Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
Water SA
versión On-line ISSN 1816-7950
versión impresa ISSN 0378-4738
Water SA vol.43 no.4 Pretoria oct. 2017
http://dx.doi.org/10.4314/wsa.v43i4.16
REVIEW
Pharmaceutical and personal care products (PPCPs) as endocrine disrupting contaminants (EDCs) in South African surface waters
Edward ArcherI, II, *; Gideon M WolfaardtI, II; Johannes H van WykIII
IDepartment of Microbiology, Faculty of Science, Stellenbosch University, Matieland 7602, Stellenbosch, South Africa
IIDepartment of Chemistry and Biology, Faculty of Science, Ryerson University, Toronto M5B 2K3, Canada
IIIDepartment of Botany and Zoology, Faculty of Science, Stellenbosch University, Matieland 7602, Stellenbosch, South Africa
ABSTRACT
Globally, water resources are under constant threat of being polluted by a diverse range of man-made chemicals, and South Africa is no exception. These contaminants can have detrimental effects on both human and wildlife health. It is increasingly evident that several chemicals may modulate endocrine system pathways in vertebrate species, and these are collectively referred to as endocrine disrupting contaminants (EDCs). Although the endocrine-disrupting effect of water pollutants has been mainly linked to agricultural pesticides and industrial effluents, other pollutants such as pharmaceuticals and personal care products (PPCPs) are largely unnoticed, but also pose a potentially significant threat. Here we present for the first time in a South African context, a summarised list of PPCPs and other EDCs detected to date within South African water systems, as well as their possible endocrine-disrupting effect in-vitro and in-vivo. This review addresses other factors which should be investigated in future studies, including endocrine disruption, PPCP metabolites, environmental toxicology, and antibiotic resistance. The challenges of removing EDCs and other pollutants at South African wastewater treatment works (WWTWs) are also highlighted. The need for focused research involving both in-vitro and in-vivo studies to detect PPCPs in water systems, and to delineate adverse outcome pathways (AOPs) of priority PPCPs to aid in environmental impact assessment (EIA), are discussed.
Keywords: pharmaceutical, endocrine disruption, wastewater, sewage water
INTRODUCTION
Fresh water is an essential resource for the survival of all life on earth. It is globally recognised that humans are creating great pressure on the quality of our water resources by means of anthropogenic (man-made) pollutants entering freshwater systems (WHO, 2012). The major sources of freshwater pollutants typically originate from industry, domestic practices, and/or agriculture (Genthe et al., 2013). These practices introduce either non-degradable and/or harmful chemicals into water systems, thereby creating health risks to both wildlife and humans. Reductions in fertility, increases in the incidence of several cancers, spontaneous abortions, and a range of in-utero physiological disorders and birth defects have been linked to contaminants found in freshwater (Soto and Sonnenschein, 2010; Robins et al., 2011).
South Africa is a developing country, with a mid-year estimated population of 56.5 million people for 2017 (Stats SA, 2017). During the last formal 2011 census, the population was estimated at 51.8 million, of which 77.7% (40.3 million) are living in formal settlements, 7.9% (4.1 million) in traditional settlements, and 13.6% (7.1 million) in informal settlements (StatsSA, 2011). More recent statistics for 2016 showed an increase in formal housing (79.2%), and an increase in households having access to clean water supplies (from 70.9% in 2011 to 83.5% in 2016; Stats SA, 2011). These statistics therefore not only show the rapid increase in the country's population, but also highlight the rapid rate of urbanisation within the country, both of which are directly associated with increased demand for water and sanitation services.
Apart from the provision of clean water to the South African public, water treatment facilities are faced with increased pressures for the provision of improved sanitation services. Efficient operation of wastewater treatment works (WWTWs) is therefore important to remove pathogens and pollutants from surface waters, which might impact the health of both wildlife and human ecosystems. The performance of the WWTWs to remove pathogens and pollutants depends on several factors, such as the type of deployed treatment technologies, capacity, hydraulic retention time, as well as stakeholder requirements of the plant. However, the general target factor for all water treatment facilities is to improve on the quality of the water resource, and therefore ensure the health of the populations dependent on these resources. By extension, access to clean water supplies and proper sanitation services is therefore dependent on the performance of these facilities in order to adhere to water quality standards. As with many countries worldwide, although most treatment processes are developed to successfully eliminate or lower the levels of pathogens and chemical pollutants to safe levels, this is not always the case in South African water treatment facilities. In a survey of 986 WWTWs in South Africa, it was shown that 50% of the plants are receiving less than 0.5 ML per day, 32% between 0.5 and 10 ML per day, and 17% more than 10 ML per day (Snyman et al., 2006). A suggested explanation for the occurrence of inefficient pathogen and micro-pollutant removal is the inadequate human resources for maintenance and operation of the plants.
To assess the South African situation, the South African Department of Water and Sanitation (DWS) launched a Green Drop (GD) certification programme in 2008, to evaluate the performance of the country's wastewater works. This initiative was mandated to improve the quality of discharged effluent from wastewater treatment operations by awarding the operating bodies with a GD status if they comply with the DWS criteria for quality wastewater treatment (DWS, 2012). This initiative aimed to provide annual assessment reports on the operating efficiency of the plants, by awarding a cumulative risk rating (CRR) based on the design capacities (and hydraulic loading into receiving waters), operational flow relative to plant capacity, compliance/non-compliance of effluent quality being discharged into receiving waters, and compliance/non-compliance of technical skills utilised at the WWTWs (DWS, 2012, 2013). In 2012, the GD report has shown that of the 831 WWTWs assessed nationwide, 323 of these plants (39%) did not comply with the DWS standards, and 153 to 212 (18-26%) of all WWTWs received a critical and high-risk rating (DWS, 2012). Furthermore, some of the plants were reported to have unknown design capacities and/or not measure the plant influent at the required frequency (DWS, 2012), creating difficulties in reporting on the water quality these plants are treating. Although the 2013 GD report has shown an improvement in the overall CCRs of the assessed WWTWs for the 2012/13 year, it was still estimated that 49.6% of the WWTWs are still below 50% compliance (DWS, 2013). Furthermore, the DWS also awarded a Purple Drop status (critical state) to the 30.1% of the assessed WWTWs that achieved < 30% compliance (DWS, 2013). Taken from these reports, the high percentage of non-compliance with water quality and service delivery criteria therefore increases the risk for higher pathogen and harmful chemical loads in environmental waters. Although it is reported that the assessed WWTWs receiving a Purple Drop status in the 2013 GD report will be placed under regulatory surveillance (under the Water Services Act, Act 108 of 1997), the ongoing non-compliance of these treatment facilities creates great pressure on general surface water quality. This emphasizes the need to conduct environmental risk assessments (ERAs) to monitor both influents and effluents of water treatment facilities. Apart from the problem that some WWTWs in South Africa do not comply with water quality standards and service delivery, another problem exists in that untreated river water is also not subjected to such water quality guideline initiatives, as this is not regarded as a drinking water resource in South Africa (Genthe et al., 2013). However, several rural communities depend on water taken directly from rivers for general daily activities, such as washing, cooking and consumption, as well as for agricultural purposes.
Due to the complexity and sheer volume of pollutants potentially present in natural water systems, global regulating bodies, such as the United States Environmental Protection Agency (USEPA) and the World Health Organisation (WHO), have set out a framework to investigate and identify environmental pollutants in freshwater systems (USEPA, 1997; WHO, 2012). These approaches consist of four main steps to be followed when doing impact assessment of water pollutants: (i) identifying the hazard to the environment, (ii) conducting dose-response assessments, (iii) exposure assessment of the pollutants to non-target organisms, and (iv) implementing risk characterisation for possible pollutants entering freshwater ecosystems (Fig. 1).
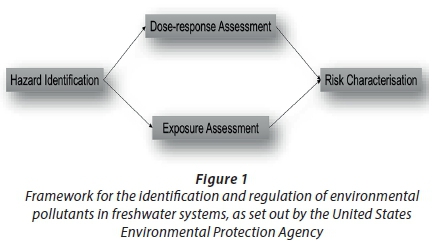
By adhering to these approaches, the first line of investigation should include hazard identification of environmental pollutants entering freshwater systems. It is evident from literature that the identification of problematic areas in South Africa where water systems may be subjected to various pollutants coming from the abovementioned human sources (households, industry, and agriculture) is much needed. Also, water treatment facilities need to be a focus point for monitoring freshwater pollutants, especially in a developing country such as South Africa, as these facilities can provide information regarding the origin of freshwater pollutants in areas of interest. In regard to the pollution of our natural water resources by human activities, some insight can be obtained by observing the wellbeing (health status) of wildlife populations within contaminated waters. Wildlife species inhabiting polluted freshwater supplies are in first-line contact with environmental pollutants and can provide useful information on the presence of pathogens in environmental waters and long-term exposure effects. Such sentinel species therefore serve as a valuable tool for hazard identification.
Hazard identification
Endocrine disruptors and impacts on wildlife
Several micro-pollutants, or emerging contaminants (ECs), found in environmental waters have been linked to potentially causing a large variety of health effects in both invertebrates and vertebrates (Daughton and Ternes, 1999; McKinlay et al., 2008; Bolong et al., 2009). In particular, selected pollutants have been suggested to interact with endocrine system pathways of vertebrates, and are collectively referred to as endocrine-disrupting contaminants (EDCs). The USEPA defines an EDC as: 'An exogenous agent that interferes with the synthesis, secretion, transport, binding, action, or elimination of natural hormones in the body that are responsible for the maintenance of homeostasis, reproduction, development, and/or behaviour' (USEPA, 1997). Man-made compounds most frequently implicated as EDCs include pesticides, pharmaceuticals, personal care products, and industrial by-products. Classic examples of environmental endocrine disruption include studies showing the feminisation of male fish and widespread occurrence of anti-androgenic ligands within UK rivers which receive effluent from connected WWTWs (Liney et al., 2006; Jobling et al., 2009). Guillette and co-workers published a series of accounts confirming the disruption of the male reproductive system in juvenile male alligators in several lakes (especially Lake Apopka) situated within Florida, USA (Guillette et al., 1996; 1999). Reproductive deformities, ranging from reduced penis size to altered plasma testosterone (T) levels were associated with extensive agricultural use of the insecticide DDT and other persistent organic pollutants (POP) leading towards non-point source pollution in water systems flowing into the lakes (Guillette et al., 1996; 1999). Along with the concerns about the general disruption of human reproductive systems leading to various detrimental effects such as ovarian cancer, breast cancer and declined sperm quality, international concerns were voiced regarding the potential subtle disruption of the endocrine systems of humans and wildlife during the organisational window during development (Colborn et al. 1993). The documented reports on the occurrence of endocrine disruption within natural wildlife populations have raised international awareness of the harmful effects which man-made pollutants can exert on surface water quality for reuse.
EDCs are known to modulate either one of the three major axes of the endocrine system, namely the hypothalamus-pituitary-gonad (HPG), hypothalamus-pituitary-thyroid (HPT), and hypothalamus-pituitary-adrenal (HPA) axes (Fig. 2). Within these pathways, several hormones, metabolic enzymes and receptors are responsible for the dispersal, activity and function of various physiological traits in vertebrates. Due to the vast cross-talk between endocrine system axes, disruption of a particular component within one endocrine axis may also cause modulation of other endocrine systems. It is therefore evident that a cocktail of EDCs present in the environment can have a range of negative effects on vertebrate health through modulating various endocrine system pathways.
Environmental contaminants causing disruption of the reproductive endocrine system have been the focus of many EDC studies around the world, including South Africa, where varying concentrations of contaminants having known oestrogenic endocrine-disrupting effects have been found in surface waters (Aneck-Hahn et al., 2009; Bornman et al., 2007; Slabbert et al., 2007; Genthe et al., 2013). Such studies may be only the tip of the iceberg, as increasing number of ECs are shown to have endocrine-disrupting activities. Although termed 'emerging contaminants', many of these contaminants have only recently been screened for their presence in the environment, despite being used for years; therefore, the full extent of their presence and associated risk is not fully understood.
Studies on gonadal abnormalities in wildlife living within polluted water systems have been done in South Africa, similar to those done at Lake Apopka in the United States. The presence of intersexuality in Sharptooth Catfish (Clarias gariepinus) has been observed at two impoundments at the Rietvlei Nature Reserve in the Gauteng Province (Barnhoorn et al., 2004; Kruger et al., 2013). Among the intersexual fish, the presence of testicular oocytes was observed, in which the possible cause was linked to the presence of an industrial pollutant, p-nonylphenol (NP), in the water. The endocrine-disrupting activity of NP has been linked to its lipophilic properties and persistence in the environment (Lech et al., 1996; Folmar et al., 2002). Since the detection of endocrine disruption in freshwater fish, as well as the presence of POPs in the Rietvlei Nature Reserve, this area has been identified as a national priority area to monitor the presence of EDCs (Bornman and Bouwman, 2012). Further examples include populations of C. gariepinus in the Hartebeespoort Dam in the Gauteng province, where testicular abnormalities in male fish have been linked to the presence of POPs detected in the dam (Wagenaar et al., 2012). Intersex fish were also found in Mozambique Tilapia populations (Oreochromis mossambicus) at three impoundments in the Limpopo Province, which are also situated within an area that is intensively sprayed with DDT to combat malaria transmission (Barnhoorn et al., 2010). Sampling of O. mossambicus in the Loskop Dam (Mpumalanga province), which receives water from the Olifants River (a highly polluted river system), showed elevated plasma thyroxine (T3) hormone levels and enlarged thyroid gland follicles, indicating potential thyroid-modulating EDCs in the water. In African Clawed Frog populations (Xenopus laevis), the presence of testicular ovarian follicles was observed in male frogs caught in the north-eastern region of South Africa, which are situated in areas of high agricultural pesticide usage (Du Preez et al., 2009). Male X. laevis frogs collected within impoundments in the Western Cape also situated near agricultural practices also showed modulation of testicular spermatogenic development and altered plasma steroid- and thyroid hormone levels (Van Wyk et al., 2014). As these studies only aimed to link the presence of endocrine disruption in wildlife to pesticide contamination in water systems, the presence of other contaminants, such as pharmaceuticals and personal care products (PPCPs) or synthetic steroid hormones was most probably overlooked.
Although the harmful effects of man-made pollutants on wildlife species are well documented (Heath and Classen, 1999; Barnhoorn et al., 2004; Bornman et al., 2009; Wagenaar et al., 2012; Kruger et al., 2013; Van Wyk et al., 2014), no national monitoring programmes or water quality guidelines have been implemented in South Africa to assess and monitor the occurrence and frequency of pollutants affecting endocrine pathways of non-target organisms (Jooste, 2008). The clinical implications of EDC contamination in surface waters have also received little attention in South Africa, and the importance of using sentinel species as bio-indicators of water pollution is regularly overlooked, especially by assessing the health of these organisms up- and downstream of water treatment processes.
It is evident that most studies link the endocrine-disrupting effect observed in wildlife and water sources to the usage of agricultural pesticides. This assumption is supported by the fact that agriculture comprises a large percentage of a country's gross produce, and therefore also utilises large quantities of available surface water. Because of the notable dependence of food production on pesticides, various point (identifiable) or non-point (diffuse) pollution sources for surface and groundwater are anticipated. However, the presence of PPCPs is regularly overlooked. These chemicals are used on a daily basis for improved healthcare, personal hygiene and/or as daily supplements. It can therefore only be assumed that the presence of PPCPs in environmental waters may contribute even more towards EDC pollution in freshwater resources than pesticides used in households or agriculture. Although it is globally recognised and recorded that several classes of PPCPs are present in environmental waters (Kasprzyk-Hordern et al., 2009; Petrie et al., 2015; Blair et al., 2015), not many studies have been done on PPCP pollutants present in South African waters (especially PPCPs acting as EDCs), and this therefore needs to be addressed in future studies.
Risk characterisation
Sources and emission routes of pharmaceuticals into the environment
Pharmaceuticals can enter the environment by various routes, including wastewater/sewage effluents and sludge from water treatment facilities, improper disposal of unused pharmaceuticals, in faeces and urine from livestock feedlots, and from waste products in PPCP-producing industries (GWRC, 2003). The two main sources of pharmaceuticals entering the environment are sewage from urbanised areas, ending up in sewage treatment works (STWs), and livestock feedlots using pharmaceuticals for growth promotion and disease control (Maletz et al., 2013). Through these pathways of exposure, several types of PPCPs (such as anti-inflammatory drugs, antibiotics, anti-epileptics, anti-depressants, skin care products, disinfectants, etc.) eventually end up at water treatment facilities with the hope that these chemicals are effectively removed before being discharged into rivers and impoundments downstream. Although the amount of PPCP waste products from producers is relatively low (GWRC, 2003), several other industries, such as hospitals and clinics, can contribute greatly to the discharge of PPCPs through sewage and wastewater into the environment (Maletz et al., 2013; Al Aukidy et al., 2014). One way of estimating the levels of PPCPs in the environment is to gather information regarding the usage of PPCPs by the general public in the area of concern.
Human pharmaceutical use in South Africa
In South Africa, as with many developing countries, the information about the presence of pharmaceuticals in environmental and drinking waters is limited to a few studies. These studies have been restricted to certain regions in the country, without multiple studies confirming the occurrence of PPCPs in the same areas. A national survey of pharmaceutical compounds present in South African waters has therefore not yet been conducted. However, the limited amount of studies done on the presence of pharmaceuticals in environmental and drinking water provides a good indication on the type of compounds present in water bodies, and also gives an indication of priority PPCPs for future screening.
Pharmaceutical usage may vary in the ratio of prescription and over-the-counter medication issued by the private vs. public health sectors. A study by Osunmakinde et al. (2013) listed 50 of the most prescribed pharmaceuticals in both the public and private health sectors of South Africa. From these lists, the analgesic paracetamol (acetaminophen) is shown to be the most prescribed drug in both sectors. Other pharmaceutical compounds included in the list are antibiotics amoxicillin, ampicillin, ceftriaxone, chloramphenicol, trimethoprim and sulfamethoxazole, the beta-blocker atenolol, and contraceptives containing levonorgestrel and the synthetic oestrogen ethynyl-oestradiol (EE2) (Osunmakinde et al., 2013). In the private health sector, analgesics are the most prescribed, followed by antihistamines, bronchodilators, and antibiotics at second, third and fourth, respectively (Osunmakinde et al., 2013). In the public health sector, analgesics are also the most prescribed, followed by hypotensives, antiretrovirals (ARVs), and antibiotics at second, third and fourth, respectively (Osunmakinde et al., 2013). For both the public and private health sectors, it is shown that hypertension medication, analgesics, ARVs, antibiotics, non-steroidal anti-inflammatory drugs (NSAIDs), antidiabetics and antihistamines are the most common prescribed medications in South Africa. Therefore, it can be expected that water systems may contain a large amount of different types of pharmaceutical compounds in South Africa.
Pharmaceuticals and steroid hormones detected in South African waters
Initial detection studies of EDCs in South Africa consisted of steroid hormone detection (especially oestrogens) in water systems (Table 1). This is due to the ubiquitous usage of synthetic oestrogens as contraceptives and hormone replacement therapy (HRT) by a large percentage of the population. These hormones were shown to originate from human excretions and improper disposal of pharmaceuticals into sewage (Swart and Pool, 2007; Manickum et al., 2011; Swart et al., 2011; Manickum and John, 2014). However, it is increasingly becoming known that several types of PPCPs are also accumulating in water systems to the same extent as contraceptive medications. These compounds can serve as EDCs and are not completely removed during water treatment (Ncube et al., 2012). From these contaminants, pharmaceuticals stand out as one of the sources which might potentially cause endocrine-disrupting activities in non-target organisms. Although it has been globally recognised that pharmaceutical compounds do enter surface waters, the detection of PPCPs in water systems has only recently been done in the country (Table 1). To our knowledge, this summarised table is novel on both a local- and African scale by depicting the current knowledge and research to date regarding trace levels of PPCPs and hormones in South African surface waters. These detections provide valuable information regarding the presence of pharmaceutical drugs in South African waters.
Although there has been limited information linking these contaminants to wildlife and human health disorders locally, the mechanism of physiological action is well established for all of these compounds. A worrying factor from these studies is that these contaminants are still being detected after wastewater treatment, as well as within environmental waters in rivers. Such trends of persistence of priority emerging contaminants after wastewater treatment are also recorded globally for PPCPs (Kasprzyk-Hordern et al., 2009; Petrie et al., 2015; Blair et al., 2015). Contaminants which are not removed from water treatment processes are therefore destined to end up back in the environment (therefore affecting wildlife), or might possibly end up in drinking water, as shown in reports by De Jager et al. (2013) and Patterton (2013). This emphasises the need to further conduct comprehensive monitoring studies in South African surface water systems to report on the fate of priority ECs to assist with environmental risk assessment.
Environmental Risk Assessment (ERA) and Adverse Outcome Pathways (AOPs)
Although the presence and recalcitrance of several PPCPs has already been demonstrated within surface water systems on a global scale, the implementation of such monitoring studies to predict environmental risk needs more scrutiny. Conventional methods for environmental risk assessment (ERA) are based on acute and/or chronic toxicity studies which assess toxicity towards the most sensitive organisms within ecosystems. These test organisms include several trophic levels, such as bacteria, algae, crustaceans, and vertebrate species. Acute toxicity data is based on short-term toxicity effects (< 24 h) which are expressed as EC50 (concentration which shows an effect in 50% of the experimental population) or LD50 (lethal dose at 50% of the experimental population), whereas chronic toxicity data are based on long-term toxicity effects (> 24 h) which are expressed as NOEC (concentration which shows no effect in the experimental population) or LOEC (lowest concentration which shows an effect in the experimental population). These toxicity endpoints are then corrected by an assessment factor to calculate the predicted no-effect concentration (PNEC) of the EC of interest. This PNEC is then compared to either predicted environmental concentrations (PEC) or measured environmental concentrations (MEC) of the EC to obtain a risk quotient (RQ). A RQ value calculated larger or equal to 1 then reflects the EC to be of environmental concern. Although such ERAs using RQ estimation for pollutants are valuable to assess toxicity risks in the environment, there are several limitations to this conventional calculation of ERA, namely:
•The relevance for selecting only specific test organisms to calculate PNEC in the environment is an underestimation of the total ecological impacts of the ECs
•Using animal models is timely and not cost-effective to calculate risk for the vast majority of ECs on the market and in environmental waters
•Ethical concerns using animal bio-indicators for toxicity testing
•Sub-lethal and chronic (long-term; multigenerational) toxicity (such as endocrine disruption, behavioural effects and other physiological modulation) are not estimated
•The prediction of environmental risk is also unknown for pollutants in highly complex chemical mixtures in the environment, which leads to the need to establish more defined ERAs which are not chemical-specific
Due to these constraints that are shown for conventional ERA, it is necessary to construct more accurate and thorough models for risk assessment, constituting both lethal and sub-lethal toxicity. In-vitro screening assays, molecular screening technologies and bioinformatics are just a few examples to be included in the decision-making process in predictive ecotoxicology. To add to the understanding of evolving 21st century toxicity testing and risk assessment, an adverse outcome pathway (AOP) framework has been proposed (Ankley et al., 2010; Villeneuve et al., 2014; Garcia-Reyero, 2015). This framework is structured upon existing knowledge based on the relationships between physiological pathways, spanning from molecular initiating events (MIEs), which in turn causes a perturbation in normal biological functioning, therefore impairing a sequence of measurable key events (KEs), ranging from cellular- to organism level (Edwards et al., 2016). Each KE is further linked with key event relationships (KERs) based on a weight-of-evidence approach. This downstream series of KEs are then coupled with a particular adverse outcome (AO) on a population level, which can be used for regulatory decision-making (Fig. 3). The AOP framework is well described by several authors (Edwards et al., 2016; Villeneuve et al., 2014; Gracia-Reyero, 2015), highlighting the advances made since its establishment by Ankley and colleagues (2010). Advancements of the AOP framework are still ongoing, which includes the broader AOP Knowledge Base (AOP-KB; https://aopkb.org/), containing the AOP Wiki (https://aopwiki.org/wiki/index.php/Main_Page). This initiative is led by several global regulating bodies, namely the USEPA, the Organisation for Economic Co-operation and Development (OECD), the European Commission Joint Research Centre (JRC), and the US Army Engineer Research and Development Centre (ERDC).
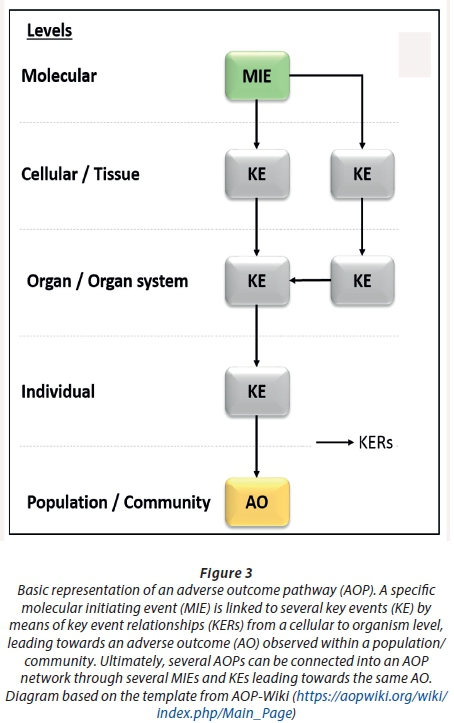
Although the AOP framework is not considered to be part of risk assessment, nor constructed to show chemical-specific outcomes, it helps with the extrapolation of possible AOs which might occur when specific MIEs or KEs are altered. Moreover, this initiative can assist with the understanding of further downstream pathways which could be modulated when a specific KE is shown to be altered by micro-pollutants. Studies indicating the environmental risk caused by micro-pollutants is still lacking in South Africa. Incorporating ERAs and the AOP framework in a local context will aid in the re-establishment of the National Toxicity Monitoring Programme (NTMP), which is only concentrated on a few pollutants and does not address more recent 'emerging contaminants' such as PPCPs (Jooste, 2008). In order to contribute to a more thorough national programme, it is clear that more information is needed on the chronic, sub-lethal level of toxicity using wildlife species and several other bio-markers for more accurate ERA analysis. This can be achieved by adopting a tiered screening approach to quantify and organise/categorise both lethal and sub-lethal toxicity data on both in-vitro and in-vivo screening approaches.
Endocrine disruption of detected pharmaceutical contaminants
Although studies indicating the monitoring of PPCPs within South African waters are on the increase (Table 1), there is still a lack of research effort towards correlating these levels of micro-pollutants with potential sub-lethal toxicological endpoints (such as endocrine disruption) for risk assessment. As discussed earlier, the potential for correlating MECs with known disruptions of KEs within an AOP framework can assist in demonstrating potential disruption of vertebrate endocrine systems, therefore serving as early-warning biomarkers of environmental health. Pollutants such as PPCPs are regularly shown to be present in water systems, either as their breakdown products, or to persist as their active ingredient, depending on several environmental factors and physiochemical properties of the compounds. Furthermore, several in-vitro and in-vivo toxicological studies have shown the potential of PPCPs to alter endocrine system pathways (Table 2), and many of these compounds have been detected within South African environmental waters and WWTW effluents (Table 1). Even more alarming, the levels of certain PPCPs detected within South African waters are well within, or close to, the levels reported to disrupt specific endocrine system pathways (as shown in Table 2).
It is apparent that several PPCPs can exert a range of MIEs and KEs according to the in-vitro and in-vivo studies shown in Table 2. The potential of these commonly-detected PPCPs in environmental waters to exert endocrine-disrupting activities therefore raise concerns for their impact upon environmental and human health. A further concern is that these compounds have been detected in broader environmental water systems, such as direct point sources of drinking water for human consumption. A study by Patterton and colleagues (2013) detected pharmaceutical compounds in drinking water from taps in Johannesburg (Gauteng Province) and Bloemfontein (Free State Province), South Africa. In particular, the anticonvulsant drug carbamazepine was detected in 63% of tap water tested in these regions (Table 1; Patterton, 2013). Anticonvulsant drugs, such as carbamazepine, levetiracetam, lamotrigine and valproate, have been shown to cause several reproductive endocrine system side-effects in men and women suffering from epilepsy (Table 2; Rättyä et al., 2001; Svalheim et al., 2009; Harden et al., 2010), as well as in fish species exposed to carbamazepine (Galus et al., 2013). In men using levetiracetam and valproate as treatment, it has been shown that these drugs can lead to increased T and steroid hormone-binding globulin (SHBG) levels, which is responsible for the transport of steroid hormones in blood plasma (Rättyä et al., 2001; Harden et al., 2010). In the same studies, it was shown that men treated with carbamazepine also evidenced increased levels of SHBG, pituitary FSH and LH (Herzog et al., 2005; Svalheim et al., 2009). Therefore, it might be possible that anticonvulsant compounds found in drinking water resources can lead to altered steroidogenesis in men. Alternatively, carbamazepine treatment in women has been shown to lead to higher SHBP levels and lower levels of P and T steroid hormones (Löfgren et al., 2006; Svalheim et al., 2009). These endocrine-disrupting effects of anticonvulsant drugs were shown in wildlife as well, including modulation of steroidogenesis and ovarian malformations in ovarian follicular cells (Briggs and French, 2004; Taubøl et al., 2006).
Another group of pharmaceuticals that are frequently prescribed and also detected in South African waters are NSAIDs. A study by Amdany and colleagues (2014) detected varying levels of naproxen and ibuprofen in the influents and effluents of two WWTWs in the Gauteng province of South Africa (Table 1). These compounds have been shown to alter endocrine systems in non-target vertebrate species. In a full life-cycle study, exposing Japanese Medaka Fish (Oryzias latipes) to ibuprofen concentrations as low as 0.1 µg/L resulted in delayed hatchling success, while a concentration of 1 mg/L resulted in increased blood plasma levels of the glycoprotein vitellogenin (VTG) (Table 2; Han et al., 2010). This protein molecule is the precursor for egg yolk, and has been validated as a biomarker to express oestrogenic endocrine disruption in egg-laying vertebrate species. In the same study, the exposure of ibuprofen to a human adrenocortical carcinoma cell line (H295R) resulted in an increase in E2 hormone levels at concentrations of 2 and 20 mg/L, and also increased aromatase enzyme activity at concentrations of 0.2 and 2 mg/L (Table 2; Han et al., 2010). Aromatase is the enzyme responsible for the metabolism of T to E2 in steroidogenic pathways. Apart from the possible gonadal endocrine-disrupting activity of ibuprofen, exposure of X. laevis larvae to concentrations ranging between 30.7 and 39.9 mg/L leads to malformations in the development of these larvae, indicating teratogenic effects of ibuprofen as well (Richards and Cole, 2006).
Another NSAID that has been investigated for its endocrine-disrupting effect is diclofenac. In South Africa, diclofenac has been detected in a KwaZulu-Natal Province river system at concentrations varying between 1.1 µg/L and 15.6 µg/L (Table 1; Agunbiade and Moodley, 2014). The exposure of X. laevis embryos to diclofenac has been shown to cause teratogenicity at a concentration of 4 mg/L (Chae et al., 2015). Furthermore, diclofenac exposure in male O. latipes fish showed that concentrations as low as 1 µg/L can increase the gene expression for VTG in the liver, thereby showing estrogenic effects (Hong et al., 2007). Furthermore, assessment of patients using diclofenac as an NSAID has shown a reduction in serum T3 levels (Table 2; Bishnoi et al., 1994), which is the more active thyroid hormone responsible for growth, development and metabolism in the body. The other NSAID that has also been found in South African waters, naproxen, has also been shown to cause a reduction in serum T3 levels in patients taking this medication (Bishnoi et al., 1994). However, according to our knowledge, little is known about the endocrine-disrupting activity of naproxen pollution into the environment, and the effects of this compound on non-target organisms. The dose-dependent response of thyroid disruption by naproxen exposure still needs to be assessed in future studies. Although some of the endocrine-disrupting effects shown above may only occur at high levels of exposure to these NSAIDs, it is important to note that a mixture of different pharmaceuticals and other contaminants might accumulate in the water system. The presence of NSAIDs such as ibuprofen, naproxen and diclofenac may therefore contribute to endocrine disruption caused by other water pollutants as well. Furthermore, these compounds have been confirmed to be present in South African surface waters (Table 1), showing that they are not completely removed from the water system after treatment. The above-mentioned studies imply that NSAIDs, such as ibuprofen, naproxen and diclofenac, have the possibility to alter both gonadal and thyroid endocrine system pathways, and also possibly cause teratogenicity at environmentally relevant concentrations.
The PPCPs which are the most frequently detected in surface waters worldwide are antibiotics and biocides. Regularly-prescribed antibiotic pharmaceuticals, such as ampicillin, chloramphenicol, ciprofloxacin, erythromycin, nalidixic acid, streptomycin, sulfamethoxazole, tetracycline, and tylosin, have all been detected in South African river systems (Table 1; Agunbiade and Moodley, 2014). These compounds have all been shown to have endocrine-disrupting effects. The semi-synthetic macrolide antibiotic tylosin, which is used in veterinary medicine, has been shown to increase the expression of the aldosteronogenic gene (CYP11β2), and decrease the production of T and E2 at a concentration of 3 mg/L in an H295R steroidogenic assay, showing that this chemical can serve as both an anti-estrogenic and anti-androgenic EDC (Table 2; Gracia et al., 2007). In the same study, another macrolide antibiotic, erythromycin, showed an increase in the expression of CYP11β2 and a reduction in T production at a concentration of 3 mg/L, but caused increased production of E2 and P in the assay (Table 2; Gracia et al., 2007). Exposure of erythromycin in a recombinant yeast oestrogen screen (YES) showed that this compound may be a minor mimic of E2 in binding to the oestrogen receptor in a dose-dependent manner, therefore having oestrogenic effects (Archer et al. unpublished). This shows that, although tylosin and erythromycin share the same macrolide ring in their chemical composition, the endocrine-disrupting effect differs between these two compounds, and therefore complicates environmental endocrine disruption studies if, for example, both these two types of chemicals are present in environmental samples. A study by Garcia and colleagues (2007) also showed that tetracyclines, exposed at a concentration of 81µg/L to H295R cells, can increase the expression of CYP19 enzymes and 3βHSD2 genes (Table 2), which are responsible for T-E2 metabolism and the production of P, respectively. Although these antibiotics were not detected in the range which showed endocrine system modulation in an in-vitro assay, their effect on wildlife through long-term exposure within environmental waters is currently unknown. Furthermore, due to the extensive usage of antibiotics in both humans and livestock, the expected concentrations of these chemicals in the environment may be underestimated, and may also have a cumulative endocrine-disrupting effect in the water if they accumulate in mixtures with other pollutants. It is therefore evident that antibiotic chemical pollutants should receive high priority in environmental screening in water systems and water treatment facilities in South Africa.
Apart from the regularly-prescribed antibiotic pharmaceuticals detected in environmental waters, it is shown that compounds in personal care products can also have endocrine-disrupting properties. One of the most well documented compounds is the biocide triclosan (TCS), which is used as a disinfectant in soaps, detergents, toothpastes, mouthwash, and more (Raut and Angus, 2010). This compound also shows a high partition coefficient (Kow) value (Log Kow 4.66; KOWWIN v. 1.67, EPI Suite), which indicates that TCS is highly lipid-soluble and does not readily dissolve in water. For this reason, TCS can be regarded as a POP, which can accumulate in the fat tissue of exposed organisms, and can also be transported in water bodies over great distances. This has been shown in a study demonstrating high levels of TCS in the breast milk of pregnant Swedish women (Allmyr et al., 2006). The pollution of TCS in the environment can therefore be assessed in a similar way to the exposure of organochloride insecticides in environmental waters, such as DDT and endosulfan, which are also shown to accumulate in the fat tissue of both wildlife and humans.
Although the use of TCS has been phased out in several personal care products in developed countries, it is still found in South African consumer products, and therefore detected in surface waters (Amdany et al., 2014; Madikizela et al., 2014). Amdany and colleagues (2014) showed varying levels of TCS in influents and effluents from two WWTWs in the Gauteng province of South Africa. These levels ranged from 78.4 to 127.7 µg/L in influent samples, and 10.7 to 22.9 µg/L in effluent samples (Amdany et al., 2014). Although the concentrations of TCS are significantly reduced after water treatment, these levels are still high if sub-lethal effects are taken into account. Exposure of North American bullfrog tadpoles (Rana catesbeiana) to TCS showed that concentrations as low as 0.3 µg/L can significantly lower tadpole body mass and decrease thyroid hormone receptor (TR) gene expression (Table 2; Veldhoen et al., 2006). Exposure of TCS at 20 µg/L has also been shown to induce hepatic VTG levels in male Japanese Medaka (Oryzias latipes) (Table 2; Ishibashi et al., 2004). Exposure of mature male Western Mosquitofish (Gambusia affinis) to TCS at 101.3 µg/L can cause decreased sperm counts, and also elevate VTG gene expression (Table 2; Raut and Angus, 2010). TCS exposure in MDA-kb2 breast cancer cells showed that a concentration of 289 µg/L significantly induces cell proliferation, and a concentration as low as 290 ng/L caused an elevated androgenic response when treated along with dihydrotestosterone (DHT), which is a more metabolically active androgen than testosterone (Table 2; Christin et al., 2012). These results show that TCS serves as an androgen agonist by binding to the androgen receptor in a human cell-based bioassay (Christin et al., 2012). These concentrations of endocrine disruption are either equivalent or lower than levels observed in South African waters (Table 1). Therefore, wildlife species living either upstream or downstream of WWTWs may be affected by levels of TCS in the environment. Bearing these studies in mind, it is possible that exposure to low concentrations of TCS over a long period of time (chronic exposure) may modulate both gonadal and thyroid endocrine systems in humans and other wildlife species at concentrations currently being detected in environmental waters.
Due to the regular detection and known endocrine-disrupting effect of TCS, it is also important to investigate other compounds found in personal care products and detected in South African waters. Based on chemical analyses and endocrine disruption studies done elsewhere in the world, it is evident that compounds used as preservatives, disinfectants and UV filters have not received much attention as priority environmental pollutants and EDCs in South Africa. Preservatives such as parabens (methylparaben, propylparaben, octylparaben), other biocides such as trichlorocarban (TCC), and UV filters in sunscreens (4-MBC, OMC) have all been shown to accumulate in wastewater systems and cause potential endocrine disruption. Several paraben compounds, as well as their metabolites, have been shown to have both oestrogenic and anti-androgenic effects in-vitro and in-vivo (Table 2). These studies imply that contaminants such as parabens can affect multiple endocrine pathways, and are therefore of environmental concern.
Biocides such as TCC are regularly included in several cosmetic and personal care products to deter microbial organisms. Although the endocrine disrupting effect of the biocide TCS has been well documented, and also found at high concentrations in the environment (Table 1, Halden and Paull, 2005), limited data are available on the endocrine-disrupting effect of TCC. Exposure to TTC in human cell-based bioassays, and exposure of rodents to TTC, indicated that the biocide does not have endocrine-disrupting activity on its own, but rather enhances the action and binding affinity of steroid hormones (Table 2; Ahn et al., 2008; Chen et al., 2008; Christen et al., 2010; Yueh et al., 2012). This shows a potentiating mechanism of endocrine disruption, as well as an alternative mode of endocrine disruption other than direct modulation of endocrine pathways. Several compounds used as UV filters in sunscreens, such as benzophenones, benzedrone (4-MBC), and octyl methoxycinnamate (OMC) have been shown to agonistically bind to the human oestrogen receptor (hER) in human cell-based bioassays, and to increase VTG production in female rats and male fish species (Table 2). These compounds are therefore regarded as oestrogenic contaminants, which might persist for long periods of time in the environment due to their low water solubility. The abovementioned compounds are all used as either 'wash-off' or 'application' personal care products. Therefore, it can be assumed that products containing biocides, UV screens, and preservatives will either be washed down in drain water, or will be absorbed through the skin after application. These compounds thus have multiple routes of exposure to either humans or other non-target organisms in water. Also, due to the fact that these chemicals are regularly used in personal care products, and the fact that these compounds have low solubility in water, their presence and persistence in the environment can be high. Paraben concentrations as high as 11 mg/L have been detected in a UK river system, with concentrations as high as 30 mg/L in wastewater influents (Kasprzyk-Hordern et al., 2009). TCC concentrations of 6 µg/L have been documented in a US river system (Halden and Paull, 2005). Environmental concentrations of UV filters as high as 13 mg/L have been reported in wastewater influents (Kasprzyk-Hordern et al., 2009), with 6 mg/L in wastewater effluents (Kasprzyk-Hordern et al., 2009), 266 ng/L in swimming pools (Cuderman and Heath, 2007), and in seawater at concentrations of 3.3 µg/L (Sánchez-Rodríguez et al., 2015). These compounds have not been screened for their presence in South African water systems, which therefore highlights the importance of screening for these chemicals to evaluate their fate within water treatment facilities.
Future perspectives
Monitoring studies focusing on the presence and fate of EDCs and PPCPs in surface waters have been comprehensive internationally. However, based on the available information on South African toxicological studies, there are several aspects which still need to be addressed. Apart from conventional EDC investigations, there are also a large variety of other topics which need to be addressed. A few of these topics are mentioned below, and will provide a significant contribution towards the understanding of the fate and presence of chemical pathogens in environmental waters. Such interdisciplinary studies should receive high priority for future research, as they are all interlinked into the larger scope of environmental water pollution investigations in South Africa.
Pharmaceutical metabolites and conjugates in WWTWs
Most pharmaceutical detection studies concentrate on the detection of parental pharmaceutically active ingredients. However, it is known that some pharmaceutical compounds are rapidly metabolised in the body after consumption, resulting in their breakdown products being the predominant component of wastewater. Also, after their excretion and/or discharge into wastewater, some PPCPs may be further transformed through biotic (microbial metabolism) and/or abiotic (photodegradation, etc) factors which could affect the drug's stability and fate. It is therefore possible that some toxicologically active compounds may be overlooked when screening for pharmaceutical residues in water bodies, due to the metabolic processing of the parental compound. For some pharmaceuticals, it is known that it is rather the major metabolite products from the parental compound which exert the physiological effect. For example, the analgesic compound tramadol will undergo hepatic metabolism by desmethylation to produce the primary metabolite O-desmethyltramadol, which is a more potent and persistent opioid than tramadol itself. The anti-epileptic compound carbamazepine is also almost completely metabolised in the liver to produce the more potent carbamazepine-10,11-epoxide. These metabolites are then excreted at higher levels than the parental compounds. Regardless, the metabolites of pharmaceutical compounds are regularly ignored in environmental screening of water systems and need to be included in future studies. The environmental consequences of pharmaceutical metabolites in water systems may be even more detrimental than the presence of their parental compounds. Several metabolites of parental compounds of other types of environmental pollutants (mostly pesticides) have been shown to have more severe endocrine-disrupting effects on non-target organisms than their parental compounds. For example, the dithiocarbamate fungicide mancozeb is shown to cause thyroid-modulating effects, but it is rather its metabolite, ethylene thiourea (ETU) which exerts this thyroid-modulating, and possible carcinogenic, activity (USEPA, 2005; Opitz et al., 2006). The dicarboximide fungicide vinclozolin is also shown to be an anti-androgenic EDC, but it is rather its metabolites that have the greater half-lives and mobility in water necessary to cause endocrine-disrupting effects (Bayley et al., 2003). Furthermore, metabolites from parental EDCs may modulate other endocrine system pathways as well, such as the organochloride insecticide DDT, which is a known oestogenic EDC, but its metabolite p,p'-dichlorodiphenyldichloroethylene (p,p'-DDE) is shown to rather have anti-androgenic activity (Mills et al., 2001). Also, although some breakdown products of EDCs may not have any endocrine-disrupting activity, these components might contribute towards an elevated pathogenic effect of another EDC (i.e. potentiating mixture interactions) in water bodies, as a large mixture of different EDCs might be present in water bodies. The potential of parental compounds and metabolites (breakdown products) having endocrine-disrupting activities might therefore cause several toxicological mixture interactions in environmental waters (Hendricks and Pool, 2012).
Apart from the potential of pharmaceutical metabolites to exert higher health impacts if they are present in the environment, the occurrence of negative mass balances for pharmaceuticals and their metabolites has also been detected at several WWTWs globally (Kasprzyk-Hordern et al., 2009; Blair et al., 2015). This trend in negative mass balances is defined as higher concentrations of ECs being detected in WWTW effluents compared to raw wastewater entering the plant. One possibility is that the parental compounds of ECs are not detected within raw sewage, as the metabolite form of these compounds is more prevalent. It may be possible that these metabolites might then be re-transformed to their parental compounds by the microbial communities present in the treatment plant, or by abiotic factors such as photolytic processes (Bonvin et al., 2013; Aris et al., 2014; Blair et al., 2015). This may therefore indicate that some compounds may bio-accumulate or transform within WWTWs, either through enzymatic metabolism or other abiotic pathways, and then be discharged into environmental waters. In particular, it is therefore important to further the knowledge regarding the metabolic capabilities of microbial communities to consume or transform xenobiotics in water treatment facilities.
Mixture interactions of environmental pollutants
It is recognised on a global scale that numerous xenobiotic chemicals accumulate in complex mixtures in the environment. Although the concentrations of pollutants range from mg/L to ng/L, the chemical interaction between pollutants can be great (Carvalho et al., 2014). It is regularly found that chemical mixture studies do not always conform to conventional predicted ecotoxicological mixture interactions. These mixture interactions are largely dependent on the individual chemical's general mode of action (MOA). The MOA of chemicals having gonadal endocrine-disrupting activities, for example, are grouped as being oestrogenic, anti-oestrogenic, androgenic, and anti-androgenic (Behrends et al., 2010). In ideal mixture interactions of environmental pollutants, it is generally assumed that compounds having the same MOA (e.g. oestrogenic + oestrogenic) will generate additive mixture interactions, meaning that the chemical mixture acts jointly to generate a larger physiological or toxicological response than their individual counterparts. This is generally known as the additivity null hypothesis (Christiansen et al., 2009). In contrast, chemicals having dissimilar MOAs (e.g. oestrogenic + androgenic) are proposed to act independently from one another in regard to a measured physiological or toxicological endpoint. This is referred to as independent action (Christiansen et al., 2009). However, it is not as simple as grouping chemicals according to a general endocrine outcome. Chemicals having the same MOA (e.g. anti-oestogenic) may have dissimilar mechanisms exerting the same MOA, for example, modulating steroid receptor binding or inhibiting steroidogenic enzyme functions. Both of these mechanisms have the same MOA, but act in a dissimilar manner, which can cause complex mixture interactions. This complexity in mixture interactions has been highlighted in several studies (Kjærstad et al. 2010; Ermler et al., 2011; Archer and Van Wyk, 2015). Therefore, recent mixture interaction studies refer not to the general outcome of the MOAs (oestrogenic, androgenic, etc.), but rather to their mechanisms of action (steroid receptor agonism/antagonism, steroidogenesis inhibition/stimulation, enzyme inhibition/modulation). Bearing in mind that a vast majority of xenobiotic compounds from agriculture, industries and domestic waste accumulate in water systems, it is expected that a large variety of compounds having both similar and dissimilar MOAs are present in the water matrix. This opens up the possibility of other ecotoxicological mixture interactions to occur, such as potentiation, synergism and antagonism. Furthermore, several compounds are known for having multiple MOAs for a large variety of physiological and toxicological endpoints, therefore creating further complications in mixture interaction studies. Regardless, from the retrospective information present to date, along with continuing research being done on this topic, the knowledge regarding mixture interactions of environmental pollutants is complex and needs to be addressed in future environmental studies.
Antibiotic resistance
Apart from the harmful endocrine-disrupting effects of several pharmaceutical contaminants in wildlife and human populations, the presence of pharmaceuticals, especially antibacterial agents, may influence the type and persistence of bacterial communities (harmless or pathogenic) in the environment and in societies. The most common types of water-related infectious diseases include gastroenteritis, amoebiasis, salmonellosis, dysentery, cholera, typhoid fever, hepatitis-A and diarrhoea. These infectious diseases have been reported to spread due to untreated water supplies and/or poor sanitation (WHO, 2004).
Pathogenic bacteria are renowned for developing resistance to antimicrobial compounds (Levy et al., 1998). Bacterial pathogens can come from several sources, depending on the type of species. Some strains are waterborne, coming from human and animal faecal matter (Gerba and Smith, 2005). Other multidrug-resistant bacteria are known for their specific occurrence in hospitals. Due to the fact that most pathogenic bacteria are prone to infect immune-compromised individuals, these micro-organisms are not only responsible for their associated illnesses, but have also been shown to increase the death rates in patients with other communicable diseases (Levy et al., 1998). The control of pathogenic bacterial colonisation in public healthcare institutions, such as hospitals, is therefore vital to improve the health of the population. Resistance generated by pathogenic bacteria can also lead to insufficient response to antibacterial therapy, and to the implementation of further alternative drugs which might also eventually create resistance. In a South African study, Essack and colleagues (2005) identified 24 pathogenic bacterial strains in 16 hospitals (2 tertiary, 9 regional and 5 district) in the KwaZulu-Natal province. Of the 1 270 bacterial isolates retrieved from patients at the different hospitals, 3% were sensitive to all 24 antibiotics tested, and 91% were resistant to multiple antibiotics. However, less resistance was observed in isolates treated with cephalosporins and fluoroquinolones, which are regarded as 'newly developed' antibiotics. Fluoroquinolones have also been detected in a WWTW effluent in the Western Cape Province (Table 1; Hendricks and Pool, 2012) and, although this pharmaceutical has high sensitivity as an antibacterial treatment, its increased occurrence in water systems might also lead to bacterial adaptation and future resistance.
With the known occurrence of pharmaceutical contaminants in water systems, it is also possible that the presence of low levels of these contaminants may improve the antibiotic resistance of undesired and/or pathogenic bacterial communities and/or influence the structure and occurrence of bacterial pathogens in the water system, therefore making it more difficult to eradicate these pathogens from the waters. These problems are the reason for increased pressure on water treatment facilities to remove both pathogens and toxins from the water system. Although water treatment facilities assure that bacterial pathogens are removed from the water, it is important to note that not all water treatment facilities operate at their expected levels (DWS, 2012). Although some WWTW facilities in South Africa comply with the recommended limits of physiochemical parameters in the effluent, they fail to adhere to other target standards (Odjadjare et al., 2012). A study by Odjadjare et al. (2012) detected several antibiotic-resistant Pseudomonas species in South African WWTW effluents. These strains did not only show resistance to antibiotics, but also to chemical treatments in the water treatment process, such as chlorination. It has also been noted that chlorination may increase the resistance of bacteria to ampicillin and cephalotin (Murray et al., 1984). Also, informal settlements situated above water treatment facilities might utilise water resources directly from the source, which might be prior to treatment. Although the levels of antibiotics in environmental waters might be below their concentrations to exert an antibiotic effect, these pollutants might serve as a 'primer' for further anti-microbial resistance (AMR) development. Taking all these factors into consideration, the development of AMR needs to be investigated further, by incorporating the data generated from environmental pharmaceutical chemical analyses, to assess whether low concentrations of antibiotics detected in environmental waters might induce further antibiotic resistance in pathogenic bacteria.
Environmental biofilms (epilithon) as biomarkers of micro-pollutants
In addition to the development of AMR in bacteria caused by selective evolutionary pressure (such as exposure to antibiotics), morphological and physical characteristics of microbial communities also play a role. It is known that microorganisms can adhere to solid surfaces where they deviate from their planktonic state, and grow and multiply to form biofilms. These biofilms typically constitute a community of multiple types of microorganisms, potentially including bacteria, fungi, and algae (Edwards and Kjellerup, 2013). The adherence of microbial communities to surfaces is assisted by the formation of extracellular polymeric substances (EPS) which keep the biofilm community intact. This facilitates a niche for the microorganisms to proliferate and colonise new surfaces by either single cell detachment, or multiple cell detachment, which is transported within the liquid environment (Ghadakpour et al., 2014). Biofilms may contain both harmless and pathogenic micro-organisms, and are challenging to eradicate in both the environment as well as WWTWs (Flemming et al., 2016). Therefore, pathogenic microbes may proliferate in the environment if favourable conditions are present for them to form biofilms, making them hard to remove at water treatment plants.
Due to several obligate and opportunistic pathogens that have developed resistance to antibiotic chemicals, it is becoming even more important to prevent the formation of pathogenic biofilms in the environment. Such pathogenic microorganisms do not only proliferate in contaminated environmental waters, but can also spread to areas where humans are in direct contact with these pathogens. This has been highlighted by Hota et al. (2009), who investigated the incidence of 36 patients infected with a multidrug-resistant strain of P. aeruginosa in a Canadian hospital during 2004 and 2006. The research showed that a biofilm containing the pathogenic strain was lined around sink piping situated close to patient beds. Although the sinks were treated with antibacterial agents for disinfection, this did not inhibit the dispersal of the bacteria when accidental splashing from the sink occurred (Hota et al., 2009). Such incidences are very likely to occur in South African hospitals as well, as most public sector hospitals do not meet basic sanitation and hygiene requirements. The outbreak studied by Hota et al. (2009) highlights the importance of controlling and limiting the possible source points of adaptive pressure enabling these bacterial pathogens to develop resistance. If antibacterial pharmaceuticals are not effectively removed from drinking water supplies, these chemicals might accumulate and increase the antimicrobial resistance of pathogenic bacterial biofilms. Also, the continued usage of certain antibiotic compounds leads to the development of resistance by the pathogens against that specific compound, and therefore leads to the development or usage of alternative antibiotic compound/s. These are, however, short-term and cyclic solutions, since they ultimately increase the suite of genetic adaptation mechanisms available to the microbial community. This is of particular concern in freshwater drinking sources, as it is found on a daily basis that more and more antibiotic pollutants are found in our freshwater systems, even after water treatment processes.
Despite the detrimental effects of pathogenic biofilm communities, the presence of biofilms in environments is not always harmful. Biofilm communities can be found on streambed sediment surfaces in environmental waters, and are a critical element of the chemical and nutrient cycling in aquatic systems (Writer et al., 2011). Biofilms and microbial flocs are one of the primary means of carbon and nitrogen removal in wastewater treatment plants, responsible for facilitating clean water and preventing eutrophication of our water bodies (Sheng et al., 2010). Biofilms are also used for bioremediation and biotransformation of toxic compounds at water treatment facilities (see Edwards and Kjellerup, 2013; Table 2 for a summary of biofilm-based treatment techniques). Therefore, biofilms may be used as a screening tool to investigate the accumulation and removal of harmful chemicals (including PPCPs and other EDCs) in surface waters. It is shown that steroid hormones and alkylphenols (such as NP) can partition very rapidly into the organic matter of biofilms (Writer et al., 2011). Biofilms can also contribute to the oxidation of organic material, such as converting E2 to E1 (Writer et al., 2011), thereby changing the chemical profiles in the water system. Thus, biofilms can serve as a bio-indicator of EDC presence in the environment, as organic compounds can be retained within the biofilm community and undergo transformation processes (Edwards and Kjellerup, 2013). However, other environmental factors may also influence the retention capacity of organic compounds in biofilms, such as temperature, light, competing carbon substrates and oxygen concentrations. Regardless, biofilm communities in environmental waters may serve as a valuable tool for EDC detection studies. Several aspects of biofilm interaction with environmental EDCs can be investigated in the future. Pharmaceuticals in environmental waters might exert pressure on the structure and composition (physical and community) of microbial biofilms in the environment, due to possible antimicrobial resistance or other chemical interactions with the microbial communities. This might have beneficial or detrimental effects on the fate and persistence of EDCs in the environment, as biofilms may assist in the bioaccumulation and/or biodegradation of organic pollutants, either making them less harmful or changing them into more biologically active metabolites. Thus, both antibiotics and broader pharmaceutical compounds will have a modulating effect on the microbiological ecology of both wastewater treatment works and freshwater systems, as well as the endocrine-disrupting effects discussed above.
A tiered approach to endocrine disruption of PPCPs of environmental concern
Taking all environmental and socio-economic factors mentioned in the current review into consideration, as well as research to date on the accumulation of EDCs and other ECs in surface waters, and their implications towards the health of humans and wildlife (AMR and EDCs), it is important to implement a tiered approach towards identifying and categorising possible pathogens and routes of exposure in environmental and treated water systems.
Several global regulatory bodies such as the Endocrine Disruptor Screening and Testing Advisory Committee (EDSTAC) managed by the USEPA, and a task force on Endocrine Disrupters Testing and Assessment (EDTA) by the OECD, have mandated the development and validation of testing (screening) methods for standardised assessments. The tiered approach suggested by USEPA was accepted globally and includes a battery of assays to screen or test for endocrine interactions and to identify and evaluate the potential of contaminants serving as EDCs (EDSTAC, 1998). First-tier assays were chosen to act as high-throughput screens to identify and prioritise ECs for second-tier testing, which aids in the understanding of the specific physiological MOA which are modulated by the EC of interest (EDSTAC, 1998). Second-tier screens aim to evaluate the results obtained from the first-tier screens in multi-generational or long-term in-vivo studies to gain further support for a compound or mixture of contaminants to serve as environmental EDCs (EDSTAC, 1998). First- and second-tier screening can therefore provide biologically relevant information, which can be used to support ERAs and to establish AOPs for more detailed eco-toxicological assessment.
CONCLUSION
Based on the current status of micro-pollutant understanding and analyses described in this review, it is evident that there is a large source of information available which can aid in the prioritisation of emerging contaminants for environmental risk assessment. Several key points were discussed and should therefore receive priority in future studies to ensure sustainability of our freshwater resources, namely:
•Further reports on the occurrences of PPCPs and their metabolites in surface waters
•Establishing the possible endocrine-disrupting effects of commonly-detected PPCPs and other micro-pollutants through a tiered eco-toxicological approach
•Investigating the contribution of environmental micro-pollutants towards the global epidemic of AMR
•Report on the effectiveness of WWTWs to remove priority micro-pollutants (such as EDCs), as well as biological pathogens
•Raising public awareness of the consequences of liberal and irresponsible PPCP use and disposal
•Establish and/or improve initiatives such as the National Toxicity Monitoring Programme (NTMP) to assist with environmental risk assessment through the use of AOP networks
•Developing more effective water treatment technologies to eradicate persistent micro-pollutants from the water system in order to deem the system safe for reuse
ACKNOWLEDGEMENTS
We acknowledge funding from the East Rand Water Care Company (ERWAT) and the Water Research Commission (WRC Project Mo. K5/2733/3)K5/2733//3).
REFERENCES
AGUNBIADE FO and MOODLEY B (2014) Pharmaceuticals as emerging organic contaminants in Umgeni River water system, KwaZulu-Natal, South Africa. Environ. Monit. Assess. 44 (1) 7273-7291. https://doi.org/10.1007/s10661-014-3926-z [ Links ]
AGUNBIADE FO and MOODLEY B (2016) Occurrence and distribution pattern of acidic pharmaceuticals in surface water, wastewater and sediment of the Msunduzi River, Kwazulu-Natal, South Africa. Environ. Toxicol. Chem. 35 (1) 36-46. https://doi.org/10.1002/etc.3144 [ Links ]
AHN K-C, ZHAO B, CHEN J, CHEREDNICHENKO G, SANMARTI E, DENISON MS, LASLEY B, PESSAH IN, KÜLTZ D, CHANG DPY and co-authors (2008) In vitro biologic activities of the antimicrobials triclocarban, its analogs, and triclosan in bioassay screens: Receptor-based bioassay screens. Environ. Health Perspect. 116 (9) 1203-1210. https://doi.org/10.1289/ehp.11200 [ Links ]
AL AUKIDY M, VERLICCHI P and VOULVOULIS N (2014) A framework for the assessment of the environmental risk posed by pharmaceuticals originating from hospital effluents. Sci. Total Environ. 493 (1) 54-64. https://doi.org/10.1016/j.scitotenv.2014.05.128 [ Links ]
ALLMYR M, ADOLFSON-ERICI M, MCLACHLAN MS and SANDBORGH-ENLUND G (2006) Triclosan in plasma milk from Swedish nursing mothers and their exposure via personal care products. Sci. Total Environ. 372 (1) 87-93. https://doi.org/10.1016/j.scitotenv.2006.08.007 [ Links ]
AMDANY R, CHIMUKA L and CUKROWSKA E (2014) Determination of naproxen, ibuprofen and triclosan in wastewater using the polar organic chemical integrative sampler (POCIS): A laboratory calibration and field application. Water SA 40 (3) 407-414. https://doi.org/10.4314/wsa.v40i3.3 [ Links ]
ANECK-HAHN NH, BORNMAN MS and DE JAGER C (2009) Oestrogenic activity in drinking waters from a rural area in the Waterberg District, Limpopo Province, South Africa. Water SA 35 (3) 245-251. [ Links ]
ANKLEY GT, BENNETT RS, ERICKSON RJ, HOFF DJ, HORNUNG MW, JOHNSON RD, MOUNT DR, NICHOLS JW, RUSSOM CL, SCHMIEDER PK and co-authors (2010) Adverse outcome pathways: A conceptual framework to support ecotoxicology research and risk assessment. Environ. Toxicol. Chem. 29 (3) 730-741. https://doi.org/10.1002/etc.34 [ Links ]
ARCHER E and VAN WYK JH (2015) The potential anti-androgenic effect of agricultural pesticides used in the Western Cape: In vitro investigation of mixture effects. Water SA 41 (1) 129-137. https://doi.org/10.4314/wsa.v41i1.16 [ Links ]
ARCHER E, PETRIE B, KASPRZYK-HORDERN B and WOLFAARDT GM (2017) The fate of pharmaceuticals and personal care products (PPCPs), endocrine disrupting contaminants (EDCs), metabolites and illicit drugs in a WWTW and environmental waters. Chemosphere 174 437-446. https://doi.org/10.1016/j.chemosphere.2017.01.101 [ Links ]
ARIS AZ, SHAMSUDDIN AS and PRAVEENA SM (2014) Occurrence of 17α-ethynylestradiol (EE2) in the environment and effect on exposed biota: A review. Environ. Int. 69 104-119. https://doi.org/10.1016/j.envint.2014.04.011 [ Links ]
BARNHOORN IEJ, BORNMAN MS, PIETERSE GM and VAN VUREN JHJ (2004) Histological evidence of intersex in feral sharptooth catfish (Clarias gariepinus) from an estrogen-polluted water source in Gauteng, South Africa. Environ. Toxicol. 19 (6) 603-608. https://doi.org/10.1002/tox.20068 [ Links ]
BARNHOORN IEJ, VAN DYK JC, PIETERSE GM and BORNMAN MS (2010) Intersex in feral indigenous freshwater Oreochromis mossambicus, from various parts in the Luvuvhu River, Limpopo Province, South Africa. Ecotoxicol. Environ. Saf. 73 (7) 1537-1542. https://doi.org/10.1016/j.ecoenv.2010.07.026 [ Links ]
BARSE AV, CHAKRABARTI T, GHOSH TK, PAL AK, KUMAR N, RAMAN RP and JADHAO SB (2010) Vitellogenin induction and histo-metabolic changes following exposure of Cyprinus carpio to methyl paraben. Asian-Aust. J. Anim. Sci. 23 (12) 1557-1565. https://doi.org/10.5713/ajas.2010.10118 [ Links ]
BAYLEY M, LARSEN PF, BÆKGAARD H and BAATRUP E (2003) The effects of vinclozolin, an anti-androgenic fungicide, on male Guppy secondary sex characters and reproductive success. Biol. Reprod. 69 (6) 1951-1956. https://doi.org/10.1095/biolreprod.103.017780 [ Links ]
BEHRENDS T, URBATZKA R, KRACKOW S, ELEPFANDT A and KLOAS W (2010) Mate calling behaviour of male South African clawed frogs (Xenopus laevis) is suppressed by the antiandrogenic endocrine disrupting compound flutamide. Gen. Comp. Endocrinol. 168 (2) 269-274. https://doi.org/10.1016/j.ygcen.2010.01.017 [ Links ]
BLAIR B, NIKOLAUS A, HEDMAN C, KLAPER R and GRUNDL T (2015) Evaluating the degradation, sorption, and negative mass balances of pharmaceuticals and personal care products during wastewater treatment. Chemosphere 134 395-401. https://doi.org/10.1016/j.chemosphere.2015.04.078 [ Links ]
BISHNOI A, CARLSON HE, GRUBER BL, KAUFMAN LD, BOCK JL and LIDONNICI K (1994) Effects of commonly prescribed nonsteroidal anti-inflammatory drugs on thyroid hormone measurements. Am. J. Med. 96 (3) 35-238. https://doi.org/10.1016/0002-9343(94)90148-1 [ Links ]
BOBERG J, TAXVIG C, CHRISTIANSEN S and HASS U (2010) Possible endocrine disrupting effects of parabens and their metabolites. Reprod. Toxicol. 30 (2) 301-312. https://doi.org/10.1016/j.reprotox.2010.03.011 [ Links ]
BOLONG N, ISMAIL AF, SALIM MR and MATSUURA T (2009) A review of the effects of emerging contaminants in wastewater and options for their removal. Desalination 239 229-246. DOI: 10.1016/j.desal.2008.03.020. https://doi.org/10.1016/j.desal.2008.03.020 [ Links ]
BONVIN F, OMLIN J, RUTLER R, SCHWEIZER WB, ALAIMO PJ, STRATHMANN TJ, MCNEILL K and KOHN T (2013) Direct photolysis of human metabolites of the antibiotic sulfamethoxazole: Evidence for abiotic back-transformation. Environ. Sci. Technol. 47 (13) 6746-6755. https://doi.org/10.1021/es303777k [ Links ]
BORNMAN MS, VAN VUREN JHJ, BOUWMAN H, DE JAGER C, GENTHE B and BARNHOORN IEJ (2007) Endocrine disruptive activity and the potential health risk in an urban nature reserve. WRC Report No. 1505/1/07. Water Research Commission, Pretoria. [ Links ]
BORNMAN R and BOUWMAN H (2012) Environmental pollutants and diseases of sexual development in humans and wildlife in South Africa: Harbingers of impact on overall health? Reprod. Domest. Anim. 47 (4) 327-332. https://doi.org/10.1111/j.1439-0531.2012.02094.x [ Links ]
BRIGGS DE and FRENCH JA (2004) Levetiracetam safety profiles and tolerability in epilepsy patients. Expert Opin. Drug Saf. 3 (5) 415-424. https://doi.org/10.1517/14740338.3.5.415 [ Links ]
CARVALHO RN, ARUKWE A, AIT-AISSA S, BADO-NILLES A, BALZAMO S, BAUN A, BELKIN S, BLAHA L, BRION F, CONTI D and co-authors (2014) Mixtures of chemical pollutants at European legislation safety concentrations: How safe are they? Toxicol. Sci. 141 (1) 218-233. https://doi.org/10.1093/toxsci/kfu118 [ Links ]
CHAE J-P, PARK MS, HWANG Y-S, MIN B-H, KIM S-H, LEE H-S and PARK M-J (2015) Evaluation of developmental toxicity and teratogenicity of diclofenac using Xenopus embryos. Chemosphere 120 (1) 52-58. https://doi.org/10.1016/j.chemosphere.2014.05.063 [ Links ]
CHRISTEN V, CRETTAZ P, OBERLI-SCHRÄMMLI A and FENT K (2010) Some flame retardants and the antimicrobials triclosan and triclocarban enhance the androgenic activity in vitro. Chemosphere 81 (10) 1245-1252. https://doi.org/10.1016/j.chemosphere.2010.09.031 [ Links ]
CHRISTIANSEN S, SCHOLZE M, DALGAARD M, VINGGAARD AM, AXELSTAD M, KORTENKAMP A and HASS U (2009) Synergistic disruption of external male sex organ development by a mixture of four antiandrogens. Environ. Health Perspect. 117 (12) 1839-1846. https://doi.org/10.1289/ehp.0900689 [ Links ]
CHEN J, CHANG AHN K, GEE NA, AHMED MI, DULEBA AJ, ZHAO L, GEE SJ, HAMMOCK BD and LASLEY BL (2008) Triclocarban enhances testosterone action: A new type of endocrine disruptor? Endocrinology 149 (3) 1173-1179. https://doi.org/10.1210/en.2007-1057 [ Links ]
COLBORN T, VOM SAAL FS and SOTO AM (1993) Developmental effects of endocrine-disrupting chemicals in wildlife and humans. Environ. Health Perspect. 101 (5) 378-384. https://doi.org/10.1289/ehp.93101378 [ Links ]
CROFTON KM, PAUL KB, DE VITO MJ and HEDGE JM (2007) Short-term in vivo exposure to the water contaminant triclosan: Evidence for disruption of thyroxine. Environ. Toxicol. Pharmacol. 24 (2) 194-197. https://doi.org/10.1016/j.etap.2007.04.008 [ Links ]
CUDERMAN P and HEATH E (2007) Determination of UV filters and antimicrobial agents in environmental water samples. Anal. Bioanal. Chem. 387 (4) 1343-1350. https://doi.org/10.1007/s00216-006-0927-y [ Links ]
DAUGHTON CG and TERNES TA (1999) Pharmaceuticals and personal care products in the environment: Agents of subtle change? Environ. Health Perspect. 107 (6) 907-938. https://doi.org/10.1289/ehp.99107s6907 [ Links ]
DE JAGER C, ANECK-HAHN NH, SWART P, TRUEBODY B and VAN ZIJL MC (2013) Estrogenic activity and endocrine disrupting chemical (EDC) status in water obtained from selected distribution points in Pretoria and Cape Town. WRC Report No. KV317/13. Water Research Commission, Pretoria. [ Links ]
DU PREEZ LH, KUNENE N, HANNER R, GIESY JP, SOLOMON KR, HOSMER A and VAN DER KRAAK GJ (2009) Population-specific incidence of testicular ovarian follicles in Xenopus laevis from South Africa: A potential issue in endocrine testing. Aquat. Toxicol. 95 (1) 10-16. https://doi.org/10.1016/j.aquatox.2009.07.018 [ Links ]
DWS (Department of Water and Sanitation, South Africa) (2012) Green Drop progress report of 2012. URL: https://www.dwaf.gov.za/dir_ws/GDS/Docs/DocsDefault.aspx (Accessed 20 November 2016). [ Links ]
DWS (Department of Water and Sanitation, South Africa) (2013) Green Drop report of 2013. URL:https://www.dwa.gov.za/Documents/Executive%20Summary%20for%20the% 202013%20Green%20Drop%20Report.pdf (Accessed 23 December 2016). [ Links ]
EDSTAC (Endocrine Disruptor Screening and Testing Advisory Committee) (1998) Endocrine Disruptor Screening and Testing Advisory Committee Final Report. URL: http://www.epa.gov/endo/pubs/edspoverview/finalrpt.htm (Accessed 20 November 2016). [ Links ]
EDWARDS SJ and KJELLERUP BV (2013) Applications of biofilms in bioremediation and biotransformation of persistent organic pollutants, pharmaceuticals/personal care products, and heavy metals. Appl. Microbiol. Biotechnol. 97 (23) 9909-9921. https://doi.org/10.1007/s00253-013-5216-z [ Links ]
EDWARDS SW, TAN Y-M, VILLENEUVE DL, MEEK ME and MCQUEEN CA (2016) Adverse outcome pathways - Organizing toxicological information to improve decision making. J. Pharmacol. Exp. Ther. 356 170-181. https://doi.org/10.1124/jpet.115.228239 [ Links ]
ERMLER S, SCHOLZE M and KORTENKAMP A (2011) The suitability of concentration addition for predicting the effects of multi-component mixtures of up to 17 anti-androgens with varied structural features in an in vitro AR antagonist assay. Toxicol. Appl. Pharmacol. 257 (2) 189-197. https://doi.org/10.1016/j.taap.2011.09.005 [ Links ]
ESSACK SY, CONNOLLY C and STURM AW (2005) Antibiotic use and resistance in public-sector hospitals in KwaZulu-Natal. S. Afr. Med. J. 95 (11) 865-870. [ Links ]
FLEMMING HC, WINGENDER J, SZEWZYK U, STEINBERG P, RICE SA and KJELLEBERG S (2016) Biofilms: an emergent form of bacterial life. Nat. Rev. Microbiol. 14 (9) 563-575. https://doi.org/10.1038/nrmicro.2016.94 [ Links ]
FOLMAR LC, HEMMER MJ, DENSLOW ND, KROLL K, CHEN J, CHEEK A, RICHMAN H, MEREDITH H and GRAU EG (2002) A comparison of the estrogenic potencies of estradiol, ethynylestradiol, diethylstilbestrol, nonylphenol and methoxychlor in vivo and in vitro. Aquat. Toxicol. 60 (1-2) 101-110. https://doi.org/10.1016/S0166-445X(01)00276-4 [ Links ]
GALUS M, JEYARANJAAN J, SMITH E, LI H, METCALFE C and WILSON JY (2013) Chronic effects of exposure to a pharmaceutical mixture and municipal wastewater in zebrafish. Aquat. Toxicol. (132-133) 212-222. https://doi.org/10.1016/j.aquatox.2012.12.016 [ Links ]
GARCIA-REYERO N (2015) Are adverse outcome pathways here to stay? Environ. Sci. Technol. 49 3-9. https://doi.org/10.1021/es504976d [ Links ]
GENTHE B, LE ROUX WJ, SCHACHTSCHNEIDER K, OBERHOLSTER PJ, ANECK-HAHN NH and CHAMIER J (2013) Health risk implications from simultaneous exposure to multiple environmental contaminants. Ecotoxicol. Environ. Saf. 93 171-179. https://doi.org/10.1016/j.ecoenv.2013.03.032 [ Links ]
GERBA CP and SMITH JE (2005) Sources of pathogenic microorganisms and their fate during land application of wastes. J. Environ. Qual. 34 (1) 42-48. [ Links ]
GHADAKPOUR M, BESTER E, LISS SN, GARDAM M, DROPPO I, HOTA S and WOLFAARDT GM (2014) Integration and proliferation of Pseudomonas aeruginosa PA01 in multispecies biofilms. Microbiol. Ecol. 68 (1) 121-131. https://doi.org/10.1007/s00248-014-0398-1 [ Links ]
GRACIA T, HILSCHEROVA K, JONES PD, NEWSTED JL, HIGLEY EB, ZHANG X, HECKER M, MURPHY MB, YU RMK, LAM PKS and co-authors (2007) Modulation of steroidogenic gene expression and hormone production of H295R cells by pharmaceuticals and other environmentally active compounds. Toxicol. Appl. Pharmacol. 225 (2) 142-153. DOI: 10.1016/j.taap.2007.07.013. https://doi.org/10.1016/j.taap.2007.07.013 [ Links ]
GUILLETTE Jr. LJ, PICKFORD DB, CRAIN DA, ROONEY AA and PERCIVAL HF (1996) Reduction in penis size and plasma testosterone concentrations in juvenile alligators living in a contaminated environment. Gen. Comp. Endocrinol. 101 (1) 32-42. https://doi.org/10.1006/gcen.1996.0005 [ Links ]
GUILLETTE Jr. LJ, WOODWARD AR, CRAIN DA, PICKFORD DB, ROONEY AA and PERCIVAL HF (1999) Plasma steroid concentrations and male phallus size in juvenile alligators from seven Florida lakes. Gen. Comp. Endocrinol. 116 (3) 356-372. https://doi.org/10.1006/gcen.1999.7375 [ Links ]
GWRC (Global Water Research Coalition) (2003) Endocrine disrupting compounds: An overview of the sources and biological methods for measuring EDCs. Global Water Research Coalition. BTO 2003.008. GWRC, London. [ Links ]
HALDEN RU and Paull DH (2005) Co-occurrence of triclocarban and triclosan in U.S. water resources. Environ. Sci. Technol. 39 (6) 1420-1426. https://doi.org/10.1021/es049071e [ Links ]
HAN S, CHOI K, KIM J, JI K, KIM S, AHN B, YUN J, CHOI K, KHIM JS, ZHANG X and co-authors (2010) Endocrine disruption and consequences of chronic exposure to ibuprofen in Japanese medaka (Oryzias latipes) and freshwater cladocerans Daphnia magna and Moina macrocopa. Aquat. Toxicol. 98 (3) 256-264. https://doi.org/10.1016/j.aquatox.2010.02.013 [ Links ]
HARDEN CL, NIKLOV B, KANDULA P, LABAR D and PANNULLO S (2010) Effect of levetiracetam on testosterone levels in male patients. Epilepsia 51 (11) 2348-2351. https://doi.org/10.1111/j.1528-1167.2010.02732.x [ Links ]
HEATH RGM and CLAASSEN M (1999) An overview of the pesticide and metal levels present in populations of the larger indigenous fish species of selected South African rivers. WRC Report No. 428/1/99. Water Research Commission, Pretoria. [ Links ]
HENDRICKS R and POOL EJ (2012) The effectiveness of sewage treatment processes to remove faecal pathogens and antibiotic residues. J. Environ. Sci. Health Part A 47 (2) 289-297. https://doi.org/10.1080/10934529.2012.637432 [ Links ]
HERZOG AG, DRISLANE FW, SCHOMER DL, PENNELL PB, BROMFIELD EB, DWORETZKY BA, FARINA EL and FRYE CA (2005) Differential effects of antiepileptic drugs on sexual function and hormones in men with epilepsy. Neurology 65 (7) 1016-1020. https://doi.org/10.1212/01.wnl.0000178988.78039.40 [ Links ]
HONG HN, KIM HN, PARK KS, LEE S-K and GU MB (2007) Analysis of the effects diclofenac has on Japanese medaka (Oryzias latipes) using real-time PCR. Chemosphere 67 (11) 2115-2121. https://doi.org/10.1016/j.chemosphere.2006.12.090 [ Links ]
HOTA S, HIRJI Z, STOCKTON K, LEMIEUX C, DEDIER H, WOLFAARDT G and GARDAM MA (2009) Outbreak of multidrug-resistant Pseudomonas aeruginosa colonization and infection secondary to imperfect intensive care unit room design.Infect. Control Hosp. Epidemiol. 30 (1) 25-33. https://doi.org/10.1086/592700 [ Links ]
INUI M, ADACHI T, TAKENAKA S, INUI H, NAKAZAWA M, UEDA M, WATANABE H, MORI C, IGUCHI T and MIYATAKE K (2003) Effect of UV screens and preservatives on vitellogenin and choriogenin production in male medaka (Oryzias latipes). Toxicology. 194 43-50. https://doi.org/10.1016/S0300-483X(03)00340-8 [ Links ]
ISHIBASHI H, MATSUMURA N, HIRANO M, MATSUOKA M, SHIRATSUCHI H, ISHIBASHI Y, TAKAO Y and ARIZONO K (2004) Effects of triclosan on early life stages and reproduction of medaka Oryzias latipes and induction of hepatic vitellogenin. Aquat. Toxicol. 67 (2) 167-179. https://doi.org/10.1016/j.aquatox.2003.12.005 [ Links ]
JI K, CHOI L, LEE S, PARK S, KHIM JS, JO EH, CHOI K, ZHANG X and GIESY JP (2010) Effects of sulfathiazole, oxytetracycline and chlortetracycline on steroidogenesis in the human adrenocarcinoma (H295R) cell line and freshwater fish Oryzias latipes. J. Hazardous Mater. 182 (1-3) 494-502. https://doi.org/10.1016/j.jhazmat.2010.06.059 [ Links ]
JOBLING S, BURN RW, THORPE K, WILLIAMS R and TYLER C (2009) Statistical modelling suggests that antiandrogens in effluents from wastewater treatment works contribute to widespread sexual disruption in fish living in English rivers. Environ. Health Perspect. 117 (5) 797-802. https://doi.org/10.1289/ehp.0800197 [ Links ]
JOOSTE S, BOLLMOHR S and THWALA M (2008) National Toxicity Monitoring Program: Report on Phase 3: Pilot implementation and testing of the design. Report No. N/0000/REQ1008. Resource Quality Services. Department of Water Affairs and Forestry, Pretoria. [ Links ]
KASPRZYK-HORDERN B, DINSDALE RM and GUWY AJ (2009) The removal of pharmaceuticals, personal care products, endocrine disruptors and illicit drugs during wastewater treatment and its impact on the quality of receiving waters. Water Res. 43 (2) 363-380. https://doi.org/10.1016/j.watres.2008.10.047 [ Links ]
KJÆRSTAD MB, TAXVIG C, ANDERSEN HR and NELLEMANN C (2010) Mixture effects of endocrine disrupting compounds in vitro. Int. J. Androl. 33 (2) 425-433. https://doi.org/10.1111/j.1365-2605.2009.01034.x [ Links ]
KRUGER T, BARNHOORN I, JANSEN VAN VUREN J and BORNMAN R (2013) The use of the urogenital papillae of male feral African sharptooth catfish (Clarias gariepinus) as indicator of exposure to estrogenic chemicals in two polluted dams in an urban nature reserve, Gauteng, South Africa. Ecotoxicol. Environ. Saf. 87 98-107. https://doi.org/10.1016/j.ecoenv.2012.10.004 [ Links ]
LECH JJ, LEWIS SK and REN L (1996) In vivo estrogenic activity of nonylphenol in Rainbow trout. Toxicol. Sci. 30 (2) 229-232. https://doi.org/10.1093/toxsci/30.2.229 [ Links ]
LEVY SB (1998) The challenge of antibiotic resistance. Sci. American 278 (3) 46-53. https://doi.org/10.1038/scientificamerican0398-46 [ Links ]
LINEY KE, HAGGER JA, TYLER CR, DEPLEDGE MH, GALLOWAY TS and JOBLING S (2006) Health effects in fish of long-term exposure to effluents from wastewater treatment works. Environ. Health Perspect. 114 (1) 81-89. [ Links ].
LÖFGREN E, TAPANAINEN JS, KOIVUNEN R, PAKARINEN A and ISOJÄRVI JIT (2006) Effects of carbamazepine and oxcarbazepine on the reproductive endocrine function in women with epilepsy. Epilipsia 47 (9) 1441-1446. https://doi.org/10.1111/j.1528-1167.2006.00506.x [ Links ]
MADIKIZELA LM, MUTHWA SF and CHIMUKA L (2012) Determination of triclosan and ketoprofen in river water and wastewater by solid phase extraction and high performance liquid chromatography. S. Afr. J. Chem. 67 143-150. [ Links ]
MALETZ S, FLOEHR T, BEIER S, KLÜMPER C, BROUWER A, BEHNISCH P, HIGLEY E, GIESY JP, HECKER M, GEBHARDT W and co-authors (2013) In vitro characterization of the effectiveness of enhanced sewage treatment processes to eliminate endocrine activity of hospital effluents. Water Res. 47 (4) 1545-1557. https://doi.org/10.1016/j.watres.2012.12.008 [ Links ]
MANICKUM T, JOHN W and TERRY S (2011) Determination of selected steroid estrogens in treated sewage effluent in the Umsunduzi River water catchment area. Hydrol. Current Res. 2 (3) 1-7. [ Links ]
MANICKUM T and JOHN W (2014) Occurrence, fate and environmental risk assessment of endocrine disrupting compounds at the wastewater treatment works in Pietermaritzburg (South Africa). Sci. Total Environ. 468-469 584-597. https://doi.org/10.1016/j.scitotenv.2013.08.041 [ Links ]
MATONGO S, BIRUNGI G, MOODLEY B and NDUNGU P (2015) Pharmaceutical residues in water and sediment of Msunduzi River, KwaZulu-Natal, South Africa. Chemosphere 134 133-140. https://doi.org/10.1016/j.chemosphere.2015.03.093 [ Links ]
MCKINLAY R, PLANT JA, BELL JNB and VOULVOULIS N (2008) Endocrine disrupting pesticides: Implications for risk assessment. Environ. Int. 34 168-183. https://doi.org/10.1016/j.envint.2007.07.013 [ Links ]
MILLS LJ, GUTJAHR-GOBELL RE, HAEBLER RA, HOROWITZ DJ, JAYARAMAN S, PRUELL RJ, MCKINNEY RA, GARDNER GR and ZAROOGIAN GE (2001) Effects of estrogenic (o,p'-DDT; octylphenol) and anti-androgenic (p,p'-DDE) chemicals on indicators of endocrine status in juvenile male summer flounder (Paralichthys dentatus). Aquat. Toxicol. 52 (2) 157-176. https://doi.org/10.1016/S0166-445X(00)00139-9 [ Links ]
MÜLLER JC, IMAZAKI PH, BOARETO AC, LOURENÇO ELB, GOLIN M, VECHI MF, LOMBARDI NF, MINATOVICZ BC, SCIPPO M-L, MARTINO-ANDRADE AJ and co-authors (2012) In vivo and in vitro estrogenic activity of the antidepressant fluoxetine. Reprod. Toxicol. 34 (1) 80-85. https://doi.org/10.1016/j.reprotox.2012.04.001 [ Links ]
MURRAY GE, TOBIN RS, JUNKINS B and KUSHNER DJ (1984) Effect of chlorination on antibiotic resistance profiles of sewage-related bacteria. Appl. Environ. Microbiol. 48 (1) 73-77. [ Links ]
NCUBE EJ, VOYI K and DU PREEZ H (2012) Implementing a protocol for selection and prioritisation of organic contaminants in the drinking water value chain: Case study of Rand Water, South Africa. Water SA 38 (4) 487-504. https://doi.org/10.4314/wsa.v38i4.3 [ Links ]
NESBITT R (2011) Effects of chronic exposure to ibuprofen and naproxen on Florida flagfish (Jordanella floridae) over one complete life-cycle. Master's thesis, University of Ontario Institute of Technology, Oshawa, Canada. URL: https://ir.library.dc-uoit.ca/bitstream/10155/176/1/Nesbitt_Richard.pdf. Accessed 05 January 2016 (Accessed 12 February 2016). [ Links ]
ODJADJARE EE, IGBINOSA EO, MORDI R, IGERE B, IGELEKE CL and OKOH AI (2012) Prevalence of multiple antibiotics resistant (MAR) Pseudomonas species in the final effluents of three municipal wastewater treatment facilities in South Africa. Int. J. Environ. Res. Public Health 9 (6) 2092-2107. https://doi.org/10.3390/ijerph9062092 [ Links ]
OPITZ R, HARTMANN S, BLANK T, BRAUNBECK T, LUTZ I and KLOAS W (2006) Evaluation of histological and molecular endpoints for enhanced detection of thyroid system disruption in Xenopus laevis tadpoles. Toxicol. Sci. 90 (2) 337-348. https://doi.org/10.1093/toxsci/kfj083 [ Links ]
OSUNMAKINDE CS, TSHABALALA OS, DUBE S and NINDI MM (2013) Verification and validation of analytical methods for testing the levels of PPHCPs (pharmaceutical and personal health care products) in treated drinking water and sewage. WRC Report No. 2094/1/13. Water Research Commission, Pretoria. [ Links ]
PATTERTON HG (2013) Scoping study and research strategy development on currently known and emerging contaminants influencing drinking water quality. WRC Report No. 2093/1/13. Water Research Commission, Pretoria. [ Links ]
PETRIE B, BARDEN R and KASPRZYK-HORDERN B (2015) A review on emerging contaminants in wastewaters and the environment: Current knowledge, understudied areas and recommendations for future monitoring. Water Res. 72 3-27. https://doi.org/10.1016/j.watres.2014.08.053 [ Links ]
RÄTTYÄ J, TURKKA J, PAKARINEN AJ, KNIP M, KOTILA MA, LUKKARINEN O, MYLLYLÄ VV and ISOJÄRVI JIT (2001) Reproductive effects of valproate, carbamazepine, and oxcarbazepine in men with epilepsy. Neurology 56 (1) 31-36. https://doi.org/10.1212/WNL.56.1.31 [ Links ]
RAUT SA and ANGUS RA (2010) Triclosan has endocrine-disrupting effects in male Western mosquitofish, Gambusia affinis. Environ. Toxicol. Chem. 29 (6) 1287-1291. https://doi.org/10.1002/etc.150. [ Links ]
RICHARDS SM and COLE SE (2006) A toxicity and hazard assessment of fourteen pharmaceuticals to Xenopus laevis larvae. Ecotoxicology 15 (8) 647-656. https://doi.org/10.1007/s10646-006-0102-4 [ Links ]
ROBINS JC, MARSIT CJ, PADBURY JF and SHARMA SS (2011) Endocrine disruptors, environmental oxygen, epigenetics and pregnancy. Front. Biosci. Elite Ed. 3 690-700. [ Links ]
SÁNCHEZ RODRÍGUEZ A, RODRIGO SANZ M and BETANCORT RODRÍGUEZ JR (2015) Occurrence of eight UV filters in beaches of Gran Canaria (Canary Islands). An approach to environmental risk assessment. Chemosphere 131 85-90. https://doi.org/10.1016/j.chemosphere.2015.02.054 [ Links ]
SARAVANAN M, HUR J-H, ARUL N and RAMESH M (2014) Toxicological effects of clofibric acid and diclofenac on plasma thyroid hormones of an Indian major carp, Cirrhinus mrigala during short and long-term exposures. Environ. Toxicol. Pharmacol. 38 948-958. https://doi.org/10.1016/j.etap.2014.10.013 [ Links ]
SCHLUMPF M, COTTON B, CONSCIENCE M, HALLER V, STEINMANN B and LICHTENSTEIGER W (2001) In vitro and in vivo estrogenicity of UV screens. Environ. Health Perspect. 109 (3) 239-244. https://doi.org/10.1289/ehp.01109239 [ Links ]
SCHULTZ MM, PAINTER MM, BARTELL SE, LOQUE A, FURLONG ET, WERNER SL and SCHOENFUSS HL (2011) Selective uptake and biological consequences of environmentally relevant antidepressant pharmaceutical exposures on male fathead minnows. Aquat. Toxicol. 104 (1-2) 38-47. https://doi.org/10.1016/j.aquatox.2011.03.011 [ Links ]
SHENG GP, YU HQ and LI XY (2010) Extracellular polymeric substances (EPS) of microbial aggregates in biological wastewater treatment systems: A review. Biotechnol. Adv. 28 (6) 882-894. https://doi.org/10.1016/j.biotechadv.2010.08.001 [ Links ]
SLABBERT JL, VENTER EA, MOLETSANE M, VAN WYK JH, BLAISE C and ANECK-HAHN NH (2007) An investigation of the estrogenic activity in water from selected drinking water treatment processes. WRC Report No.1532/1/07. Water Research Commission, Pretoria. [ Links ]
SNYMAN H VAN NIEKERK AM and RAJASAKRAN N (2006) Sustainable wastewater treatment - what has gone wrong and how do we get back on track? Water Institute of Southern Africa Conference, Durban, May 2006. URL: https://www.dwa.gov.za/events/MunicipalIndaba/Sanitation/05Sustainable WasteWaterTreatment.pdf (Accessed 12 January 2015). [ Links ]
SOTO AM and SONNENSCHEIN C (2010) Environmental causes of cancer: Endocrine disruptors as carcinogens. Nat. Rev. Endocrinol. 6 (7) 363-370. https://doi.org/10.1038/nrendo.2010.87 [ Links ]
STATS SA (2011) Statistics South Africa. Formal census 2011; Population statistics. URL: http://www.statssa.gov.za/ and community survey for 2016. URL: http://cs2016.statssa.gov.za/ (Accessed 25 June 2017). [ Links ]
STATS SA (2017) Statistics South Africa. Statistical Release P0302. Mid-year population estimates for 2017. URL: http://www.statssa.gov.za/publications/P0302/P03022017.pdf (Accessed 25 June 2017). [ Links ]
SVALHEIM S, TAUBØLL E, LUEF G, LOSSIUS A, RAUCHENZAUNER M, SANDVAND F, BERTELSEN M, MØRKRID L and GJERSTAD L (2009) Differential effects of levetiracetam, carbamazepine, and lamotrigine on reproductive endocrine function in adults. Epilepsy Behav. 16 (2) 281-287. https://doi.org/10.1016/j.yebeh.2009.07.033 [ Links ]
SWART N and POOL EJ (2007) Rapid detection of selected steroid hormones from sewage effluents using an ELISA in the Kuils River water catchment area, South Africa, J. Immunoass. Immunochem. 28 (4) 395-408. https://doi.org/10.1080/15321810701603799 [ Links ]
SWART JC, POOL EJ and VAN WYK JH (2011) The implementation of a battery of in vivo and in vitro bioassays to assess river water for estrogenic endocrine disrupting chemicals. Ecotoxicol. Environ. Saf. 74 (1) 138-143. https://doi.org/10.1016/j.ecoenv.2010.09.006 [ Links ]
TAUBØLL E, GREGORASZCZUK EL, TWORZYDØ A, WÓJTOWICZ AK and ROPSTAD E (2006) Comparison of reproductive effects of levetiracetam and valproate studied in prepubertal porcine ovarian follicular cells. Epilepsia 47 (9) 1580-1583. https://doi.org/10.1111/j.1528-1167.2006.00668.x [ Links ]
USEPA (United States Environmental Protection Agency) (1997) Special report on environmental endocrine disruption: An effects assessment and analysis. USEPA Office of Research and Development, Washington, DC. URL: http://www.epa.gov/raf/publications/pdfs/ENDOCRINE.PDF (Accessed 12 January 2015). [ Links ]
USEPA (United States Environmental Protection Agency) (2005) Prevention, Pesticides and Toxic Substances (7508C). EPA 738-F-05-XX. September 2005. USEPA, Washington DC. URL: http://envirocancer.cornell.edu/turf/pdf/mancozeb_fs.pdf. (Accessed 12 January 2015). [ Links ]
VAN WYK JH, ARCHER E, BABALOLA OO, TRUTER JC, VAN RENSBURG EJ and DABROWSKI J (2014) Pesticides as endocrine disruptors in South Africa: Laboratory and field studies. WRC Report No. 1932/1/14. Water Research Commission, Pretoria. [ Links ]
VAN ZIJL MC, ANECK-HAHN NH, SWART P, HAYWARD S, GENTHE B and DE JAGER C (2017) Estrogenic activity, chemical levels and health risk assessment of municipal distribution point water from Pretoria and Cape Town, South Africa. Chemosphere 186 305-313. https://doi.org/10.1016/j.chemosphere.2017.07.130 [ Links ]
VELASCO-SANTAMARÍA YM, KORSGAARD B, MADSEN SS and BJERREGAARD P (2011) Bezafibrate, a lipid-lowering pharmaceutical, as a potential endocrine disruptor in male zebrafish (Danio rerio). Aquat. Toxicol. 105 (1-2) 107-118. https://doi.org/10.1016/j.aquatox.2011.05.018 [ Links ]
VELDHOEN N, SKIRROW RC, OSACHOFF H, WIGMORE H, CLAPSON DJ, GUNDERSON MP, VAN AGGELEN G and HELBING CC (2006) The bactericidal agent triclosan modulates thyroid hormone-associated gene expression and disrupts postembryonic anuran development. Aquat. Toxicol. 80 (1) 217-227. https://doi.org/10.1016/j.aquatox.2006.08.010 [ Links ]
VILLENEUVE DL, CRUMP D, GARCIA-REYERO N, HECKER M, HUTCHINSON TH, LALONE CA, LANDESMANN B, LETTIERI T, MUNN S, NEPELSKA M and co-authors (2014) Adverse Outcome Pathway (AOP) development I: strategies and principles. Toxicol. Sci. 142 312-320. https://doi.org/10.1093/toxsci/kfu199 [ Links ]
WAGENAAR G, BOTHA T and BARNHOORN I (2012) Sperm motility and testicular histology as reproductive indicators in Clarias gariepinus from an eutrophic impoundment, South Africa. J. Appl. Ichthyol. 28 (6) 990-997. https://doi.org/10.1111/jai.12065 [ Links ]
WHO (World Health Organisation) (2012) State of the science of endocrine disrupting chemicals. URL: http://apps.who.int/iris/bitstream/10665/78102/1/WHO_HSE_PHE_IHE_2013.1_eng.pdf (Accessed 5 November 2014). [ Links ]
WOOD TP, DUVENAGE CSJ and ROHWER E (2015) The occurrence of anti-retroviral compounds used for HIV treatment in South African surface water. Environ. Pollut. 199 235-243. https://doi.org/10.1016/j.envpol.2015.01.030 [ Links ]
WRITER JH, BARBER LB, RYAN JN and BRADLEY PM (2011) Biodegradation and attenuation of steroidal hormones and alkylphenols by stream biofilms and sediments. Environ. Sci. Technol. 45 (10) 4370-4376. https://doi.org/10.1021/es2000134 [ Links ]
YUEH M-F, LI T, EVANS RM, HAMMOCK B and TUKEY RH (2012) Triclocarban mediates induction of xenobiotic metabolism through activation of the constitutive androstane receptor and the estrogen receptor alpha. PLoS ONE 7 (6) e37705. https://doi.org/10.1371/journal.pone.0037705 [ Links ]
Received 27 January 2017
Accepted in revised form 11 October 2017
* To whom all correspondence should be addressed. +27 (0)21 808 5805. e-mail: earcher@sun.ac.za
ABBREVIATIONS
4-MBC Benzedrone
ACTH Adrenocorticotropic hormone
AMR Anti-microbial resistance
AOP Adverse outcome pathway
ARV Antiretroviral
CHO Chinese hamster ovary
CRH Corticotrophin-releasing hormone
CRR Cumulative risk ratio
CYP Cytochrome P450
DDE Dichlorodiphenyldichloroethylene
DDT Dichlorodiphenyltrichloroethane
DHT Dihydrotestosterone
DWS Department of Water and Sanitation
E1 Oestrone
E2 Oestradiol
EC Emerging contaminant
EC50 Effect concentration for 50% of test organisms
EDC Endocrine disrupting contaminant
EDSTAC Endocrine Disruptor Screening and Testing Advisory Committee
EDTA Endocrine disrupters testing and assessment
EE2 Ethynyl-oestradiol
EPS Extracellular polymeric substance
ERA Environmental risk assessment
ERDC U.S. Army Engineer Research and Development Centre
ERα Oestrogen receptor alpha
ETU Ethylenethiourea
FSH Follicle-stimulating hormone
GD Green Drop
GnRH Gonadotropin-releasing hormone
GWRC Global Water Research Coalition
hAR Human androgen receptor
hER Human oestrogen receptor
HPA Hypothalamic-pituitary adrenal
HPG Hypothalamic-pituitary gonadal
HPT Hypothalamic-pituitary thyroid
HRT Hormone replacement therapy
JRC European Commission Joint Research Centre
KE Key event
KER Key event relationships
Kow Octanol-water coefficient
LD50 Lethal dose for 50% of test organisms
LH Luteinizing hormone
LOEC Lowest observed effect concentration
MEC Measured environmental concentration
MIE Molecular initiating event
MOA Mode of action
mRNA Messenger ribonucleic acid
NOEC No-observed effect concentration
NP Nonylphenol
NSAID Non-steroidal anti-inflammatory drug
NTMP National Toxicity Monitoring Programme
OECD Organisation for economic co-operation and development
OMC Octyl-methoxycinnamate
P Progesterone
PEC Predicted environmental concentration
PNEC Predicted no-effect concentration
POP Persistent organic pollutants
PPCP Pharmaceuticals and personal care products
RQ Risk quotient
SAID Steroidal anti-inflammatory drug
SHBG Steroid hormone binding globulin
STW Sewage treatment works
T Testosterone
T3 Triiodothyronine
T4 Thyroxine
TCC Triclorocarbanilide
TCS Triclosan
TR Thyroid hormone receptor
TRH Thyrotropin-releasing hormone
TSH Thyroid-stimulating hormone
USEPA United States Environmental Protection Agency
VTG Vitellogenin
WHO World Health Organisation
WWTW Wastewater treatment works














