on a potentiometric alternating biosensing (PAB) transducer. Sensor Actuat. B-Chem. 83 (1-3) 48-52. https://doi.org/10.1016/S0925-4005(01)01027-9 [ Links ]
EUROPEAN COMISSION (n.d.). Research Directorate-General - Growth Programme: Microfluidic analysis offers low-cost solution for water quality research. European Commission, Brussels. [ Links ]
GAO A-R, LIU X, GAO X-L, LI T, GAO H-M, PING Z and WANG Y-L (2011) A low voltage driven digital-droplet-transporting-chip by electrostatic force. Chin. Phys. Lett. 28 (8) 084706. https://doi.org/10.1088/0256-307X/28/8/084706 [ Links ]
GARCÍA-ALJARO C, CELLA L, SHIRALE D, PARK M, MUÑOZ F, YATES M and MULCHANDANI A (2010) Carbon nanotubes-based chemiresistive biosensors for detection of microorganisms. Biosens. Bioelectron. 26 (4) 1437-1441. https://doi.org/10.1016/j.bios.2010.07.077 [ Links ]
GONG J and KIM C (2008) All-electronic droplet generation on-chip with real-time feedback control for EWOD digital microfluidics. Lab Chip 8 (8) 898-906. https://doi.org/10.1039/b717417a [ Links ]
GONG J and KIM C-J (2005) Two-dimensional digital microfluidic system by multi-layer printed circuit board. In: Proceedings of the 18th IEEE International Conference on MEMS, 30 January-3 February 2005, Miami Beach. [ Links ]
GWIMBI P (2011) The microbial quality of drinking water in Manonyane community: Maseru District (Lesotho). Afr. Health Sci. 11 (3) 474-480. [ Links ]
HEINEMAN W and KISSINGER P (1996) Large amplitude controlled potential techniques. In: Kissinger P and Heineman W (eds) Laboratory Techniques in Electroanalytical Chemistry. Marcel Dekker, Inc., New York. [ Links ]
HOSSAIN S, OZIMOK C, SICARD C, AGUIRRE S, ALI M, LI Y and BRENNAN J (2012) Multiplexed paper test strip for quantitative bacterial detection. Anal. Bioanal. Chem. 403 (6) 1567-1576. https://doi.org/10.1007/s00216-012-5975-x [ Links ]
HUNT H and ARMANI A (2010) Label-free biological and chemical sensors. Nanoscale 2 (9) 1544-1559. https://doi.org/10.1039/c0nr00201a [ Links ]
JAYASENA S (1999) Aptamers: An emerging class of molecules that rival antibodies in diagnostics. Clin. Chem. 45 (9) 1628-1650. [ Links ]
JUNCKER D, SCHMID H, DRESCHLER U, WOLF H, WOLF M, MICHEL B, DE ROOIJ N and DELAMARCHE E (2002) Autonomous microfluidic capillary system. Anal. Chem. 74 (24) 6139-6144. https://doi.org/10.1021/ac0261449 [ Links ]
KENANAKIS G, VERNARDOU D, KOUDOUMAS E and KATSARAKIS N (2009) Growth of c-axis oriented ZnO nanowires from aqueous solution: The decisive role of a seed layer for controlling the wires' diameter. J. Cryst. Growth 311 (23-24) 4799-4804. https://doi.org/10.1016/j.jcrysgro.2009.09.026 [ Links ]
KESSELS W, VAN DELFT J, DINGEMANS G and MANDOC M (n.d.) Review on the prospects for the use of Al2O3 for high-efficiency solar cells. Eindhoven University of Technology. [ Links ]
KIM P, KOHLI N, HASSLER B, DOTSON N, MASON A, WORDEN R and OFOLI R (2003) An electrochemical interface for integrated biosensors. In: Proceedings of the IEEE Sensors 2003 Conference, 22-24 October 2003, Toronto. https://doi.org/10.1109/ICSENS.2003.1279100 [ Links ]
KÖNNÖLÄ P (2006) TESTNET WP1 Future Outlook on Water Quality Monitoring Technologies. VTT Technical Research Centre of Finland, Espoo, Finland. [ Links ]
KOYUN A, AHLATCIOĞLU E and İPEK Y (2012) Biosensors and their principles. In: Kara S (ed.) A Roadmap of Biomedical Engineers and Milestones. InTech. ISBN: 978-953-51-0609-8. https://doi.org/10.5772/48824 [ Links ]
KRISHNAMOORTHYA S and ILIADISA A (2008) Properties of high sensitivity ZnO surface acoustic wave sensors on SiO2 (1 0 0) Si substrates. Solid State Electron. 52 1710-1716. https://doi.org/10.1016/j.sse.2008.06.039 [ Links ]
KUMAR V and SHARMA N (2012) SU-8 as hydrophobic and dielectric thin film in electrowetting-on-dielectric based microfluidics device. J. Nanotechnol. 2012 1-6. https://doi.org/10.1155/2012/312784 [ Links ]
KUSWANDI B, NURIMAN, HUSKENS J and VERBOOM W (2007) Optical sensing systems for microfluidic devices: A review. Anal. Chim. Acta 601 (2) 141-155. https://doi.org/10.1016/j.aca.2007.08.046 [ Links ]
LAGARDE F and JAFFREZIC-RENAULT N (2011) New trends in biosensors for water monitoring. In: Somerset V (ed.) Environmental Biosensors. InTech. ISBN: 978-953-307-486-3. https://doi.org/10.5772/19945 [ Links ]
LAZCKA O, DEL CAMPO F and MUÑOZ F (2007) Pathogen detection: A perspective of traditional methods and biosensors. Biosens. Bioelectron. 22 (7) 1205-1217. https://doi.org/10.1016/j.bios.2006.06.036 [ Links ]
LI Q, YU Y-H, BHATIA C, MARKS L, LEE S and CHUNG Y (2000) Low-temperature magnetron sputter-deposition, hardness, and electrical resistivity of amorphous and crystalline alumina thin films. J. Vac. Sci. Technol. A 18 2333. https://doi.org/10.1116/1.1286715 [ Links ]
LI Y, AFRASIABI R, FATHI F, WANG N, XIANG C, LOVE R, SHE Z and KRAATZ H-B (2014) Impedance based detection of pathogenic E. coli O157:H7 using a ferrocene-antimicrobial peptide modified biosensor. Biosens. Bioelectron. 58 193-199. https://doi.org/10.1016/j.bios.2014.02.045 [ Links ]
LIU G and LIN Y (2005) Electrochemical sensor for organophosphate pesticides and nerve agents using zirconia nanoparticles as selective sorbents. Anal. Chem. 77 (18) 5894-5901. https://doi.org/10.1021/ac050791t [ Links ]
LIU K, WU R, CHUANG Y, KHOO H, HUANG S and TSENG F (2010) Microfluidic systems for biosensing. Sensors 10 (7) 6623-6661. https://doi.org/10.3390/s100706623 [ Links ]
LOMER P (1950) The dielectric strength of aluminum oxide films. P. Phys. Soc. B 63 (10) 818. https://doi.org/10.1088/0370-1301/63/10/110 [ Links ]
LU X, ZHANG Y, NI Y, ZHANG Q and CHEN J (2008). Porous nanosheet-based ZnO microspheres for the construction of direct electrochemical biosensors. Biosens. Bioelectron. 24 (1) 93-98. https://doi.org/10.1016/j.bios.2008.03.025 [ Links ]
LUO J, FU Y, LI Y, DU X, FLEWITT A, WALTON A and MILNE W (2009) Moving-part-free microfluidic systems for lab-on-a-chip. J. Micromech. Microeng. 19 (5) 054001. https://doi.org/10.1088/0960-1317/19/5/054001 [ Links ]
LUO X and DAVIS J (2013) Electrical biosensors and the label free detection of protein disease biomarkers. Chem. Soc. Rev. 42 (13) 5944-5962. [ Links ]
MAAS MB, MAYBERY G, PEROLD WJ, NEVELING DP and DICKS LMT (2017) Borosilicate glass fiber-optic biosensor for the detection of Escherichia coli. Curr. Microbiol. DOI: 10.1007/s00284-017-1359-7. [ Links ]
MAIRAL T, OZALP V, LOZANO SÁNCHEZ P, MIR M, KATAKIS I. and O'SULLIVAN C (2008) Aptamers: Molecular tools for analytical applications. Anal. Bioanal. Chem. 390 (4) 989-1007. https://doi.org/10.1007/s00216-007-1346-4 [ Links ]
MAIRHOFER J, ROPPERT K and ERTL P (2009) Microfluidic systems for pathogen sensing: a review. Sensors 9 (6) 4804-4823. https://doi.org/10.3390/s90604804 [ Links ]
MINISTRY OF HEALTH AND SOCIAL WELFARE and WHO (2002) Lesotho - Health indicators - June 5, 2002. [ Links ]
MITA Y, LI Y, KUBOTA M, MORISHITA S, PARKES W, HAWORTH LI. FLYNN BW, TERRY JG, TANG T-B, RUTHVEN AD, SMITH S and WALTON AJ (2009) Demonstration of a wireless driven MEMS pond skater that uses EWOD technology. Solid State Electron. 53 (7) 798-802. https://doi.org/10.1016/j.sse.2009.02.020 [ Links ]
MUHAMMAD-TAHIR Z and ALOCILJA E (2003) A conductometric biosensor for biosecurity. Biosens. Bioelectron. 18 (5-6) 813-819. https://doi.org/10.1016/S0956-5663(03)00020-4 [ Links ]
MURA S, GREPPI G, MARONGIU ML, ROGGERO PP, RAVINDRANATH SP, MAUER LJ, SCHIBECI N, PERRIA F, PICCININI M, INNOCENZI P and IRUDAYARAJ J (2012) FTIR nanobiosensors for Escherichia coli detection. Beilstein J. Nanotechnol. 3 485-492. https://doi.org/10.3762/bjnano.3.55 [ Links ]
NELSON W and KIM C-J (2012) Droplet actuation by electrowetting-on-dielectric (EWOD): a review. J. Adhesion Sci. Technol. 26 1747-1771. https://doi.org/10.1163/156856111X599562 [ Links ]
NEVELING DP, VAN DEN HEEVER TS, PEROLD WJ and DICKS LMT (2014) Development of a ZnO nanowire-array biosensor for the detection and quantification of immunoglobulins. Sensor. Actuat. B-Chem. 203 102-110. [ Links ]
O'TOOLE M and DIAMOND D (2008) Absorbance based light emitting diode optical sensors and sensing devices. Sensors 8 (4) 2453-2479. https://doi.org/10.3390/s8042453 [ Links ]
OHK S-H and BHUNIA A (2013) Multiplexfiber optic biosensor for detection of Listeria monocytogenes, Escherichia coli O157:H7 and Salmonella enterica from ready-to-eat meat samples. Food Microbiol. 33 (2) 166-171. https://doi.org/10.1016/j.fm.2012.09.013 [ Links ]
PARK H-Y, GO H-Y, KALME S, MANE R, HAN S-H and YOON M-Y (2009) Protective antigen detection using horizontally stacked hexagonal ZnO platelets. Anal. Chem. 81 (11) 4280-4284. https://doi.org/10.1021/ac900632n [ Links ]
PEI S and WU M (2011) Light-actuated digital microfluidics for large-scale droplet manipulation. University of California at Berkeley, Electrical Engineering and Computer Sciences. [ Links ]
PLATE D, STRASSMANN B and WILSON M (2004) Water sources are associated with childhood diarrhoea prevalence in rural east-central Mali. Trop. Med. Int. Health 9 (3) 416-425. https://doi.org/10.1111/j.1365-3156.2004.01200.x [ Links ]
POLLACK M, FAIR R and SHENDEROV A (2000) Electrowetting-based actuation of liquid droplets for microfluidic applications. Appl. Phys. Lett. 77 (11) 1725-1726. https://doi.org/10.1063/1.1308534 [ Links ]
PRASAD H, PRASAT R and HASHIM U (2012) A review on the label free nanowire based biosensor. J. Appl. Sci. Res. 8 (9) 4759-4769. [ Links ]
QIN Y, YANG R and WANG Z (2008) Growth of horizontal ZnO nanowire arrays on any substrate. J. Phys. Chem. C 112 (48) 18734-18736. https://doi.org/10.1021/jp808869j [ Links ]
QUEIRÓS R, NORONHA J, MARQUES P and SALES M (2012). Emerging (bio)sensing technology for assessing and monitoring freshwater contamination - methods and applications. In: Voudouris K (ed.) Ecological Water Quality - Water Treatment and Reuse. InTech. URL: http://www.intechopen.com/books/ecological-water-quality-water-treatment-and-reuse/emerging-biosensing-technology-for-assessing-and-monitoring-freshwater-contamination-methods-and-ap. ISBN: 978-953-51-0508-4. [ Links ]
RADKE S and ALOCILJA E (2005) A high density microelectrode array biosensor for detection of E. coli O157:H7. Biosens. Bioelectron. 20 (8) 1662-1667. https://doi.org/10.1016/j.bios.2004.07.021 [ Links ]
RAJ B, DHINDSA M, SMITH N, LAUGHLIN R and HEIKENFELD J (2009) Ion and liquid dependent dielectric failure in electrowetting systems. Langmuir 25 (20) 12387-12392. https://doi.org/10.1021/la9016933 [ Links ]
RAMADAN Q and GIJS M (2012) Microfluidic applications of functionalized magnetic particles for environmental analysis: Focus on waterborne pathogen detection. Microfluid. Nanofluid. 13 (4) 529-542. https://doi.org/10.1007/s10404-012-1041-4 [ Links ]
RAMANATHAN K, BANGAR M, YUN M, CHEN W, MYUNG N and MULCHANDANI A (2005) Bioaffinity sensing using biologically functionalized conducting-polymer nanowire. J. Am. Chem. Soc. 127 (2) 496-497. https://doi.org/10.1021/ja044486l [ Links ]
RIJAL K, LEUNG A, SHANKAR P and MUTHARASAN R (2005) Detection of pathogen Escherichia coli O157:H7 AT 70 cells/mL using antibody-immobilized biconical tapered fiber sensors. Biosens. Bioelectron. 21 (6) 871-880. https://doi.org/10.1016/j.bios.2005.02.006 [ Links ]
RODRIGUEZ-MOZAZ S, LOPEZ DE ALDA M and BARCELÓ D (2006) Biosensors as useful tools for environmental analysis and monitoring. Anal. Bioanal. Chem. 386 (4) 1025-1041. https://doi.org/10.1007/s00216-006-0574-3 [ Links ]
SABS (2011) South African National Standard 241-1: Drinking water Part 1: Microbiological, physical, aesthetic and chemical determinands. South African Bureau of Standards, Pretoria. [ Links ]
SADANA A (2006) Binding and Dissociation Kinetics for Different Biosensor Applications Using Fractals. Elsevier Science, Amsterdam. [ Links ]
SAEKI F, BAUM J, MOON H, YOON J-Y, KIM C-J and GARRELL R (2001) Electrowetting on dielectrics (EWOD): reducing voltage requirements for microfluidics. Polymeric Mater. Sci. Eng. 85 12-13. [ Links ]
SAERENS D, HUANG L, BONROY K and MUYLDERMANS S (2008) Antibody fragments as probe in biosensor development. Sensors 8 (8) 4669-4686. https://doi.org/10.3390/s8084669 [ Links ]
SAHA S, GUPTA V, SREENIVAS K, TAN H and JAGADISH C (2010) Third generation biosensing matrix based on Fe-implanted ZnO thin films. Appl. Phys. Lett. 97 133704. https://doi.org/10.1063/1.3496456 [ Links ]
SASSOLAS A, BLUM L and LECA-BOUVIER B (2012) Immobilization strategies to develop enzymatic biosensors. Biotechnol. Adv. 30 (3) 489-511. https://doi.org/10.1016/j.biotechadv.2011.09.003 [ Links ]
SONG J and LIM S (2007) Effect of seed layer on the growth of ZnO nanorods. J. Phys. Chem. 111 (2) 596-600. https://doi.org/10.1021/jp0655017 [ Links ]
SPICHIGER-KELLER U (1998) Chemical and biochemical sensors. In: Spichiger-Keller U (ed.) Chemical Sensors and Biosensors for Medical and Biological Applications. Wiley-VCH, Weinheim. https://doi.org/10.1002/9783527612284.ch2 [ Links ]
SQUIRES T and QUAKE S (2005) Microfluidics: Fluid physics at the nanoliter scale. Rev. Mod. Phys. 77 (3) 977-1026. https://doi.org/10.1103/RevModPhys.77.977 [ Links ]
TAN W and DESAI T (2005) Microscale multilayer cocultures for biomimetic blood vessels. J. Biomed. Mater. Res. A 72 (2) 146-160. https://doi.org/10.1002/jbm.a.30182 [ Links ]
TENG Y, ZHANG X, FU Y, WANG Z, JIN L and ZHANG W (2011) Optimized ferrocene-functionalized ZnO nanorods for signal amplification in electrochemical immunoassay of Escherichia coli. Biosens. Bioelectron. 26 (12) 4661-4666. https://doi.org/10.1016/j.bios.2011.04.017 [ Links ]
THÉVENOT D, TOTH K, DURST R and WILSON G (2001) Electrochemical biosensors: Recommended definition and classification. Biosens. Bioelectron. 16 (1-2) 121-131. https://doi.org/10.1016/S0956-5663(01)00115-4 [ Links ]
TOKAS J, BEGUM R, JAIN S and YADAV H (n.d.) Biosensor: General principles and applications. Presentation. URL: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1 &ved=0ahUKEwj6spKIhd_ QAhWKCsAKHVMeCYMQFgggMAA&url=http%3A%2F%2Fwww.pitt.edu%2F~super4 %2F36011-37001%2F36421.ppt&usg=AFQjCNGhovLKt6PDH1BJLS4NWdNAKCynSw (Accessed 17 January 2017). [ Links ]
TOPOGLIDIS E, PALOMARES E, ASTUTI Y, GREEN A, CAMPBELL C and DURRANT J (2005) immobilization and electrochemistry of negatively charged proteins on modified nanocrystalline metal oxide electrodes. Electroanalysis 17 (12) 1035-1041. https://doi.org/10.1002/elan.200403211 [ Links ]
TROJANOWICZ M (2001) Miniaturized biochemical sensing devices based on planar bilayer lipid membranes. Fresinius J. Anal. Chem. 371 (2) 246-260. https://doi.org/10.1007/s002160101005 [ Links ]
VO-DINH T and CULLUM B (2000) Biosensors and biochips: Advances in biological and medical diagnostics. Fresenius J. Anal. Chem. 366 (6-7) 540-551. https://doi.org/10.1007/s002160051549 [ Links ]
WAGTECH WTD (n.d.) Portable water quality test kits: Datasheet. Wagtech, Berkshire, UK. [ Links ]
WATER SUPPLY AND SANITATION TECHNOLOGY PLATFORM (2006) Strategic research agenda: Water Research - a necessary investment in our common future. Water Supply and Sanitation Technology Platform, URL: http://www.eugris.info/contactform.asp [ Links ]
Wilhelmsen Ships Service (n.d.) Marine Chemical: Nalfleet potable water test solution. [ Links ]
WHO and United Nations Children's Fund (2000) Global Water Supply and Sanitation Assessment 2000 Report. WHO & United Nations Children's Fund, Geneva. [ Links ]
WHO and UNICEF (2006) Meeting the MDG drinking water and sanitation target: The urban and rural challenge of the decade. World Health Organization & UNICEF, Geneva. [ Links ]
WHO and UNICEF (2008) Progress on Drinking Water and Sanitation: Joint Monitoring Programme - Special Focus on Sanitation. World Health Organization & UNICEF, Geneva. [ Links ]
WHO (1996) Guidelines for Drinking-Water Quality - Second Edition - Volume 2 - Health Criteria and Other Supporting Information. World Health Organization, Geneva. [ Links ]
WHO (1997) Guidelines for drinking-water quality. Second edition. Volume 3 - Surveillance and control of community supplies. World Health Organization, Geneva. [ Links ]
WHO (2001) Water Quality: Guidelines, Standards and Health. IWA Publishing, Geneva. [ Links ]
World Health Organisation (2003) The World Health Report: Shaping the future. World Health Organization, Geneva. [ Links ]
WU Z, GUO M, ZHANG S, CHEN C, JIANG J, SHEN G and YU R (2007) Reusable eletrochemical sensing platform for high sensitive detection of small molecules based on structure-switching signalling aptamers. Anal. Chem. 79 (7) 2933-2939. https://doi.org/10.1021/ac0622936 [ Links ]
XIA B, YANG P, SUN Y, WU Y, MAYERS B, GATES B, YIN Y, KIM F and YAN H (2003) One-dimensional nanostructures: synthesis, characterization, and applications. Adv. Mater. 15 (5) 353-389. https://doi.org/10.1002/adma.200390087 [ Links ]
XIAO Y and LI C (2008) Nanocomposites: from fabrications to electrochemical bioapplications. Electroanalysis 20 (6) 648-662. https://doi.org/10.1002/elan.200704125 [ Links ]
YAKIMOVA R, SELEGARD L, KHRANOVSKYY V, PEARCE R, SPETZ A and UVDAL K (2012) ZnO materials and surface tailoring for biosensing. Front. Biosci. 4 254-278. https://doi.org/10.2741/e374 [ Links ]
YANG P, YAN H, MAO S, RUSSO R, JOHNSON J, SAYKALLY R, MORRIS N, PHAM J, HE R and CHOI H-J (2002) Controlled growth of ZnO nanowires and their optical properties. Adv. Funct. Mater. 12 (5) 323-331. https://doi.org/10.1002/1616-3028(20020517)12:5<323::AID-ADFM323>3.0.CO;2-G [ Links ]
YANG Z, ZONG X, YE Z, ZHAO B, WANG Q and WANG P (2010) The application of complex multiple forklike ZnO nanostructures to rapid and ultrahigh sensitive hydrogen peroxide biosensors. Biomaterials 31 (29) 7534-7541. https://doi.org/10.1016/j.biomaterials.2010.06.019 [ Links ]
YOON J-Y and KIM B (2012) Lab-on-a-chip pathogen sensors for food safety. Sensors 12 (8) 10713-10741. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3472853/ [ Links ]
YOU D, GESHELL K and YOON J-Y (2011) Direct and sensitive detection of foodborne pathogens within fresh produce samples using a field-deployable handheld device. Biosens. Bioelectron. 28 (1) 399-406. https://doi.org/10.1016/j.bios.2011.07.055 [ Links ]
YUN Y-H, ETESHOLA E, BHATTACHARYA A, DONG Z, SHIM J-S, CONFORTI L, KIM D, SCHULZ MJ, AHN CH and WATTS N (2009) Tiny medicine: nanomaterial-based biosensors. Sensors 9 (11) 9275-9299. https://doi.org/10.3390/s91109275 [ Links ]
ZANG J, LI C, CUI X, WANG J, SUN X, DONG H and SUN C (2007) Tailoring zinc oxide nanowires for high performance amperometric glucose sensor. Electroanalysis 19 (9) 1008-1014. https://doi.org/10.1002/elan.200603808 [ Links ]
ZENG J and KORSMEYER T (2004) Principles of droplet electrohydrodynamics for lab-on-a-chip. Lab Chip 4 (4) 265-277. https://doi.org/10.1039/b403082f [ Links ]
ZENGERLE R and DUCRÉE J (2004) The future of microfluidics: low-cost technologies and microfluidic platforms. IMTEK, University of Freiburg. [ Links ]
ZHANG F, WANG X, AI S, SUN S, WAN Q, ZHU Z, XIAN Y, JIN L and YAMAMOTO K (2004) Immobilization of uricase on ZnO nanorods for a reagentless uric acid biosensor. Anal. Chim. Acta 519 (2) 155-160. https://doi.org/10.1016/j.aca.2004.05.070 [ Links ]
ZHANG W, TANG H, GENG P, WANG Q, JIN L and WU Z (2007) Amperometric method for rapid detection of Escherichia coli by flow injection analysis using a bismuth nano-film modified glassy carbon electrode. Electrochem. Commun. 9 (4) 833-838. https://doi.org/10.1016/j.elecom.2006.11.019 [ Links ]
ZHANG X, QIN G and CUI D (2009) Recent advances in nanotechnology applied to biosensors. Sensors 9 (2) 1033-1053. https://doi.org/10.3390/s90201033 [ Links ]
ZHAO W, CHIUMAN W, BROOK MA and LI Y (2007) Simple and rapid colorimetric enzyme sensing assays using non-crosslinking gold nanoparticle aggregation. Chembiochem 8 727-731. https://doi.org/10.1002/cbic.200700014 [ Links ]
ZHOU J, XU N and WANG Z (2006) Dissolving behavior and stability of ZnO Wires in biofluids: a study on biodegradability and biocompatibility of ZnO nanostructures. Adv. Mater. 18 (18) 2432-2435. https://doi.org/10.1002/adma.200600200 [ Links ]
ZHU H, SIKORA U and OZCAN A (2012) Quantum dot enabled detection of Escherichia coli using a cell-phone. Analyst 137 (11) 2541-2544. https://doi.org/10.1039/c2an35071h [ Links ]
Received 23 January 2017
Accepted in revised form 12 October 2017
* To whom all correspondence should be addressed. e-mail: wjperold@sun.ac.za














