Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
Water SA
versión On-line ISSN 1816-7950
versión impresa ISSN 0378-4738
Water SA vol.43 no.4 Pretoria oct. 2017
http://dx.doi.org/10.4314/wsa.v43i4.08
BTEX compounds in water - future trends and directions for water treatment
OM FayemiwoI; MO DaramolaII; K MoothiI, *
IDepartment of Chemical Engineering, Faculty of Engineering and the Built Environment, University of Johannesburg, Doornfontein 2028, Johannesburg, South Africa
IISchool of Chemical and Metallurgical Engineering, Faculty of Engineering and the Built Environment, University of the Witwatersrand, Wits 2050, Johannesburg, South Africa
ABSTRACT
BTEX (benzene, toluene, ethylbenzene, and xylene) compounds are common water resource and potable water pollutants that are often left undetected and untreated by municipal treatment systems in spite of the negative repercussions associated with their ingestion. The US EPA has classified these pollutants as priority pollutant, yet they are persistently present in a variety of water resources. In this review paper, we highlight the sources and reported concentrations of BTEX compounds in water and explore historical remediation techniques that have been applied such as bioremediation and natural attenuation. We also highlight emerging possibilities and future directions for remediation techniques, such as nanotechnology-based materials and novel green materials (tannins) that can be applied to ensure removal of these compounds in water.
Keywords: adsorbents, biosorbents, BTEX, remediation, nanotechnology, water quality.
INTRODUCTION
Mono-aromatic hydrocarbons - benzene, toluene, ethylbenzene and xylenes (BTEX) - are common pollutants found in groundwater plumes and in other water resources, as a result of the disposal of contaminated industrial effluents and accidental events such as oil spills and oil pipeline leakages (Alberici et al., 2002, Castillo et al., 1998; Mazzeo et al., 2010; Costa et al., 2012). The negative health effects that result from the consumption of these compounds include cancer, liver lesions, drowsiness and irritation of organs (Zhang et al., 2012; Tunsaringkarn et al., 2012). Mitra and Roy (2011) further reported that human exposure to BTEX compounds over a long period of time results in skin and sensory irritation, adverse respiratory health effects and central nervous system irritation. In spite of the negative effects they pose to human health, BTEX compounds remain overlooked and untreated in municipal systems, thereby increasing the risk of water-related diseases through their ingestion.
Recent remediation efforts have focused mainly on the compounds contained in heavy oil fractions, i.e., the poly-aromatic hydrocarbons (PAHs) which are known to cause conspicuous environmental degradation, and are more noticeable at pollution sites (Bojes and Pope, 2007; Tronczynski et al., 2004). BTEX compounds on the other hand are often overlooked in remediation efforts due to their obscure nature in water.
Although their presence in water may not be as easily discernible as with PAHs, BTEX compounds are more abundant in the environment and can be found in a variety of sources (El-Naas et al., 2014), some of which include petrochemical industry waste streams, household wastes and municipal landfills (Chriac et al., 2007; Slack et al., 2005), as well as groundwater plumes, especially when located at a considerable distance from an oil spill site (Bekins et al., 2001; Chapelle, 1999; Davis et al., 1999). In addition, studies such as Grady and Casey (2001), Schmidt et al. (2004), Mitra and Roy (2011), and Reddy et al. (2012) have reported the presence of BTEX compounds in drinking water, indicating extensive health risks that may not be immediately evident. Dutta et al. (2009) and Zhang et al. (2012) have also highlighted the persistent presence of BTEX compounds in air, and have reported the transportation of these compounds from air into water bodies as a result of rainfall. As a result, it is imperative that the remediation of these compounds in water is prioritized in future water treatment systems.
There has been a reported variety of remediation techniques applied to BTEX compounds, especially at oil-spill sites (Röling et al., 2002; Carmody et al., 2007; Boonsaner et al., 2011). These techniques, which can mostly be classified under bioremediation and natural attenuation, have been reported to be inadequate if the intention is to achieve timeous removal of these compounds from water systems (Vidali, 2001). Novel strides in science have emerged with new possibilities that range from the use of nanoparticles and nanocomposites to the use of membrane technology. Advancements in the field of nanotechnology have resulted in an evolvement from sole applications to common water pollutants and pathogens (Tiwari et al., 2008), to applications in large-scale environmental remediation efforts, including the remediation of non-reactive compounds and even soluble and insoluble organic pollutants (Theron et al., 2008; Qu et al., 2013). In membrane technology, novel materials are being discovered and reported, with some not yet applied to BTEX compounds or even PAHs, due to lack of sufficient understanding. It is only by understanding the potential applications of these materials that prospective research can be directed at removal of these pollutants from water to ensure the delivery of truly clean and safe water.
The purpose of this paper is to provide a review of historical remediation techniques applied to BTEX compounds, detailing the advantages and disadvantages of these techniques. The following sections are aggregations of knowledge available on the properties of BTEX compounds, their sources and reported concentrations, the historical remediation techniques that have been successfully and unsuccessfully applied for their removal from water, and the emerging techniques and materials that can be focused on in future.
BTEX COMPOUNDS: PROPERTIES AND REPORTED CONCENTRATIONS IN WATER
Properties of BTEX compounds
Benzene, toluene, ethylbenzene and xylenes are mono-aromatic ring compounds with a 6-carbon benzene ring as their core structure. Due to their closed structures, these compounds, especially benzene, are generally considered to be non-reactive species; Bunnett and Zahler (1953); however, it is well-known that these compounds have the ability to undergo hydrogenation and certain substitution reactions. At oil-spill sites, BTEX compounds can be transported through several metres under favourable redox conditions causing them to be persistent pollutants in both soil and water (Cozzarelli et al., 2001; Camilli et al., 2010). Their ability to dissolve in water, relative to their poly-aromatic counterparts, is due to low octanol-water partition coefficient (Kow) values (Anderson, 2000; Pruden et al., 2003; Poulsen et al., 1992) which favour hydrophilic dissolution, in spite of their hydrophobic nature.
Furthermore, BTEX compounds are mostly found to co-exist with one another or with methyl tert-butyl ether (MTBE) - a common fuel oxygenate reported to be a common organic pollutant in water. The co-existence of BTEX compounds with one another suggests that their toxicity is amplified through their interactions with one another; however, such interaction models have not been verified (Aivalioti et al., 2010). Table 1 summarizes the physical and chemical properties of BTEX compounds as adapted from El-Naas et al. (2014).
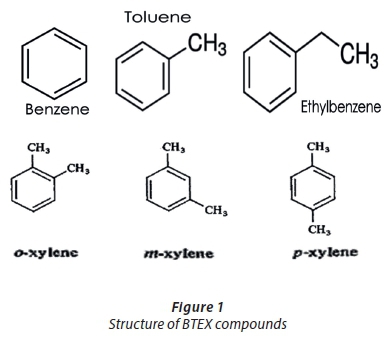
Interestingly, BTEX compounds are among the most abundantly produced chemicals in the world, in spite of their ill effects on human health and resultant environmental degradation. They are utilized as solvents in many industrial processes, as well as used in many household products including insecticides and paints.
Sources and reported concentrations of BTEX compounds in water
BTEX compounds are priority pollutants according to the United States Environmental Protection Agency (US EPA) (Abumaizar et al., 1998). These volatile organic compounds (VOCs) are often present in air, especially in areas where oil spillages have occurred (Leusch and Bartkow, 2010), and in closely-located groundwater plumes. They are also found to be dissolved in high concentrations in oilfield produced water - an oil and gas industry effluent (Neff, 2002; Veil et al., 2004). Industries that utilize petrochemicals for the production of paints, adhesives, inks and rubber also produce BTEX compounds in their effluents, although extensive studies have not been conducted to identify BTEX compounds in these effluent streams or characterize their concentrations (Castillo et al., 1998).
BTEX compounds are more abundant in the environment than their poly-aromatic counterparts. They are present in either gaseous or liquid media in the environment, and their presence has been reported in a number of studies. Most commonly reported is the detection of these compounds in groundwater as a result of oil spills and oil pipeline leakages. Mitra and Roy (2011) reported that the main source of BTEX contamination in water is the release of petroleum products ranging from gasoline and diesel fuel to heating oil from leaking oil tanks. Meniconi et al. (2002) characterised the composition of hydrocarbons and other compounds released into water in different regions of Brazil as a result of oil spills. The study reported that the concentration and persistence of BTEX compounds was directly proportional to the scale and duration of the oil spill, highlighting high BTEX concentrations in large-scale, long-duration oil spills. Lu et al. (1999) reported the migration and dissolution of BTEX compounds in groundwater due to a leak at a petroleum oil facility.
Camilli et al. (2010) tracked the hydrocarbon transport at the site of the Deepwater Horizon oil spill and reported that a continuous and persistent plume of oil, more than 35 km in length (at a depth greater than 1 100 m from the surface of the water) was present. Samples obtained from this oil plume contained high concentrations of mono-aromatic compounds, including BTEX compounds. Wang et al. (2002) characterized near-surface groundwater (0 - 5 m), and deeper-level groundwater (15 - 60 m), and reported BTEX concentrations of 155 µg/kg of water, and 2.6 µg/kg of water, respectively. This report showed that in spite of the depth of groundwater, BTEX compounds were still present, albeit at low concentrations. Reddy et al. (2012) examined the composition and fate of oil and gas that flowed from the Macondo well during the Deepwater Horizon oil spill. The study highlighted that the most abundant hydrocarbons present in the water were BTEX compounds (up to78 µg/L). These findings have been reiterated in various studies, such as Essaid et al. (2003), Margesin et al. (2003), and Das and Chandran (2011).
The persistence of BTEX compounds in groundwater plumes is worrisome, especially in vulnerable regions like Africa and South-East Asia where the direct reliance on groundwater in the form of drinking wells and boreholes for potable and other uses is high. The health risks posed as a result of such chemical persistence cannot be overstated.
In addition to groundwater, other water sources, including industrial effluents and drinking water, have been investigated for the presence of BTEX compounds (Arambarri et al., 2004; Delzer et al., 1996; Edwards, 2004; Fontenot et al., 2013; Goss et al., 1998; Grady and Casey, 2001; Schmidt et al., 2004; Kelley et al., 1997; Gross et al., 2013). In industrial effluents, BTEX compounds are undeniably present, especially in the effluents of industries that utilize one or more of the BTEX compounds as solvents for their processes. The most commonly reported source of BTEX in terms of industrial effluents is oilfield-produced water - an oil and gas effluent that is usually discharged into coastal bodies and other surface water bodies, in regions where environmental regulations are not well-enforced. Ranck et al. (2005) reported the presence of BTEX compounds in produced water at high concentrations. Dórea et al. (2007) reported concentrations of BTEX compounds in produced water obtained from Brazil ranged from 96.7 to 1 397 µg/L. The values reported in Dórea et al., (2007) flirt rather closely with the acceptable limits for these compounds (1.0 mg/L for benzene, 1.3 mg/L for toluene, 1.0 mg/L for ethylbenzene and 1.4 mg/L for xylenes) according to Australia's Water Corporation (Australian Water Corporation, 2017). It can be estimated from the reported concentrations of these compounds in produced water that the concentrations of these compounds are very often exceeded in other industrial effluents from petrochemical, inks and adhesives, as well as rubber industries, though there are no studies reported in this regard.
In drinking water, Goss et al. (1998) investigated the concentration of BTEX compounds in farmstead domestic wells, often used as drinking water sources, and reported that about 40% of the farm wells investigated contained one or more of the BTEX pollutants in concentrations that exceeded the normal limit (1 µg/L in most European potable water guidelines) (Serrano and Gallego, 2004). In drinking water samples, Serrano et al. (2007) reported concentrations of benzene between 1 and 30 µg/L, which exceed reported acceptable limits (Table 2). In 2004, it was reported by Schmidt et al. that Lake Zurich, the largest supplier of drinking water to the biggest Swiss city, contained BTEX compounds in addition to other fuel oxygenates. Grady and Casey (2001) reported the presence of BTEX compounds to be higher than 20 µg/L in finished drinking water in the north-east and mid-Atlantic regions of the United States. This exceeds the acceptable limit for BTEX compounds. Table 2 shows the acceptable limits for BTEX compounds in drinking and environmental waters as set by the Australian Drinking Water Guidelines (ADWG: NHMRC, 2011) in addition to other regulatory institutions.
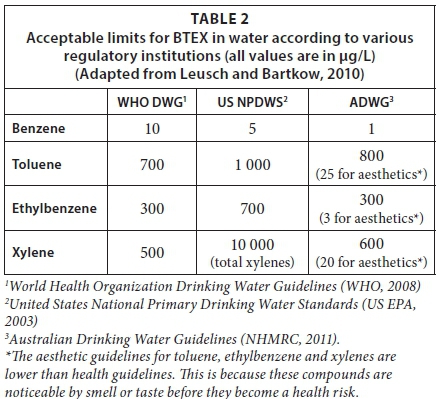
According to the Australian Drinking Water Guidelines (NHMRC, 2011), the benzene concentration in drinking water should not exceed 1 µg/L, while the other three BTEX compounds are permissible in the range of 300 - 800 µg/L in water. The higher permissibility for toluene, ethylbenzene and xylene is attributed to their strong odour, which often makes them easily detectable (Leusch and Bartkow, 2010). However, the reliance on the allowance of such high concentrations is questionable given the earlier-stated health effects that can result from the ingestion of these compounds. It has been estimated by Leusch and Bartkow (2010) that the daily intake of BTEX compounds from drinking water is up to 10 µg for benzene, 43 µg for toluene, 20 µg for ethylbenzene and 24 µg for xylenes, per kg of water, assuming a person consumes 2 L of water per day (Table 3). There have been reports of bioaccumulation of benzene derivatives in marine organisms (Neff, 2002; Neff and Sauer, 2012; Lotfinasabasl et al., 2013), albeit in low concentrations. Still, the health risks posed to human health through the ingestion of these compounds, considering benzene is a confirmed carcinogenic, cannot by any means be exaggerated.
Remarkably, household hazardous wastes have also been reported to contain high concentrations of BTEX compounds. Ro et al. (2005) revealed that household products such as garden pesticides, pharmaceuticals, paints, some detergents and personal care products contain BTEX compounds, hence resulting in an increase in the concentration of these compounds in municipal waste streams. Chiriac et al. (2007) also reported the presence of BTEX compounds in municipal solid wastes. A study by Robinson et al. (2005) highlighted the high pollution potential of trace organic compounds such as BTEX compounds in household wastes that are disposed in landfills. These compounds can readily dissolve in water and be carried in run-off to surface water bodies, thus posing major risks to water resource health, marine life and subsequently human health.
From the above it is evident that BTEX compounds can easily be encountered in the environment. Although reported concentrations in treated drinking water may seem low, the bioaccumulation effect cannot be ruled out, nor can the subsequent health effects that may result. The presence of these compounds in groundwater plumes, and their ability to persistently be transported to great depths in groundwater, increases the risks of water-related illnesses and deaths in highly vulnerable regions like some parts of Africa and South-East Asia, where municipal treatment systems do not exist and residents rely solely on groundwater for all their needs.
Historical remediation techniques for BTEX compounds in water
The remediation of water contaminated with BTEX compounds utilizing a variety of techniques has been reported. Some of these techniques include adsorption, ozonation, natural attenuation, phytoremediation, and bioremediation (Lu et al., 1999; Mackay et al., 2006; Weishaar et al., 2009; Cunningham et al., 2001; Andreoni and Gianfreda, 2007). Of all these, natural attenuation and bioremediation are the most commonly reported techniques and are the focus of this section.
Natural attenuation
Natural attenuation was first proposed as a non-intrusive, cost-effective alternative to other expensive remediation techniques (Wiedemeier et al., 1999). The process 'refers to the observed reduction in contaminant concentration as contaminants migrate from the source in environmental media' (Wiedemeier et al., 1999 p. 2). Due to its 'non-intrusive' nature, natural attenuation has been the preferred technique for a wide range of pollutants in water. Its application to hydrocarbons, especially at oil-spill sites, was reported in studies conducted by Cozzarelli et al. (2001), Bekins et al. (2001) and Dellile and Pelletier (2002).
In 1997, Cho et al. reported the continuous decrease in BTEX concentrations after 18 months of active remediation of a jet fuel release site as a result of natural attenuation. Lu et al., (1999) reported the decrease in BTEX concentration as a result of natural processes within groundwater. Kao and Prosser (2001) estimated the natural attenuation rate of BTEX compounds at a gasoline spill site to be 0.036% per day. Suarez and Rifai (2002) evaluated the use of natural attenuation for BTEX removal at a coastal facility and reported that the bulk of BTEX was lost via biodegradation, whilst 'other mechanisms' were responsible for further loss. The degradation rate estimated was ~0.0002/day, with clean-up time projected to take roughly 200 years.
Although natural attenuation is widely accepted for its cost-effectiveness, the ill effects of this process on the environment are worrisome. Some of these include the depletion of dissolved oxygen which will negatively impact marine life, depletion of nutrients such as nitrate and sulphate, the production of dissolved ferrous iron in water, production of sulphide and carbon dioxide, as well as an undesirable shift in pH values (Kao et al., 2006). All of these factors eventually result in water resource degradation. Furthermore, the duration of natural attenuation processes, as stated in studies by Suarez and Rifai (2002) make it an undesirable remediation technique. Mulligan and Yong (2004) quantified that it will take around 250 years of natural attenuation to remediate an initial concentration of 900 mg/L benzene. In addition, Da Silva and Alvarez (2002) reported that the natural attenuation of BTEX compounds may be affected by the use of ethanol as a fuel oxygenate. Ethanol enhances the dissolution of BTEX in water and its subsequent transport to deeper level of the water table or coastal body; hence rendering natural attenuation ineffective.
It is important to note that natural attenuation does not necessarily imply the removal of BTEX compounds in water; rather, it is a dilution of the compounds as they travel from the source, sometimes into groundwater, and sometimes into deeper levels of coastal bodies, depending on the source. Therefore, it is erroneous to assume that natural attenuation removes BTEX compounds from contaminated media. Taking into consideration the level of degradation and exposure to health risks posed by this technique, it is an undesirable remediation method to apply in recent times where many regions are facing water shortages and extensive water resource degradation. A review by Seagren and Becker (2002) highlighted that natural attenuation in itself is not a remediation mechanism that is effective on its own, especially when dealing with resistant, recalcitrant compounds such as BTEX. The destructive mechanism often responsible for the reduction in contaminant concentration is in fact bioremediation.
Bioremediation
Bioremediation has gained a lot of research momentum due to the belief that it does not result in any undesirable consequences after the remediation process has been completed (Bowlen et al., 1995; Lovley, 1997; Chapelle, 1999; Margesin et al., 2003; Andreoni and Gianfreda, 2007). The ability of microbes to remediate BTEX compounds is dependent on their metabolic preferences, which informs their ability to use the compounds of concern as carbon sources (Vidali, 2001). The advantage of bioremediation is the fact that it can be conducted in-situ - a technique preferable for most of the BTEX contaminant media. The in-situ biodegradation of BTEX compounds at oil-spill sites has been reported under various environmental conditions (Farhadian et al., 2008; Chapman et al., 1997; Wolicka et al., 2009; Yeh et al., 2010).
The group of microorganisms mostly reported for use as bioremediation agents are bacteria; however, fungal species have also been reported to be able to degrade BTEX compounds for use as carbon and energy sources. Prenafeta-Boldú et al. (2004) reported the use of a toluene-metabolising fungus to degrade BTEX compounds under various pH conditions. Alvarez and Vogel (1995) highlighted the ability of indigenous bacteria to degrade BTEX compounds without suffering as much metabolic inhibition as micro-organisms from other sources. Kao and Wang (2000) reported the control of BTEX migration using bioremediation within controlled environmental zones. Jin et al. (2013) also described the isolation of a BTEX-degrading bacterium. Bioremediation occurs under varying conditions, depending on the preferences of the microorganisms. The ability to degrade highly toxic pollutants such as BTEX compounds is occasionally dependent on the microbes' ability to produce secondary metabolites such as biosurfactants (Chirwa et al. 2013). This has been reiterated by Margesin et al. (2003) and Lu et al. (2006), where synthesis of rhamnolipid biosurfactants has been reported to aid the uptake and degradation of BTEX compounds. The effective uptake and degradation of BTEX compounds, however, is due to certain environmental conditions, with some beneficial to the process, while others seem to impede it.
According to Kao and Wang (2000), iron-reducing conditions are the most favourable conditions for the biodegradation of BTEX compounds. Denitrification and the use of oxygen as an electron acceptor were also highlighted as preferable conditions. Aerobic conditions have also been reported as favourable to the bioremediation process. Wolicka et al. (2009) reported that BTEX biodegradation is faster under aerobic conditions; however, many studies have been published which report that BTEX biodegradation under anaerobic conditions can be enhanced by injecting nutrients such as sulphate, nitrate and Fe (III) (Lovley, 1997; Cunningham et al., 2001; Schreiber and Bahr, 2002; Anderson and Lovley, 2000; De Nardi et al., 2005). Cunningham et al. (2001) enhanced the bioremediation of BTEX-contaminated groundwater by injecting nitrate and sulphate into the water plume. An analogous study by Schreiber and Bahr (2002) showed that the addition of nitrate resulted in the loss of toluene, ethylbenzene and xylenes after an initial lag period of 9 days.
Although bioremediation is highly preferred because of its lack of harmful residues and cost-effectiveness, there are factors that make the process undesirable. One such factor is the duration of the bioremediation process. Schreiber and Bahr (2002) reported an initial lag phase of 9 days for toluene, ethylbenzene and xylene, while degradation of benzene was not achieved after 60 days of incubation. Cunningham et al. (2001) reported that degradation of benzene only began to occur toward the end of a 15-month study period. In-situ bioremediation is also an unpredictable process, as it is difficult to ascertain if the microbial community within the environmental media will utilize the BTEX compounds (Head, 1998). In this regard, the toxicity of the compounds to the microbial communities also comes into play (Langenhoff et al., 1996; Gray, 1998; Thacker and Ford, 1999), especially in in-situ bioremediation.
EMERGING REMEDIATION TRENDS FOR THE FUTURE
Nanotechnology
At a time when environmental factors such as global warming, changing patterns of precipitation and run-off are increasingly playing a role, as well as the emergence of new water pollutants and pathogens affecting the quality of water resources, the use of nanotechnology-based materials as treatment agents is being investigated on a large scale for improved water treatment (Goyal et al., 2011; Theron et al., 2008; Kanchi, 2014; Baruah et al., 2015). Nanoparticles, nano-powders, and nanomembranes have been found to have extensive applications in the water sector (Theron et al., 2008; Li et al., 2008; Pendergast and Hoek, 2011; Qu et al., 2013; Maphutha et al., 2013); as a result, interest in these materials for water treatment has increased significantly over the past decade (Roco 2011; De Volder et al., 2013; Humplik et al., 2011). The ability of these nano-materials to remove pathogens in water, adsorb priority water pollutants and degrade chlorination by-products makes them highly desirable for water treatment (Tiwari et al., 2008).
Various metals have been utilised for nanoparticle synthesis and their subsequent applications for various forms of water treatment have been investigated. Some of the common metals reported include titanium, manganese, silver, gold and iron nanoparticles, of which titanium, gold and silver are the most common (FeiFang et al., 2008; Qu et al., 2013). Silver nanoparticles have been investigated mainly for their anti-microbial properties. Rai et al. (2009) report that the use of silver nanoparticles in water treatment is a welcome development as many waterborne pathogens have developed resistance to antibiotics. Morones et al. (2005) reported the antibacterial properties of silver nanoparticles, highlighting their possible application in the removal of pathogens during wastewater treatment. The ability of silver nanoparticles to remove biological pathogens has been attributed to a mode of action that attacks the cell membranes of microbes, resulting in changes that affect the cell, such as a disruption in the ion-efflux system, and lead to cell death (Pal et al., 2007; Hwang et al., 2008). On the other hand, Hwang et al. (2008) reported that gold nanoparticles were unable to disrupt the cell membranes of Escherichia coli - a common water pathogen often used as an indicator for the presence of faecal coliforms. This finding was later disproved by Cui et al. (2012), who reported that gold nanoparticles are effective against a variety of gram-negative bacteria (including E. coli). The mode of action was reported to be an inhibition of ATPase activities which resulted in the reduction of ATP levels and an inhibition in ribosomal bonding.
These studies indicate that silver and gold nanoparticles have anti-pathogenic characteristics and may become the future replacement for chlorine as disinfectants. Chlorination byproducts are currently pollutants of interest which pose major risks to human health; hence the possibility of anti-pathogenic nanoparticles as chlorine replacements is not far-fetched, given that they can be successfully recovered after disinfection and possibly reused.
In addition to silver and gold nanoparticles, titanium nanoparticles are of great interest due to their ability to degrade/split organic pollutants (Mahmoodi et al., 2007; Ménesi et al., 2008). Titanium nanoparticles are best activated by light; hence the mode of action of these nanoparticles is photocatalytic. Orlov et al. (2006) reported the degradation of MTBE in water using titanium oxide nanoparticles. Studies show that titanium nanoparticles act as reaction catalysts and aid degradation of organic pollutants in water in the presence of UV light (Nosaka et al., 2005; Mahmoodi et al., 2007). Also of great interest in water treatment are iron nanoparticles. Modifications of these particles suggest that they may be able to combat more chemical water pollutants than any other metallic or metallic oxide nanoparticles. Wang et al. (2010) highlighted that magnetism of iron nanoparticles made them viable agents for the removal of organic pollutants in polluted media. Shih et al. (2011) reported the reduction of hexachlorobenzene under various pH and temperature conditions using nanoscale iron particles. Nanoparticles, when solely applied, may be considered inefficient in treating polluted water completely, due to the variety of contaminants that may be present. Most nanoparticles are specific in their mode of action (e.g. silver for pathogens and titanium for chlorinated hydrocarbons); hence, the development of nanocomposites has become a widespread evolution of nanotechnology applications in water treatment.
The use of nanocomposites to remove harmful compounds in water is aimed at enhancing the remediation potential of the material by increasing selectivity and adsorption capacity while reducing retention time of the polluted water (Qu et al., 2013). The application of nanoparticles in isolation may not be sufficiently effective in removing persistent recalcitrant compounds in water; therefore, the combination of nanoparticles, nanotubes and a suitable polymer to form nanocomposites has become a widely used technique for improving the efficacy of water treatment (Jones et al., 2007; Patel et al., 2006; Crock et al., 2013). Shawky et al. (2011) reported the synthesis of a CNT-polyamide polymer nanocomposite for water treatment. The composite was reported to have a high tensile strength, and was able to prevent the influx of key tested contaminants such as sodium and organic matter. Chu and Pan (2012) reported the synthesis of Fe-C nanocomposites for the removal of oils in water. Han et al. (2012) reported the use of Fe-graphene oxide nanocomposites in the solid-phase extraction of hydrocarbons in polluted water. The study reported removal efficiencies for five different hydrocarbons within the range of 76.8% to 103.2%. Emerging trends suggest that such modifications and combinations can result in nanocomposites that can be utilised to remove dissolved organics in potable water, PAHs in industrial effluents, and possibly used to clean up oil spill and oil pipeline leakage sites (Bruna et al., 2012; Chen et al., 2008; Yang et al., 2006).
Although successful applications of nanocomposites have been conveyed in various reports, it is important to note that most of these studies have focused on common water pollutants which can be treated through municipal systems, while emerging pollutants and recalcitrant hydrocarbons such as PAHs and BTEX are hardly investigated, and could be the focus of future studies. The improved activity of nanocomposites is often dependent on the high adsorption properties of materials such as carbon nanotubes and graphene sheets. Carbon nanotubes (CNTs) (Moothi et al., 2012; Moothi et al., 2015; Yah et al., 2011) and graphene sheets are known for their high adsorption capacity (Chen et al, 2007). This is mainly due to their small size and large surface area (Xu et al., 2008). Studies such as Peng et al. (2003), Lu et al. (2005); Kosynkin et al. (2009), Gupta et al. (2011) and Zhao et al. (2011) have reported the removal of persistent aromatic hydrocarbons, trihalomethanes and 1, 2-dichloro-benzene using CNTs and graphene sheets.
Lu et al. (2005) reported the removal of trihalomethanes from water using CNTs. Peng et al. (2003) reported the removal of 1, 2-dichlorobenzene from water using CNTs for adsorption. The application of CNTs to compounds such as BTEX are sparsely reported in literature; nevertheless, studies such as Wang et al. (2008) and Su et al. (2010) indicate the potential wide-scale application of CNTs to the remediation of aqueous environments contaminated with these compounds and similar water-degrading pollutants such as PAHs. Figure 2 illustrates the various forms of CNTs such as unzipped, single-walled CNT (SWCNT) and multi-walled CNT (MWCNT).
The unzipping of CNTs to form graphene sheets has been reported (Kosynkin et al., 2009; Jiao et al., 2009; Elías et al., 2009). The prospect of this development for water treatment could be far-reaching. By unzipping CNTs, the removal of pollutants such as BTEX compounds from water resources, potable water and industrial effluents can be improved. This will directly translate to the removal of PAHs in industrial effluents, reducing the ill effects caused by the disposal of such 'semi-treated' effluents on water resources. The increased surface area of unzipped CNTs can reduce the treatment time of contaminated water and also improve the formation of nanocomposites and their efficacy in water treatment. Zhang et al. (2012) highlighted the possible application of graphene sheets (unzipped CNTs) for wastewater treatment. Mishra and Ramaprabhu (2011) reported the use of functionalized graphene sheets for the removal of arsenic from water and the desalination of seawater, and reported very high adsorption capacities. Using the Langmuir isotherm, the authors determined that the removal of arsenic derivatives (arsenite and arsenate), as well as sodium, in seawater were as high as 139 mg/g, 142 mg/g and 122 mg/g, respectively. Zhao et al. (2011) reported the use of sulfonated graphene for the removal of persistent aromatic hydrocarbons. Wang et al. (2014) also reported the removal of poly-aromatic hydrocarbons from water using graphene and graphene oxide nano-sheets. Similar studies were reported by Sun et al. (2013), Jin et al. (2015) and Yu et al. (2016). However, applications to BTEX compounds remain scarce in reported literature.
Although nanotechnology is a futuristic form for water treatment, it should be noted that most of the nanoparticles, nanocomposites and nanotubes being utilized for enhanced adsorption are metals that may pose health risks in the long run. Hence, the use of these materials in the treatment of drinking water treatment should be approached with caution (Simate et al., 2012). The possible health effects that may result from the ingestion of carbon nanotubes if these are used for drinking water treatment have not been fully investigated. Studies suggest that there are cytological and cardiovascular health effects that may result from the consumption of CNTs (Jia et al., 2005; Li et al., 2007; Smart et al., 2006). Li et al. (2007) reported that mice exposed to SWCNTs for a period of 60 days developed aorta damage, while Jia et al. (2005) reported an increase in toxicity with a direct increase in dosage of SWCNTs. Warheit et al. (2004) reported that exposure of mice to CNTs at a dosage of 5 mg per kg of body weight resulted in mortality. This limits the applications of CNTs to industrial wastewater, and even then, there is a need for applied CNT-based materials to be collected after treatment has been completed. This is very necessary in vulnerable regions where industrial effluents are disposed into surface waters after treatment, and where surrounding vulnerable communities tend to rely on direct abstraction from such water sources for potable purposes.
Futuristic water treatment materials are however not limited to nano-materials; there are possibly cheaper materials that could have better remediation than nano-materials. One such possibility is biosorbents which can be synthesized from green raw materials and green wastes. We believe these materials might be the focus of future research in water treatment within the next decade.
Tannin-based adsorbents (biosorbents)
Tannins are polyphenolic biomolecules that are anionic in nature, and are able to precipitate various proteins, amino acids and other organic compounds (Van Buren and Robinson, 1969). Although they are water-soluble, studies have reported that they can be converted into insoluble gels and matrices for widespread application (Nakano et al., 2001; Ogata et al., 2011). These compounds are known to possess high levels of antimicrobial activity (Scalbert, 1991), as well as antioxidant properties (Hagerman et al., 1998; Gil et al., 2000). Mostly investigated for their applications in nutrition and cosmetics where they are applied as astringents, tannins have not been duly explored for other possibilities such as water treatment. A recent review by Bacelo et al. (2016) highlights the possibility of using tannins as biological adsorbents for environmental applications. The report advises that tannin-based adsorbents have a naturally high affinity for heavy metals, dyes, and other organic and inorganic pollutants in aqueous solution, indicating a need to investigate the application of these compounds for the removal of mono-aromatic hydrocarbons in water.
The use of tannin-based adsorbents for the removal of BTEX compounds not only has a remediation benefit but also a waste-utilization benefit. This is because many known tannin sources are agricultural products such as leaves and tree barks that are often discarded (El Sissi et al., 1965). Interestingly, solid winery waste, (especially red grape pomace which consists of grape skins, seeds, and stalks), has been highlighted as a possible source of tannins that could be explored for the synthesis of tannin-based adsorbents which can subsequently be applied to water treatment (Lu et al., 1999; Souquet et al., 2000; Ping et al., 2011; Ping et al., 2012). It is expected that tannin-based adsorbents might prove to be safer than CNTs and previously reported nanocomposites. Future studies should therefore aim to focus on the utilization of green wastes to synthesize these adsorbents, as this could spark a new trend, even in membrane synthesis.
CONCLUSION
Current municipal water treatment systems do not detect or treat BTEX compounds, thereby creating a risk of ingestion by end users of municipal-supplied potable water. The use of groundwater (in the form of boreholes) increases the risk of these compounds being ingested as they have been reported to naturally occur in groundwater, and are present in many industrial effluents disposed into the environment (Meidl, 1997; Wallace and Kadlec, 2005). As occurrences of cancer-related deaths increase and unexplainable health defects in newborn babies rise, it is important that future water treatment technologies focus on previously-overlooked pollutants such as BTEX compounds. The use of futuristic treatment materials such as nano-materials and tannin adsorbents could create more efficient water treatment systems, and reduce risks related to the consumption of unclean water. Research trends (Fig. 3) indicate that there is still room for more studies to be conducted on the occurrence of BTEX compounds in various water systems, as well as to examine future treatment techniques that can help alleviate unpleasant health effects and possibly reduce water-related deaths.
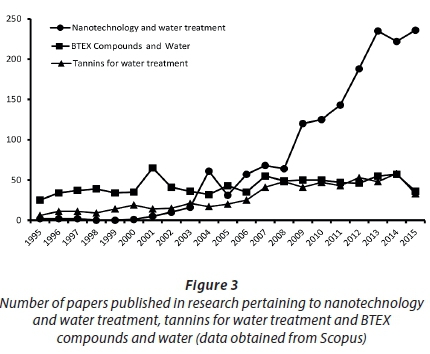
In addition to fully understanding the level of occurrence of these compounds in water, it is important to examine their chemical and physical properties, so as to better understand and optimize the mechanisms of remediation using emerging techniques and materials (Pan and Xing, 2008). The possible degradation of BTEX compounds to useful intermediates or harmless end-products can also be achieved by synthesizing materials that include degradation catalysts in the form of highly reactive nanoparticles. The successful extraction and characterization of tannins, as well as the synthesis of tannin-based adsorbents, could provide a novel platform for removal of compounds such as BTEX in water, without any environmental or human health ill effects. The global water sector has much to gain from research focused on these areas.
ACKNOWLEDGEMENTS
The authors wish to acknowledge financial support from the University of Johannesburg for the Global Excellence and Stature (GES) award that has been beneficial in starting this project.
REFERENCES
ABUMAIZAR RJ, KOCHER W and SMITH EH (1998) Biofiltration of BTEX contaminated air streams using compost-activated carbon filter media. J. Hazardous Mater. 60 (2) 111-126. [ Links ]Australian Water Corporation (2017) Trade Waste. Acceptance criteria for trade waste - Information sheet 6. URL: https://www.watercorporation.com.au/-/media/files/business/trade-waste/applying-to-discharge/acceptance-criteria.pdf (Accessed 18 January 2017). [ Links ]
AIVALIOTI M, VAMVASAKIS I and GIDARAKOS E (2010) BTEX and MTBE adsorption onto raw and thermally modified diatomite. J. Hazardous Mater. 178 (1) 136-143. [ Links ]
ALBERICI RM, ZAMPRONIO CG, POPPI RJ and EBERLIN MN (2002) Water solubilization of ethanol and BTEX from gasoline: on-line monitoring by membrane introduction mass spectrometry. Analyst 127 (2) 230-234. [ Links ]
ALVAREZ PJJ and VOGEL TM (1995) Degradation of BTEX and their aerobic metabolites by indigenous microorganisms under nitrate reducing conditions. Water Sci. Technol. 31 (1) 15-28. [ Links ]
ANDERSON MA (2000) Removal of MTBE and other organic contaminants from water by sorption to high silica zeolites. Environ. Sci. Technol. 34 (4) 725-727. https://doi.org/10.1021/es990390t [ Links ]
ANDERSON RT and LOVLEY DR (2000) Anaerobic bioremediation of benzene under sulfate-reducing conditions in a petroleum-contaminated aquifer. Environ. Sci. Technol. 34 (11) 2261-2266. https://doi.org/10.1021/es991211a [ Links ]
ANDREONI V and GIANFREDA L (2007) Bioremediation and monitoring of aromatic-polluted habitats. Appl. Microbiol. Biotechnol. 76 (2) 287-308. https://doi.org/10.1007/s00253-007-1018-5 [ Links ]
ARAMBARRI I, LASA M, GARCIA R and MILLÁN E (2004) Determination of fuel dialkyl ethers and BTEX in water using headspace solid-phase microextraction and gas chromatography-flame ionization detection. J. Chromatogr. A 1033 (2) 193-203. https://doi.org/10.1016/j.chroma.2004.01.046 [ Links ]
BACELO HA, SANTOS SC and BOTELHO CM (2016) Tannin-based biosorbents for environmental applications - a review. Chem. Eng. J. 303 575-587. https://doi.org/10.1016/j.cej.2016.06.044 [ Links ]
BARUAH S, KHAN MN and DUTTA J (2015) Nanotechnology in water treatment. Pollut. Buildings, Water Living Organisms 7 51-84. https://doi.org/10.1007/978-3-319-19276-5_2 [ Links ]
BEKINS BA, COZARELLI IM, GODSY EM, WARREN E, ESSAID HI and TUCCILLO M E (2001) Progression of natural attenuation processes at a crude oil spill site: II. Controls on spatial distribution of microbial populations. J. Contam. Hydrol. 53 (3) 387-406. https://doi.org/10.1016/S0169-7722(01)00175-9 [ Links ]
BOJES HK and POPE PG (2007) Characterization of EPA's 16 priority pollutant polycyclic aromatic hydrocarbons (PAHs) in tank bottom solids and associated contaminated soils at oil exploration and production sites in Texas. Regul. Toxicol. Pharmacol. 47 (3) 288-295. https://doi.org/10.1016/j.yrtph.2006.11.007 [ Links ]
BOONSANER M, BORRIUKWISITSAK S and BOONSANER A (2011) Phytoremediation of BTEX contaminated soil by Canna× generalis. Ecotoxicol. Environ. Saf. 74 (6) 1700-1707. https://doi.org/10.1016/j.ecoenv.2011.04.011 [ Links ]
BOWLEN GF, KOSSON DS and YOUNG L (1995) In situ processes for bioremediation of BTEX and petroleum fuel products. In Young LY and Cerniglia CE (ed.) Microbial Transformations and Degradation of Toxic Organic Chemicals. Wiley-Lis Inc., New York. 515-542. [ Links ]
BRUNA F, CELIS R, REAL M and CORNEJO J (2012) Organo/LDH nanocomposite as an adsorbent of polycyclic aromatic hydrocarbons in water and soil-water systems. J. Hazardous Mater.225 74-80. https://doi.org/10.1016/j.jhazmat.2012.04.064 [ Links ]
BUNNETT JF, DRAPER JR F, RYASON PR, NOBLE JR P, TONKYN RG and ZAHLER RE (1953) Comparative activation of nucleophilic substitution in 4-substituted-2-nitrochlorobenzenes. J. Am. Chem. Soc. 75 (3) 642-645. https://doi.org/10.1021/ja01099a036 [ Links ]
CAMILLI R, REDDY CM, YOERGER DR, VAN MOOY BA, JAKUBA MV, KINSEY JC, MCINTYRE CP, SYLVA SP and MALONEY JV (2010) Tracking hydrocarbon plume transport and biodegradation at Deepwater Horizon. Science 330 (6001) 201-204. https://doi.org/10.1126/science.1195223 [ Links ]
CARMODY O, FROST R, XI Y and KOKOT S (2007) Adsorption of hydrocarbons on organo-clays - implications for oil spill remediation. J. Colloid Interface Sci. 305 (1) 17-24. https://doi.org/10.1016/j.jcis.2006.09.032 [ Links ]
CASTILLO M, OUBIÑA A and BARCELÓ D (1998) Evaluation of ELISA kits followed by liquid chromatography-atmospheric pressure chemical ionization-mass spectrometry for the determination of organic pollutants in industrial effluents. Environ. Sci. Technol. 32 (14) 2180-2184. https://doi.org/10.1021/es971042z [ Links ]
CHAPELLE FH (1999) Bioremediation of petroleum hydrocarbon‐contaminated ground water: the perspectives of history and hydrology. Groundwater 37 (1) 122-132. https://doi.org/10.1111/j.1745-6584.1999.tb00965.x [ Links ]
CHAPMAN SW, BYERLEY BT, SMYTH DJ and MACKAY DM (1997) A pilot test of passive oxygen release for enhancement of in situ bioremediation of BTEX‐contaminated ground water. Groundwater Monit. Remed. 17 (2) 93-105. https://doi.org/10.1111/j.1745-6592.1997.tb01282.x [ Links ]
CHEN W, DUAN L and ZHU D (2007) Adsorption of polar and nonpolar organic chemicals to carbon nanotubes. Environ. Sci. Technol. 41 (24) 8295-8300. https://doi.org/10.1021/es071230h [ Links ]
CHEN W, DUAN L, WANG L and ZHU D (2008) Adsorption of hydroxyl-and amino-substituted aromatics to carbon nanotubes. Environ. Sci. Technol. 42 (18) 6862-6868. https://doi.org/10.1021/es8013612 [ Links ]
CHIRIAC R, CARRE J, PERRODIN Y, FINE L and LETOFFE JM (2007) Characterisation of VOCs emitted by open cells receiving municipal solid waste. J. Hazardous Mater. 149 (2) 249-263. https://doi.org/10.1016/j.jhazmat.2007.07.094 [ Links ]
CHIRIAC R, CARRE J, PERRODIN Y, FINE L and LETOFFE JM (2007) Characterisation of VOCs emitted by open cells receiving municipal solid waste. J. Hazardous Mater. 149 (2) 249-263. https://doi.org/10.1016/j.jhazmat.2007.07.094 [ Links ]
CHIRWA EM, MAMPHOLO T and FAYEMIWO O (2013) Biosurfactants as demulsifying agents for oil recovery from oily sludge - performance evaluation. Water Sci. Technol. 67 (12) 2875-2881. https://doi.org/10.2166/wst.2013.207 [ Links ]
CHO JS, WILSON JT, DIGIULIO DC, VARDY JA and CHOI W (1997) Implementation of natural attenuation at a JP-4 jet fuel release after active remediation. Biodegradation 8 (4) 265-273. https://doi.org/10.1023/A:1008212127604 [ Links ]
CHU Y and PAN Q (2012) Three-dimensionally macroporous Fe/C nanocomposites as highly selective oil-absorption materials. ACS Appl. Mater. Interfaces4 (5) 2420-2425. https://doi.org/10.1021/am3000825 [ Links ]
COSTA AS, ROMĂO LPC, ARAUJO BR, LUCAS SCO, MACIEL STA, WISNIEWSKI A and ALEXANDRE MDR (2012) Environmental strategies to remove volatile aromatic fractions (BTEX) from petroleum industry wastewater using biomass. Bioresour. Technol. 105 31-39. https://doi.org/10.1016/j.biortech.2011.11.096 [ Links ]
COZZARELLI IM, BEKINS BA, BAEDECKER MJ, AIKEN GR, EGANJOUSE RP and TUCCILLO ME (2001) Progression of natural attenuation processes at a crude-oil spill site: I. Geochemical evolution of the plume. J. Contam. Hydrol. 53 (3) 369-385. https://doi.org/10.1016/S0169-7722(01)00174-7 [ Links ]
CROCK CA, ROGENSUES AR, SHAN W and TARABARA VV (2013) Polymer nanocomposites with graphene-based hierarchical fillers as materials for multifunctional water treatment membranes. Water Res. 47 (12) 3984-3996. https://doi.org/10.1016/j.watres.2012.10.057 [ Links ]
CUI Y, ZHAO Y, TIAN Y, ZHANG W, LU X and JIANG X (2012) The molecular mechanism of action of bactericidal gold nanoparticles on Escherichia coli. Biomaterials 33 (7) 2327-2333. https://doi.org/10.1016/j.biomaterials.2011.11.057 [ Links ]
CUNNINGHAM JA, RAHME H, HOPKINS GD, LEBRON C and REINHARD M (2001) Enhanced in situ bioremediation of BTEX-contaminated groundwater by combined injection of nitrate and sulfate. Environ. Sci. Technol. 35 (8) 1663-1670. https://doi.org/10.1021/es001722t [ Links ]
DA SILVA ML and ALVAREZ PJ (2002) Effects of ethanol versus MTBE on benzene, toluene, ethylbenzene, and xylene natural attenuation in aquifer columns. J. Environ. Eng. 128 (9) 862--67. https://doi.org/10.1061/(ASCE)0733-9372(2002)128:9(862) [ Links ]
DAS N and CHANDRAN P (2011) Microbial degradation of petroleum hydrocarbon contaminants: an overview. Biotechnol. Res. Int. 2011 1-13. https://doi.org/10.4061/2011/941810 [ Links ]
DAVIS GB, BARBER C, POWER TR, THIERRIN J, PATTERSON BM, RAYNER JL and WU Q (1999) The variability and intrinsic remediation of a BTEX plume in anaerobic sulphate-rich groundwater. J. Contam. Hydrol. 36 (3) 265-290. https://doi.org/10.1016/S0169-7722(98)00148-X [ Links ]
DE NARDI IR, RIBEIRO R, ZAIAT M and FORESTI E (2005) Anaerobic packed-bed reactor for bioremediation of gasoline-contaminated aquifers. Process Biochem. 40 (2) 587-592. https://doi.org/10.1016/j.procbio.2004.01.035 [ Links ]
DE VOLDER MF, TAWFICK SH, BAUGHMAN RH and HART AJ (2013) Carbon nanotubes: present and future commercial applications. Science 339 (6119) 535-539. https://doi.org/10.1126/science.1222453 [ Links ]
DELILLE D and PELLETIER E (2002) Natural attenuation of diesel-oil contamination in a subantarctic soil (Crozet Island). Polar Biol. 25 (9) 682-687. [ Links ]
DELZER GC, ZOGORSKI JS, LOPES TJ and BOSSHART RL (1996) Occurrence of the gasoline oxygenate MTBE and BTEX compounds in urban stormwater in the United States, 1991-95. USGS Water-Resources Investigations Report No. 96-4145, United States Geological Survey, South Dakota. [ Links ]
DÓREA HS, BISPO JR, ARAGĂO KA, CUNHA BB, NAVICKIENE S, ALVES JP, ROMÁO LP and GARCIA CA (2007) Analysis of BTEX, PAHs and metals in the oilfield produced water in the State of Sergipe, Brazil. Microchem. J. 85 (2) 234-238. https://doi.org/10.1016/j.microc.2006.06.002 [ Links ]
DUTTA C, SOM D, CHATTERJEE A, MUKHERJEE AK, JANA TK and SEN S (2009) Mixing ratios of carbonyls and BTEX in ambient air of Kolkata, India and their associated health risk. Environ. Monit. Assess. 148 (1-4) 97-107. https://doi.org/10.1007/s10661-007-0142-0 [ Links ]
EDWARDS M (2004) Controlling corrosion in drinking water distribution systems: a grand challenge for the 21st century. Water Sci. Technol. 49 (2) 1-8. [ Links ]
EL SISSI HI, SALEH NAM, EL SHERBEINY AE and EL ANSARY MAI (1965) Local plants as potential sources of tannins and the isolation of their free and combined sugars. Qual. Plant. Mater. Veg. 12 (3) 262-268. https://doi.org/10.1007/BF01105143 [ Links ]
ELÍAS AL, BOTELLO-MÉNDEZ AR, MENESES-RODRÍGUEZ D, JEHOVÁ-GONZÁLEZ V, RAMÍREZ-GONZÁLEZ D, CI L, MUNOZ-SANDOVAL E, AJAYAN PM, TERRONES H and TERRONES M (2009) Longitudinal cutting of pure and doped carbon nanotubes to form graphitic nanoribbons using metal clusters as nanoscalpels. Nano Lett. 10 (2) 366-372. https://doi.org/10.1021/nl901631z [ Links ]
EL-NAAS MH, ACIO JA and ET-TELIB AE (2014) Aerobic biodegradation of BTEX: Progresses and prospects. J. Environ. Chem. Eng. 2 1104-1122. https://doi.org/10.1016/j.jece.2014.04.009 [ Links ]
ESSAID HI, COZZARELLI IM, EGANHOUSE RP, HERKELRATH WN, BEKINS BA and DELIN GN (2003) Inverse modeling of BTEX dissolution and biodegradation at the Bemidji, MN crude-oil spill site. J. Contam. Hydrol. 67 (1) 269-299. https://doi.org/10.1016/S0169-7722(03)00034-2 [ Links ]
FARHADIAN M, VACHELARD C, DUCHEZ D and LARROCHE C (2008) In situ bioremediation of monoaromatic pollutants in groundwater: a review. Bioresour. Technol. 99 (13) 5296-5308. https://doi.org/10.1016/j.biortech.2007.10.025 [ Links ]
FEIFANG F, JIN CHOI H and JOO J (2008) Conducting polymer/clay nanocomposites and their applications. J. Nanosci. Nanotechnol. 8 (4) 1559-1581. https://doi.org/10.1166/jnn.2008.036 [ Links ]
FONTENOT BE, HUNT LR, HILDENBRAND ZL, CARLTON JR DD, OKA H, WALTON JL, HOPKINS D, OSPRIO A, BJORNDAL B, HU QH and SCHUG KA (2013) An evaluation of water quality in private drinking water wells near natural gas extraction sites in the Barnett Shale Formation. Environ. Sci. Technol. 47 (17) 10032-10040. https://doi.org/10.1021/es4011724 [ Links ]
GIL MI, TOMÁS-BARBERÁN FA, HESS-PIERCE B, HOLCROFT DM and KADER AA (2000) Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J. Agric. Food Chem. 48 (10) 4581-4589. https://doi.org/10.1021/jf000404a [ Links ]
GOSS MJ, BARRY DAJ and RUDOLPH DL (1998) Contamination in Ontario farmstead domestic wells and its association with agriculture: Results from drinking water wells. J. Contam. Hydrol. 32 (3) 267-293. https://doi.org/10.1016/S0169-7722(98)00054-0 [ Links ]
GOYAL AS, JOHAL E and RATH G (2011) Nanotechnology for water treatment. Current Nanosci. 7 (4) 640-654. https://doi.org/10.2174/157341311796196772 [ Links ]
GRADY SJ and CASEY GD (2001) Occurrence and distribution of methyl tert-butyl ether and other volatile organic compounds in drinking water in the Northeast and Mid-Atlantic regions of the United States. 1993-98, National water quality Assessment Program, National Synthesis on Volatile Organic Compounds: Water Resources Investigation Report, 00-4228. United States Geological Survey, Denver. [ Links ]
GRAY SN (1998) Fungi as potential bioremediation agents in soil contaminated with heavy or radioactive metals. Biochem. Soc. Trans. 26 (4) 666-670. https://doi.org/10.1042/bst0260666 [ Links ]
GROSS S, AVENS HJ, BANDUCCI AM, SAHMEL J, PANKO JM and TVERMOES BE (2013) Analysis of BTEX groundwater concentrations from surface spills associated with hydraulic fracturing operations. J. Air Waste Manage. Assoc. 63 (4) 424-432. https://doi.org/10.1080/10962247.2012.759166 [ Links ]
GUPTA VK, AGARWAL S and SALEH TA (2011) Chromium removal by combining the magnetic properties of iron oxide with adsorption properties of carbon nanotubes. Water Res. 45 (6) 2207-2212. https://doi.org/10.1016/j.watres.2011.01.012 [ Links ]
HAGERMAN AE, RIEDL KM, JONES GA, SOVIK KN, RITCHARD NT, HARTZFELD PW and RIECHEL TL (1998) High molecular weight plant polyphenolics (tannins) as biological antioxidants. J. Agric. Food Chem. 46 (5) 1887-1892. https://doi.org/10.1021/jf970975b [ Links ]
HAN TH, LEE Y, CHOI MR, WOO SH, BAE SH, HONG BH, AHN JH and LEE TW (2012) Extremely efficient flexible organic light-emitting diodes with modified graphene anode. Nature Photonics 6 (2) 105-110. https://doi.org/10.1038/nphoton.2011.318 [ Links ]
HEAD IM (1998) Bioremediation: towards a credible technology. Microbiology 144 (3) 599-608. https://doi.org/10.1099/00221287-144-3-599 [ Links ]
HUMPLIK T, LEE J, O'HERN SC, FELLMAN BA, BAIG MA, HASSAN SF, ATIEH MA, RAHMAN F, LAOUI T, KARNIK R and WANG EN (2011) Nanostructured materials for water desalination. Nanotechnology 22 (29) 292001. https://doi.org/10.1088/0957-4484/22/29/292001 [ Links ]
HWANG ET, LEE JH, CHAE YJ, KIM YS, KIM BC, SANG BI and GU MB (2008) Analysis of the toxic mode of action of silver nanoparticles using stress‐specific bioluminescent bacteria. Small 4 (6) 746-750. https://doi.org/10.1002/smll.200700954 [ Links ]
JIA G, WANG H, YAN L, WANG X, PEI R, YAN T, ZHAO Y and GUO X (2005) Cytotoxicity of carbon nanomaterials: single-wall nanotube, multi-wall nanotube, and fullerene. Environ. Sci. Technol.39 (5) 1378-1383. https://doi.org/10.1021/es048729l [ Links ]
JIAO L, ZHANG L, WANG X, DIANKOV G and DAI H (2009) Narrow graphene nanoribbons from carbon nanotubes. Nature 458 (7240) 877-880. https://doi.org/10.1038/nature07919 [ Links ]
JIN HM, CHOI EJ and JEON CO (2013) Isolation of a BTEX-degrading bacterium, Janibacter sp. SB2, from a sea-tidal flat and optimization of biodegradation conditions. Bioresour. Technol. 145 57-64. https://doi.org/10.1016/j.biortech.2013.02.004 [ Links ]
JIN Z, WANG X, SUN Y, AI Y and WANG X (2015) Adsorption of 4-n-nonylphenol and bisphenol-A on magnetic reduced graphene oxides: a combined experimental and theoretical studies. Environ. Sci. Technol. 49 (15) 9168-9175. https://doi.org/10.1021/acs.est.5b02022 [ Links ]
JONES K, BOXALL C, MCCABE R, SHAW D and BUCK M (2007) Nanocomposites for water treatment. ECS Trans. 6 (9) 17-27. https://doi.org/10.1149/1.2790398 [ Links ]
KANCHI S (2014) Nanotechnology for water treatment. J. Environ. Anal. Chem. 1 (2) 1-3. https://doi.org/10.4172/2380-2391.1000e102 [ Links ]
KAO CM and PROSSER J (2001) Evaluation of natural attenuation rate at a gasoline spill site. J. Hazardous Mater. 82 (3) 275-289. https://doi.org/10.1016/S0304-3894(00)00361-7 [ Links ]
KAO CM and WANG CC (2000) Control of BTEX migration by intrinsic bioremediation at a gasoline spill site. Water Res. 34 (13) 3413-3423. https://doi.org/10.1016/S0043-1354(00)00070-1 [ Links ]
KAO CM, HUANG WY, CHANG LJ, CHEN TY, CHIEN HY and HOU F (2006) Application of monitored natural attenuation to remediate a petroleum-hydrocarbon spill site. Water Sci. Technol. 53 (2) 321-328. https://doi.org/10.2166/wst.2006.066 [ Links ]
KELLEY CA, HAMMER BT and COFFIN RB (1997) Concentrations and stable isotope values of BTEX in gasoline-contaminated groundwater. Environ. Sci. Technol. 31 (9) 2469-2472. https://doi.org/10.1021/es960635r [ Links ]
KOSYNKIN DV, HIGGINBOTHAM AL, SINITSKII A, LOMEDA JR, DIMIEV A, PRICE BK and TOUR JM (2009) Longitudinal unzipping of carbon nanotubes to form graphene nanoribbons Nature 458 (7240) 872-876. https://doi.org/10.1038/nature07872 [ Links ]
LANGENHOFF AA, ZEHNDER AJ and SCHRAA G (1996) Behaviour of toluene, benzene and naphthalene under anaerobic conditions in sediment columns. Biodegradation 7 (3) 267-274. https://doi.org/10.1007/BF00058186 [ Links ]
LEUSCH F and BARTKOW M (2010) A short primer on benzene, toluene, ethylbenzene and xylenes (BTEX) in the environment and in hydraulic fracturing fluids. Smart Water Research Centre, Griffith University. URL: https://www.ehp.qld.gov.au/management/coal-seam-gas/pdf/btex-report.pdf. (Accessed 10 October 2017). [ Links ]
LI Q, MAHENDRA S, LYON DY, BRUNET L, LIGA MV, LI D and ALVAREZ PJ (2008) Antimicrobial nanomaterials for water disinfection and microbial control: potential applications and implications. Water Res. 42 (18) 4591-4602. https://doi.org/10.1016/j.watres.2008.08.015 [ Links ]
LI Z, HULBERMAN T, SALEMN R, CHAPMAN R, LEONARD SS, YOUNG SH, SHVEDOVA A, LUSTER MI and SIMEONOVA PP (2007) Cardiovascular effects of pulmonary exposure to single-wall carbon nanotubes. Environ. Health Perspect. 115 (3) 377-382. https://doi.org/10.1289/ehp.9688 [ Links ]
LOTTFINNASABASL S, GUNALE VR and RAJURKAR NS (2013) Petroleum hydrocarbons pollution in soil and its bioaccumulation in mangrove species, Avicennia marina from Alibaug mangrove ecosystem, Maharashtra, India. Int. J. Adv. Res. Sci. Technol. 2 (2) 1-7. [ Links ]
LOVLEY DR (1997) Microbial Fe (III) reduction in subsurface environments. FEMS Microbiol. Rev. 20 (3-4) 305-313. https://doi.org/10.1111/j.1574-6976.1997.tb00316.x [ Links ]
LU C, CHUNG YL and CHANG KF (2005) Adsorption of trihalomethanes from water with carbon nanotubes. Water Res. 39 (6) 1183-1189. https://doi.org/10.1016/j.watres.2004.12.033 [ Links ]
LU G, CLEMENT TP, ZHENG C and WIEDEMEIER TH (1999) Natural attenuation of BTEX compounds: Model development and field‐scale application. Ground Water 37 (5) 707-717. https://doi.org/10.1111/j.1745-6584.1999.tb01163.x [ Links ]
MACKAY DM, DE SIEYES NR, EINARSON MD, FERIS KP, PAPPAS AA, WOOD IA, JACOBSON L, JUSTICE LG, NOSKE MN, SCOW KM and WILON JT (2006) Impact of ethanol on the natural attenuation of benzene, toluene, and o-xylene in a normally sulfate-reducing aquifer. Environ. Sci. Technol. 40 (19) 6123-6130. https://doi.org/10.1021/es060505a [ Links ]
MAHMOODI NM, ARAMI M, LIMAEE NY and GHARANJIG K (2007) Photocatalytic degradation of agricultural N-heterocyclic organic pollutants using immobilized nanoparticles of titania. J. Hazardous Mater. 145 (1) 65-71. https://doi.org/10.1016/j.jhazmat.2006.10.089 [ Links ]
MAPHUTHA S, MOOTHI K, MEYYAPPAN M and IYUKE SE (2013) A carbon nanotube-infused polysulfone membrane with polyvinyl alcohol layer for treating oil-containing waste water. Nat. Sci. Rep.3 (1509) 1-6. https://doi.org/10.1038/srep01509 [ Links ]
MARGESIN R, WALDER G and SCHINNER F (2003) Bioremediation assessment of a BTEX‐contaminated soil. Acta Biotechnol. 23 (1) 29-36. https://doi.org/10.1002/abio.200390004 [ Links ]
MAZZEO DEC, LEVY CE, DE ANGELIS DDF and MARIN-MORALES MA (2010) BTEX biodegradation by bacteria from effluents of petroleum refinery. Sci. Total Environ. 408 (20) 4334-4340. https://doi.org/10.1016/j.scitotenv.2010.07.004 [ Links ]
MEIDL JA (1997) Responding to changing conditions: how powdered activated carbon systems can provide the operational flexibility necessary to treat contaminated groundwater and industrial wastes. Carbon 35 (9) 1207-1216. https://doi.org/10.1016/S0008-6223(97)00093-6 [ Links ]
MÉNESI J, KÖRÖSI L, BAZSÓ É, ZÖLLMER V, RICHARDT A and DÉKÁNY I (2008) Photocatalytic oxidation of organic pollutants on titania-clay composites. Chemosphere 70 (3) 538-542. https://doi.org/10.1016/j.chemosphere.2007.06.049 [ Links ]
MENICONI MDF, GABARDO IT, CARNEIRO MER, BARBANTI SM, DA SILVA GC and MASSONNE CG (2002) Brazilian oil spills chemical characterization-case studies. Environ. Forensics 3 (3-4) 303-321. https://doi.org/10.1080/713848377 [ Links ]
MISHRA AK and RAMAPRABHU S (2011) Functionalized graphene sheets for arsenic removal and desalination of sea water. Desalination 282 39-45. https://doi.org/10.1016/j.desal.2011.01.038 [ Links ]
MITRA S and ROY P (2011) BTEX: A serious ground-water contaminant. Res. J. Environ. Sci. 5 (5) 394-398. https://doi.org/10.3923/rjes.2011.394.398 [ Links ]
MOOTHI K, IYUKE SE, MEYYAPPAN M and FALCON R (2012) Coal as a carbon source for carbon nanotube synthesis. Carbon 50 2679-2690. https://doi.org/10.1016/j.carbon.2012.02.048 [ Links ]
MOOTHI K, SIMATE GS, FALCON R, IYUKE SE and MEYYAPPAN M (2015) Carbon nanotube synthesis using coal pyrolysis. Langmuir 31 9464-9472. https://doi.org/10.1021/acs.langmuir.5b01894 [ Links ]
MORONES JR, ELECHIGUERRA JL, CAMACHO A, HOLT K, KOURI JB, RAMÍREZ JT and YACAMAN MJ (2005) The bactericidal effect of silver nanoparticles. Nanotechnology 16 (10) 2346. https://doi.org/10.1088/0957-4484/16/10/059 [ Links ]
MÜLLER NC and NOWACK B (2010) Nano zero valent iron - the solution for water and soil remediation. ObservatoryNANO focus report 2010. URL: www.observatorynano.eu. 34 pp. [ Links ]
MULLIGAN CN and YONG RN (2004) Natural attenuation of contaminated soils. Environ. Int. 30 (4) 587-601. https://doi.org/10.1016/j.envint.2003.11.001 [ Links ]
NAKANO Y, TAKESHITA K and TSUTSUMI T (2001) Adsorption mechanism of hexavalent chromium by redox within condensed-tannin gel. Water Res. 35 (2) 496-500. https://doi.org/10.1016/S0043-1354(00)00279-7 [ Links ]
NEFF JM (2002) Bioaccumulation in Marine Organisms: Effect of Contaminants from Oil Well Produced Water (1st edn). Elsevier Publishing, Amsterdam. [ Links ]
NEFF JM and SAUER JR TC (2012) Bioaccumulation and trophic transfer in marine food webs. In: Produced Water 2: Environmental Issues and Mitigation Technologies (1st edn). Plenum Press, New York. [ Links ]
NHMRC (National Health and Medical Research Council, Australia) (2011) Australian Drinking Water Guidelines Paper 6 National Water Quality Management Strategy. National Health and Medical Research Council, National Resource Management Ministerial Council, Commonwealth of Australia, Canberra. [ Links ]
NOSAKA Y, MATSUSHITA M, NISHINO J and NOSAKA AY (2005) Nitrogen-doped titanium dioxide photocatalysts for visible response prepared by using organic compounds. Sci. Technol. Adv. Mater. 6 (2) 143-148. https://doi.org/10.1016/j.stam.2004.11.006 [ Links ]
OGATA T, MORISADA S, OINUMA Y, SEIDA Y and NAKANO Y (2011) Preparation of adsorbent for phosphate recovery from aqueous solutions based on condensed tannin gel. J. Hazardous Mater. 192 (2) 698-703. https://doi.org/10.1016/j.jhazmat.2011.05.073 [ Links ]
ORLOV A, CHAN MS, JEFFERSON DA, ZHOU D, LYNCH RJ and LAMBERT RM (2006) Photocatalytic degradation of water-soluble organic pollutants on TiO2 modified with gold nanoparticles. Environ. Technol. 27 (7) 747-752. https://doi.org/10.1080/09593332708618685 [ Links ]
PAL S, TAK YK and SONG JM (2007) Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia coli. Appl. Environ. Microbiol. 73 (6) 1712-1720. https://doi.org/10.1128/AEM.02218-06 [ Links ]
PAN B and XING B (2008) Adsorption mechanisms of organic chemicals on carbon nanotubes. Environ. Sci. Technol. 42 (24) 9005-9013. https://doi.org/10.1021/es801777n [ Links ]
PATEL HA, SOMANI RS, BAJAJ HC and JASRA RV (2006) Nanoclays for polymer nanocomposites, paints, inks, greases and cosmetics formulations, drug delivery vehicle and waste water treatment. Bull. Mater. Sci. 29 (2) 133-145. https://doi.org/10.1007/BF02704606 [ Links ]
PENDERGAST MM and HOEK EM (2011) A review of water treatment membrane nanotechnologies. Energ. Environ. Sci. 4 (6) 1946-1971. https://doi.org/10.1039/c0ee00541j [ Links ]
PENG X, LI Y, LUAN Z, DI Z, WANG H, TIAN B and JIA Z (2003) Adsorption of 1, 2-dichlorobenzene from water to carbon nanotubes. Chem. Phys. Lett. 376 (1) 154-158. https://doi.org/10.1016/S0009-2614(03)00960-6 [ Links ]
PING L, BROSSE N, CHRUSCIEL L, NAVARETTE P and PIZZI A (2011) Extraction of condensed tannins from grape pomace for use as wood adhesives. Ind. Crops Products 33 (1) 253-257. https://doi.org/10.1016/j.indcrop.2010.10.007 [ Links ]
PING L, PIZZI A, GUO ZD and BROSSE N (2012) Condensed tannins from grape pomace: characterization by FTIR and MALDI TOF and production of environment friendly wood adhesive. Ind. Crops Products 40 13-20. https://doi.org/10.1016/j.indcrop.2012.02.039 [ Links ]
POULSEN M, LEMON L and BARKER JF (1992) Dissolution of monoaromatic hydrocarbons into groundwater from gasoline-oxygenate mixtures. Environ. Sci. Technol. 26 (12) 2483-2489. https://doi.org/10.1021/es00036a022 [ Links ]
PRENAFETA-BOLDÚ FX, BALLERSTEDT H, GERRITSE J and GROTENHUIS JTC (2004) Bioremediation of BTEX hydrocarbons: effect of soil inoculation with the toluene-growing fungus Cladophialophora sp. strain T1. Biodegradation 15 (1) 59-65. https://doi.org/10.1023/B:BIOD.0000009973.53531.96 [ Links ]
PRUDEN A, SEDRAN M, SUIDAN M and VENOSA A (2003) Biodegradation of MTBE and BTEX in an aerobic fluidized bed reactor. Water Sci. Technol. 47 (9) 123-128. [ Links ]
QU X, ALVAREZ PJ and LI Q (2013) Applications of nanotechnology in water and wastewater treatment. Water Res. 47 (12) 3931-3946. https://doi.org/10.1016/j.watres.2012.09.058 [ Links ]
RAI M, YADAV A and GADE A (2009) Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 27 (1) 76-83. https://doi.org/10.1016/j.biotechadv.2008.09.002 [ Links ]
RANCK JM, BOWMAN RS, WEEBER JL, KATZ LE and SULLIVAN EJ (2005) BTEX removal from produced water using surfactant-modified zeolite. J. Environ. Eng. 131 (3) 434-442. https://doi.org/10.1061/(ASCE)0733-9372(2005)131:3(434) [ Links ]
REDDY CM, AREY JS, SEEWALD JS, SYLVA SP, LEMKAU KL, NELSON RK, CARMICHAEL CA, MCINTYRE CP, FENWICK J, VENTURA GT and VAN MOOY BA (2012) Composition and fate of gas and oil released to the water column during the Deepwater Horizon oil spill. Proc. Natl Acad. Sci. 109 (50) 20229-20234. https://doi.org/10.1073/pnas.1101242108 [ Links ]
ROBINSON HD, KNOX K, BONE BD and PICKEN A (2005) Leachate quality from landfilled MBT waste. Waste Manage. 25 (4) 383-391. https://doi.org/10.1016/j.wasman.2005.02.003 [ Links ]
ROCO MC (2011) The long view of nanotechnology development: the National Nanotechnology Initiative at 10 years. J. Nanoparticle Res. 13 (2) 427-445. https://doi.org/10.1007/s11051-010-0192-z [ Links ]
RÖLING WF and VAN VERSEVELD HW (2002) Natural attenuation: what does the subsurface have in store? Biodegradation 13 (1) 53-64. https://doi.org/10.1023/A:1016310519957 [ Links ]
SCALBERT A (1991) Antimicrobial properties of tannins. Phytochemistry 30 (12) 3875-3883. https://doi.org/10.1016/0031-9422(91)83426-L [ Links ]
SCHMIDT TC, HADERLEIN SB, PFISTER R and FORSTER R (2004) Occurrence and fate modelling of MTBE and BTEX compounds in a Swiss Lake used as drinking water supply. Water Res. 38 (6) 1520-1529. https://doi.org/10.1016/j.watres.2003.12.027 [ Links ]
SCHREIBER ME and BAHR JM (2002) Nitrate-enhanced bioremediation of BTEX-contaminated groundwater: parameter estimation from natural-gradient tracer experiments. J. Contam. Hydrol. 55 (1) 29-56. https://doi.org/10.1016/S0169-7722(01)00184-X [ Links ]
SEAGREN EA and BECKER JG (2002) Review of natural attenuation of BTEX and MTBE in groundwater. Pract. Period. Hazardous, Toxic Radioactive Waste Manage. 6 (3) 156-172. https://doi.org/10.1061/(ASCE)1090-025X(2002)6:3(156) [ Links ]
SERANNO A and GALLEGO M (2004) Direct screening and confirmation of benzene, toluene, ethylbenzene and xylenes in water. J. Chromatogr. A1045 (1) 181-188. https://doi.org/10.1016/j.chroma.2004.06.028 [ Links ]
SERRANO A, GALLEGO M and SILVA M (2007) Enhancing sensitivity in headspace-mass spectrometric determination of BTEX in drinking water. Anal. Chem. 79 (7) 2997-3002. https://doi.org/10.1021/ac070044r [ Links ]
SHAWKY HA, CHAE SR, LIN S and WIESNER MR (2011) Synthesis and characterization of a carbon nanotube/polymer nanocomposite membrane for water treatment. Desalination 272 (1) 46-50. https://doi.org/10.1016/j.desal.2010.12.051 [ Links ]
SHIH YH, HSU CY and SU YF (2011) Reduction of hexachlorobenzene by nanoscale zero-valent iron: kinetics, pH effect, and degradation mechanism. Sep. Purif. Technol. 76 (3) 268-274. https://doi.org/10.1016/j.seppur.2010.10.015 [ Links ]
SIMATE GS, IYUKE SE, NDLOVU S, HEYDENRYCH M and WALUBITA LF (2012) Human health effects of residual carbon nanotubes and traditional water treatment chemicals in drinking water. Environ. Int. 39 (1) 38-49. https://doi.org/10.1016/j.envint.2011.09.006 [ Links ]
SLACK RJ, GRONOW JR and VOULVOULIS N (2005) Household hazardous waste in municipal landfills: contaminants in leachate. Sci. Total Environ. 337 119-137. https://doi.org/10.1016/j.scitotenv.2004.07.002 [ Links ]
SMART SK, CASSADY AI, LU GQ and MARTIN DJ (2006) The biocompatibility of carbon nanotubes. Carbon 44 (6) 1034-1047. https://doi.org/10.1016/j.carbon.2005.10.011 [ Links ]
SOUQUET JM, LABARBE B, LE GUERNEVÉ C, CHEYNIER V and MOUTOUNET M (2000) Phenolic composition of grape stems. J. Agric. Food Chem. 48 (4) 1076-1080. https://doi.org/10.1021/jf991171u [ Links ]
SU F, LU C and HU S (2010) Adsorption of benzene, toluene, ethylbenzene and p-xylene by NaOCl-oxidized carbon nanotubes. Colloids Surf. A: Physicochem. Eng. Aspects53 (1) 83-91. https://doi.org/10.1016/j.colsurfa.2009.10.025 [ Links ]
SUAREZ MP and RIFAI HS (2002) Evaluation of BTEX remediation by natural attenuation at a coastal facility. Groundwater Monit. Remediation 22 (1) 62-77. https://doi.org/10.1111/j.1745-6592.2002.tb00655.x [ Links ]
SUN Y, YANG S, ZHAO G, WANG Q and WANG X (2013) Adsorption of polycyclic aromatic hydrocarbons on graphene oxides and reduced graphene oxides. Chemistry - An Asian Journal 8 (11) 2755-2761. https://doi.org/10.1002/asia.201300496 [ Links ]
THACKER BK and FORD CG (1999) In situ bioremediation technique for sites underlain by silt and clay. J. Environ. Eng. 125 (12) 1169-1172. https://doi.org/10.1061/(ASCE)0733-9372(1999)125:12(1169) [ Links ]
THERON J, WALKER JA and CLOETE TE (2008) Nanotechnology and water treatment: applications and emerging opportunities. Crit. Rev. Microbiol. 34 (1) 43-69. https://doi.org/10.1080/10408410701710442 [ Links ]
TIWARI DK, BEHARI J and SEN P (2008) Application of nanoparticles in waste water treatment 1. World Appl. Sci. J. 3 (3) 417-433. [ Links ]
TRONCZYŃSKI J, MUNSCHY C, HÉAS-MOISAN K, GUIOT N, TRUQUET I, OLIVIER N, MEN S and FURAUT A (2004) Contamination of the Bay of Biscay by polycyclic aromatic hydrocarbons (PAHs) following the T/V "Erika" oil spill. Aquat. Living Resour. 17 (3) 243-259. https://doi.org/10.1051/alr:2004042 [ Links ]
TUNSARINGKARN T, SIRIWONG W, RUNGSIYOTHIN A and NOPPARABUNDIT S (2012) Occupational exposure of gasoline station workers to BTEX compounds in Bangkok, Thailand. Int. J. Occup. Environ. Med. 3 117-125. [ Links ]
VAN BUREN JP and ROSINSON WB (1969) Formation of complexes between protein and tannic acid. J. Agric. Food Chem. 17 (4) 772-777. https://doi.org/10.1021/jf60164a003 [ Links ]
VEIL JA, PUDER MG, ELCOCK D and REDWEIK JR RJ (2004) A white paper describing produced water from production of crude oil, natural gas, and coal bed methane. Technical report prepared for US Department of Energy National Energy Technology Laboratory under Contract W-31-109-Eng-38. Argonne National Laboratory, Illinois. [ Links ]
VIDALI M (2001) Bioremediation: an overview. Pure Appl. Chem. 73 (7) 1163-1172. https://doi.org/10.1351/pac200173071163 [ Links ]
WALLACE S and KADLEC R (2005) BTEX degradation in a cold-climate wetland system. Water Sci. Technol. 51 (9) 165-171. [ Links ]
WANG J, CHEN Z and CHEN B (2014) Adsorption of polycyclic aromatic hydrocarbons by graphene and graphene oxide nanosheets. Environ. Sci. Technol. 48 (9) 4817-4825. https://doi.org/10.1021/es405227u [ Links ]
WANG N, ZHU L, WANG D, WANG M, LIN Z and TANG H (2010) Sono-assisted preparation of highly-efficient peroxidase-like Fe3O4 magnetic nanoparticles for catalytic removal of organic pollutants with H2O2. Ultrasonics Sonochem. 17 (3) 526-533. https://doi.org/10.1016/j.ultsonch.2009.11.001 [ Links ]
WANG X, LU J and XING B (2008) Sorption of organic contaminants by carbon nanotubes: influence of adsorbed organic matter. Environ. Sci. Technol. 42 (9) 3207-3212. https://doi.org/10.1021/es702971g [ Links ]
WANG Z, LI K, FINGAS M, SIGOUIN L and MENARD L (2002) Characterization and source identification of hydrocarbons in water samples using multiple analytical techniques. J. Chromatogr. A 971 (1) 173-184. https://doi.org/10.1016/S0021-9673(02)01003-8 [ Links ]
WARHEIT DB, LAURENCE BR, REED KL, ROACH DH, REYNOLDS GA and WEBB TR (2004) Comparative pulmonary toxicity assessment of single-wall carbon nanotubes in rats. Toxicol. Sci. 77 (1) 117-125. https://doi.org/10.1093/toxsci/kfg228 [ Links ]
WEISHAAR JA, TSAO D and BURKEN JG (2009) Phytoremediation of BTEX hydrocarbons: potential impacts of diurnal groundwater fluctuation on microbial degradation. Int. J. Phytoremediation 11 (5) 509-523. https://doi.org/10.1080/15226510802656326 [ Links ]
WIEDEMEIER TH, RIFAI HS, NEWELL CJ and WILSON JT (1999) Natural Attenuation of Fuels and Chlorinated Solvents in the Subsurface. John Wiley and Sons, New York. [ Links ]
WOLICKA D, SUSZEK A, BORKOWSKI A and BIELECKA A (2009) Application of aerobic microorganisms in bioremediation in situ of soil contaminated by petroleum products. Bioresour. Technol. 100 (13) 3221-3227. https://doi.org/10.1016/j.biortech.2009.02.020 [ Links ]
XU Y, BAI H, LU G, LI C and SHI G (2008) Flexible graphene films via the filtration of water-soluble noncovalent functionalized graphene sheets. J. Am. Chem. Soc. 130 (18) 5856-5857. https://doi.org/10.1021/ja800745y [ Links ]
YAH CS, SIMATE GS, MOOTHI K, MAPHUTHA KS and IYUKE SE (2011) Synthesis of large carbon nanotubes from ferrocene: the chemical vapour deposition technique. Trends Appl. Sci. Res. 6 (11) 1270-1279. https://doi.org/10.3923/tasr.2011.1270.1279 [ Links ]
YANG K, ZHU L and XING B (2006) Adsorption of polycyclic aromatic hydrocarbons by carbon nanomaterials. Environ. Sci. Technol. 40 (6) 1855-1861. https://doi.org/10.1021/es052208w [ Links ]
YEH CH, LIN CW and WU CH (2010) A permeable reactive barrier for the bioremediation of BTEX-contaminated groundwater: microbial community distribution and removal efficiencies. J. Hazardous Mater. 178 (1) 74-80. https://doi.org/10.1016/j.jhazmat.2010.01.045 [ Links ]
YU S, WANG X, AI Y, TAN X, HAYAT T, HU W and WANG X (2016) Experimental and theoretical studies on competitive adsorption of aromatic compounds on reduced graphene oxides. J. Mater. Chem. A 4 (15) 5654-5662. https://doi.org/10.1039/C6TA00890A [ Links ]
ZHANG Y, MU Y, LIU J and MELLOUKI A (2012) Levels, sources and health risks of carbonyls and BTEX in the ambient air of Beijing, China. J. Environ. Sci. 24 (1) 124-130. https://doi.org/10.1016/S1001-0742(11)60735-3 [ Links ]
ZHAO G, JIANG L, HE Y, LI J, DONG H, WANG X and HU W (2011) Sulfonated graphene for persistent aromatic pollutant management. Adv. Mater. 23 (34) 3959-3963. https://doi.org/10.1002/adma.201101007 [ Links ]
Received 27 February 2017
Accepted in revised form 9 October 2017
* To whom all correspondence should be addressed. +27 11 559 6385; e-mail: kmoothi@uj.ac.za














