Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
Water SA
versão On-line ISSN 1816-7950
versão impressa ISSN 0378-4738
Water SA vol.43 no.1 Pretoria Jan. 2017
http://dx.doi.org/10.4314/wsa.v43i1.01
Coagulation efficiency of Dicerocaryum eriocarpum (DE) plant
JO OdiyoI; OJ BasseyII, *; A OchiengIII; L ChimukaIV
IDepartment of Hydrology and Water Resources, University of Venda, X0950, South Africa
IIDepartment of Ecology and Resources Management, University of Venda, X0950, South Africa
IIICentre for Renewable Energy and Water, Vaal University of Technology, Private Bag X021, Vanderbijlpark, South Africa
IVMolecular Science Institute, School of Chemistry, University of the Witwatersrand, P/Bag 3, WITS University, 2050, Johannesburg, South Africa
ABSTRACT
A study was conducted to investigate coagulation efficiency of the plant Dicerocaryum eriocarpum (DE) in the removal of turbidity from raw water. Widespread poor land use practices contribute to high turbidity in river water, making turbidity management or removal critical, particularly before the water is used for drinking or subjected to chemical treatment. In this study, mucilage from DE was extracted with deionized water and different chloride solutions. A coagulation efficiency of 99% using modified mucilage coagulant was achieved. The modified mucilage of potassium crude extract and sodium crude extract displayed higher coagulation efficiencies than unmodified mucilage of deionized water crude extract. An increase in coagulant dosage and initial turbidity influenced the coagulation efficiency of DE coagulant. A large reduction in turbidity levels of the treated water samples resulted in an improvement in water quality.
Keywords: coagulation, Dicerocaryum eriocarpum plant, mucilage, optimise, turbidity
INTRODUCTION
The coagulation process is independently capable of removing soluble organic and inorganic constituents or colloidal phase impurities, including microorganisms; coagulation is thus of great importance in water treatment. Natural plant coagulants are promising materials for drinking water and wastewater treatment because they offer similar functions to inorganic coagulants and are also able to satisfy the guidelines for drinking water standards (Yin, 2010). Plant coagulants are also biodegradable and thus seen as more environmentally friendly than inorganic coagulants.
Turbidity removal reported for a number of identified natural plant coagulants has been impressive in purification of both raw and prepared turbid water. Various studies have used guar gum (Pritchard et al., 2009), Moringa oleifera (Ndabigengesere et al., 1995; Okuda et al., 1999, 2001; Abaliwano et al., 2008), cactus species (Diaz et al., 1999; Zhang et al., 2006), and common beans (Antov et al., 2010). In each of the above studies, natural plant coagulants showed high performance efficiency in turbidity removal. Pritchard et al. (2010) and Abaliwailo et al. (2008) also demonstrated the potential of natural plant coagulants to reduce the number of microorganisms (coliforms) in water via their anti-microbial properties. The potential of a natural plant to act as a coagulant seems to be associated with their polyelectrolytes and biochemical properties (Yin, 2010).
Dicerocaryum eriocarpum (DE) (Pedaliaceae) is a plant species common in grasslands and is known by the common name devil's thorns' (Bassey et al., 2016). DE plants have a range of uses. Most of the plant parts, including the leaves, flowers, stem, and seed shell, contain mucilage and are slippery when exposed to water. If the plant is crushed and left overnight in water, the resultant mucilage from the leaves becomes a useful substitute for soap and shampoo (Van Wyk and Gericke, 2000). In terms of medicinal purposes, DE can be used for antibacterial (Luseba et al., 2007) and ethnoveterinary medicines (Van Der Merwe et al., 2001). Parts of the plant have been used to treat diseases in the rural areas of the Vhembe District, South Africa.
However, there is a general lack of information regarding the use of DE as a coagulant in water purification. Understanding the potential of DE in turbidity removal will enhance its application and use in water treatment in rural communities, in particular in Vhembe District, South Africa, where it is available locally. Preliminary laboratory studies showed that DE mucilage has the potential to purify water but takes days to do so. The slow rate of turbidity removal by DE coagulant highlighted the need to improve its coagulation efficiency. This study therefore focused on improving DE coagulation efficiency for turbidity removal.
MATERIALS AND METHODS
Coagulant used
DE plant materials were collected from Nzhelele Village, Limpopo Province, South Africa, from farmlands and natural vegetation. The plant sample was taken to the botanical laboratory in the School of Natural Sciences, University of Venda, and was identified by a botanist to be Dicerocaryum eriocarpum (Pedaliaceae).
Preparation of DE
Plant leaves were detached from the stem, washed with tap water and rinsed with de-ionized water to remove dirt, soil particles and debris that may have been deposited on the leaves from the soil and atmosphere.
Preparation of solution used in extracting DE mucilage
Different solutions were used in the extraction of DE mucilage. These included: de-ionized water, potassium chloride solution and sodium chloride solution. Concentration of 1 mol/L solution of KCl and NaCl solutions were prepared from their respective salts by weighing out 7.8 g of KCl and 5.8 g of NaCl salt and dissolving each salt in de-ionized water to make 100 mL of solution. Note: purity of the potassium chloride salt was 96% which is why 7.8 g was used in this study.
Extraction of DE mucilage
An appropriate amount of fresh leaves was suspended in hot (80°C) de-ionized water, NaCl solution and KCl solution, respectively, and stirred for 30 min, with the aid of a magnetic stirrer. Hot water is able to extract mucilage from DE leaves within a shorter space of time than cold water. Thereafter the mixture was allowed to stand for 1 h at room temperature. In this process, thick viscous, slimy, foamy crude mucilage was extracted from the DE leaves. The final concentration of transparent mucilage solution was recovered by filtration using 0.45 µm filter paper with the aid of a suction pump.
Determination of mucilage concentration
The filtrate mucilage in liquid form was converted into solid form to determine the mucilage concentration. Ethanol was added to the filtrate mucilage in a quantity that was three times the volume of the total filtrate, as described by Mudadi et al. (1993). The mixture was stirred for 30 min and kept overnight at a room temperature to allow it to form a precipitate. The mixture was centrifuged to produce a greyish thread-like substance (solid) as precipitate mucilage and recovered by filtration using 0.45 µm cellulose acetate filter paper. The recovered precipitate was washed again with de-ionized water, dried and ground to a fine powder. Mucilage concentration was determined as mucilage wet mass/dry mass.
This same process was applied solely and then to all the filtrates, which included: deionized-water mucilage, KCl-solution mucilage and NaCl-solution mucilage. The mucilage concentration of DCE, NCE and KCE was 6% representing 100 mL crude solution for each coagulant. The concentration varies with optimisation of coagulant dosage.
In this paper the crude mucilage extracted using different solutions is identified as follows: de-ionized water crude extract is represented as DCE, NaCl solution crude extract is represented as CE and KCl solution crude extract is represented as PCE.
Preparation of turbid water
The turbid water used in this study was collected from Mvudi River, Vhembe District, using a 5-L plastic container. The water samples were collected according to the commonly accepted sampling protocols of Hermond and Fechner-Levy (2000). The turbidity of untreated and treated raw water samples was measured using a TB200 Portable Turbidimeter (Orbeco-Hellige, Germany). The collected water samples were kept in a cooler box and transported to the laboratory. The samples were filtered in the laboratory and kept in a fridge at 4°C to prevent bacterial activity prior to application in the coagulation experiments. Tap water was used to dilute the turbid raw water in order to obtain the desired turbidity levels used in this study, as described by Ndabigengesere et al. (1995) and Okuda et al. (1999).
Coagulation test
A coagulation experiment was carried out using a series of 550 mL volumetric beakers filled with 500 ml of turbid water sample. Coagulation activities of unmodified and modified DE mucilage were assessed by addition of a known amount (optimised) of coagulant dosage to each turbid water sample. It was immediately followed by rapid agitation for 2 min at 250 r/min and then slow agitation for 30 min using a magnetic stirrer at 40 r/min. After sedimentation, 15 mL of the sample was collected from the middle of the flask. Sedimentation time was from 0-18 h. EC and pH were not adjusted throughout the experiment. Turbidity, pH and EC of the treated water samples were assessed in accordance with the drinking water standards of DWAF (1996/1999), WHO (2006) and USEPA (2009). The pH and EC of the water samples were measured using a portable Thermo Scientific Orion (5 Star Thermo Scientific Orion, Germany) equipped with a pH electrode integrated with a standard conductivity cell.
Optimisation of parameters
In order to determine the coagulation efficiency of DE, different parameters were optimised by varying one parameter while keeping the others constant.
The influence of these parameters was evaluated by calculating the turbidity removal efficiency as:
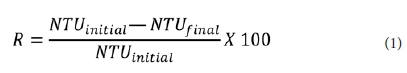
where: NTU = nephelometric turbidity units
RESULTS AND DISCUSSION
Coagulation studies
Effect of settling time
Removal of turbidity in raw water involves coagulation, sedimentation and filtration. Increase in settling time influenced coagulation efficiency of DE mucilage (Fig. 1). It was observed that after 0 to 6 h of sedimentation the turbidity removal efficiency of DE mucilage was generally low. SCE, PCE and DCE removal efficiencies were 79, 69 and 34%, respectively, after 0 to 6 h. Generally, coagulation efficiency was greatly enhanced by increase in settling time from 0 to 18 h. SCE and PCE coagulants showed the best performance in terms of turbidity removal (Fig. 1). SCE and PCE recorded coagulation efficiencies of 99% and 98%, respectively, while DCE coagulation efficiency increased greatly with an increase in settling time, to 92%. This study also confirms that sedimentation alone without coagulation can only remove large coarse suspended solids, as previously reported by Ndabigengesere et al. (1995). With addition of coagulants, the tiny colloidal particles in the raw water are entrapped. This results in the formation of insoluble precipitate (large flocs) that can easily settle; sedimentation time, therefore, plays a key role in removal of turbidity.

Coagulation efficiency of modified and unmodified mucilage
The highest turbidity removal obtained from the turbid water samples was achieved by SCE followed by PCE (Fig. 2). The final turbidity recorded after addition of SCE, PCE and DCE coagulants was 1.95, 2.84 and 15.13 NTU, respectively, from an initial turbidity of 209.9 NTU after 18 h of settling time.

There was no significant difference in the coagulation efficiency of PCE and SCE coagulants. Coagulation efficiencies of SCE and PCE coagulants were significantly higher than that of DCE coagulant. This indicates that the coagulation efficiency of DE mucilage was greatly improved by the modification of the crude extract. Similar results were reported by Okuda et al. (1999) and Ghebremichael et al. (2005) where extraction of Moringa oleifera (MO) using salt solution instead of distilled water improved its coagulation efficiency.
The increase in coagulation activity in the modified mucilage is largely attributed to the chloride salts present in the mucilage, which increase its ionic strength. PCE and SCE coagulants were able to trap the negatively-charged colloidal particles easily due to their chloride salts and to retain them inside the mucilage cell walls.
The low coagulation activity observed in unmodified mucilage (DCE) can be attributed to the repulsion force between DCE mucilage and negatively-charged colloidal particles. It can also be attributed to the low ionic strength in the DCE mucilage. Lin et al. (1989) confirmed that the most suitable method to modify an anionic polymer is by introducing chloride salts into the cell walls. In all reported cases the use of salt solutions in extraction of DE mucilage has increased its coagulation efficiency.
Influence of SCE dosage on turbidity removal
The high level of coagulation activity displayed by SCE coagulant led to it being chosen for use in further optimisation studies. Results of coagulation experiments using SCE coagulant at dosages ranging from 5 to 40 mL are displayed in Fig. 3. At dosages of 10-20 mL, optimum removal efficiency of 78.8% was recorded within 12 h of settling time. Further increase in the dosage of SCE coagulant to 40 mL decreased the removal efficiency to 74.8%. The results obtained from this study show that SCE dosage influences coagulation efficiency. The maximum removal of turbidity was achieved at between 10 and 20 mL of coagulant dosage.

The decrease in the coagulation efficiency of SCE when 40 mL dosage was added to the turbid water can be attributed to the early saturation of the mucilage sites by colloidal particles (cell walls). This leads to the dispersion of colloidal particles due to there being an insufficient number of particles to form inter-particle bridging. It also reduced the mechanical strength of SCE coagulant, reduced the formation of flocs and delayed sedimentation. The results of this study are consistent with that reported by Ndabigengesere et al. (1995) and Okuda et al. (2001).
At low coagulant dosage of 5 mL the coagulation efficiency was 65.8%, showing that little coagulation occurred at low coagulant dosages. With an increase in SCE dosage from 5 to 20 mL, the maximum reduction in turbidity was observed (78.8%). The low coagulation efficiency observed when 5 mL coagulant dosage was added can be as a result of the diffusion of mucilage into the colloidal particles. At low dosage the coagulant easily diffuses into the colloidal particles, resulting in low aggregation and less formation of flocs. While at optimum dosage, the mucilage is able to cover the surface of the colloidal particles without diffusing into it. This causes the formation of large flocs and precipitation leading to a high reduction in turbidity and rapid sedimentation.
Results of this study support the results of Lin (2008), and similar studies have also documented that coagulation efficiency of natural plant coagulants increases with increase in the coagulant dosage (Ndabigengesere and Narasiah, 1998; Zhang et al., 2006). It was observed that coagulant dosage is critical for improving the coagulation efficiency of SCE coagulant.
Effect of changes in initial turbidity
The results of optimising the level of turbidity, ranging from 65 NTU to 380 NTU, is given in Fig. 4. The worst performance was observed in less turbid water (65 NTU) resulting in a coagulation efficiency of 34.3%. Coagulation efficiency increased substantially with an increase in the initial turbidity from 220 NTU to 380 NTU, resulting in an increase in coagulation efficiency from 67% to 71%, within 12 h of settling time. Thus it was shown that SCE coagulant is more effective as a coagulant in highly turbid water than in less turbid water.
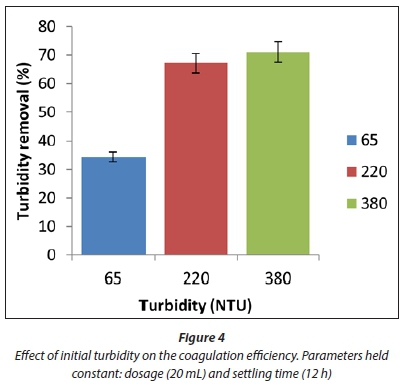
The increase in coagulation efficiency of SCE in highly turbid water (220-380 NTU) can be attributed to the increase in the number of particles in the water, which increases aggregation and floc formation. The efficiency of SCE coagulant in removal of turbidity is also dependent on the nature and amount of colloidal particles present in the turbid water. For example, water samples with high turbidity enhance the formation of large flocs, rapid sedimentation and increased coagulation efficiency.
The low coagulation efficiency observed for less turbid water (65 NTU) can be attributed to an insufficient number of colloidal particles present to form large flocs. This delays sedimentation due to the slow rate at which small particles can form large flocs. Understanding the interactions and properties of coagulant species and colloidal particles in the water sample is important in improving the coagulation efficiency of natural plant coagulants.
Most studies conducted on plant-based coagulants have reported similar results showing that natural material is more efficient in turbidity removal for highly turbid water (> 100 NTU) than for less turbid water (< 100 NTU) (Ndabigengesere et al., 1995; Pritchard et al., 2010). The exception was Okuda et al. (1999), who reported that Moringa oleifera extracted with salt solution was highly efficient in the treatment of less turbid (50 NTU) water. However, the variation in the results can be attributed to the difference in chemical composition of the same plant species at different locations.
Mechanism of coagulation
The difference in the nature of the floc particles formed by each coagulant suggests that the coagulation mechanism of mucilage extracted from various solvents differs. The flocs formed by PCE and SCE were tiny orange-coloured particles and spherical in shape, while the flocs formed by DCE coagulant were thick, brownish, and cobweb-like in structure. It was based on the above characteristics that the coagulation mechanisms of DCE, SCE and PCE were inferred in this study.
It is suggested that the mechanism of coagulation of DCE is via repulsion between particles and mucilage. According to Saenz et al. (2004), natural mucilages derived from plants are carbohydrate polymers that are negatively charged. DCE coagulant and the colloidal particles are likely to have similar charges (negative) since it has been established that clay particles suspended in water are negatively charged (Black and Sidney, 1961; Black and Manuel, 1969).
Even with a strong repulsive force between the coagulant and colloidal particles, it was still possible for some coagulation activity to occur. This can be attributed to coagulant and colloidal particles being able to overcome the repulsion after encounters with each other over time (Lin et al., 1989). This implies that particles collide many times before sticking together. This occurs between the colloidal particles and the polymer chain of the mucilage, resulting in formation of flocs inside the mucilage walls.
The above postulations also agree with the observation of Miller et al. (2008), who investigated the use of anionic mucilage from Opuntia species in removal of turbidity in water. Miller et al. (2008), however, suggested that the coagulation mechanism of Opuntia species, the mucilage from which is also negatively charged, is via adsorption and bridging, whereby clay particles do not contact one another but are bound to polymer-like material from Opuntia species.
It is also proposed that the SCE and PCE coagulation mechanisms occurred via a double-layer interaction. This seems to be achieved by charge neutralisation due to the presence of positively-charged ions Na+ and K+ in the modified mucilage. This increases the positive and negative charge interaction between particles. The increase in ionic strength of the modified mucilage leads to a weaker repulsive force between the particles.
The increase in ionic strength also leads to a reduction in the negatively-charged potentials of both mucilage and colloidal particles, and enhances rapid particle aggregation and permits closer clustering of the polymer and particles which then settles easily. Flocs in PCE- and SCE-treated water samples were thus formed via electrostatic patching while DCE flocs were formed via enmeshment. Lin et al. (1989) observed that electrostatic patching or charge neutralisation can cause coagulation faster than enmeshment, which explains the high coagulation activity of PCE and SCE relative to DCE recorded in the current study.
Water quality
The quality of the treated water samples was assessed according to the drinking water guidelines of DWAF (1996/1999), WHO (2006) and USEPA (2009), as presented in Table 1. The turbidity values show that after treatment with modified mucilage coagulants, the turbidity values exceeded the DWAF (1996/1999) guideline but were within the acceptable drinking water standard of WHO (1996) and USEPA (2009). Meanwhile the turbidity of the water samples treated with unmodified mucilage coagulant did not meet the required standards of DWAF (1996/1999), WHO (2006) and USEPA (2009). The pH value of water samples treated with DCE, SCE and PCE coagulants complied with DWAF (1996), WHO (2006) and USEPA (2009) standards. A high quality of treated water was attained, especially with the use of modified coagulants.
Comparing the performance of DE with other plants in water treatment
Dicerocaryum eriocarpum plant coagulation efficiency was able to compete with the performance of other plant coagulants, especially when it has been modified. The quality of the water treated with both modified and unmodified DE mucilage remained potable after days of treatment without the development of organic load. Similar results were obtained by Saenz et al. (2004) using Opuntia spp mucilage. This is unlike Moringa oleifera, which was reported by Ndabigengesere and Narasiah, (1998) and Okuda et al. (2001) to produce odour and organic load after days of treatment. The major disadvantage of using DE is the long settling time compared to other natural plant-derived coagulants that require a maximum of 1 h of sedimentation time. This may be due to the nature of the active agents present in the mucilage.
CONCLUSION
Modified DE has been successfully shown to have the potential to reduce turbidity with a coagulation efficiency of up to 99%. Significantly, this study has established that coagulation efficiency of DE coagulant can be improved from days to hours, with a huge decrease in the turbidity levels of the treated water after maximum sedimentation time. The study has shown that addition of chloride salts in the extraction of DE mucilage substantially improved the coagulation efficiency of DE. The chloride salts are highly recommended for use in extraction because they are cheap and do not adversely affect the pH or the EC of the treated water; they are also easy to use and require a simple process. The study established that modified DE mucilage can guarantee good water quality corresponding with potable drinking water standards, making it suitable for drinking and ensuring no ill-health impacts on humans. However, further study is required to investigate the active agent of coagulation in DE plants.
ACKNOWLEDGEMENTS
The authors would like to thank the Research and Publication Committee of the University of Venda for providing financial support for this research.
REFERENCES
ABALIWANO JK, GHEBREMICHAEL KA and AMY GL (2008) Application of the purified Moringa oleifera coagulant for surface water treatment. Water Mill. Working Paper Series No 5. Institute for Water Education, Delft, Netherlands. [ Links ]
ANTOV MG, ŠĆIBANA, MB and PETROVIĆA NJ (2010) Proteins from common bean (Phaseolus vulgaris) seed as a natural coagulant for potential application in water turbidity removal. Bioresour. Technol. 101 (7) 2167-2172. https://doi.org/10.1016/j.biortech.2009.11.020 [ Links ]
BASSEY OJ, ODIYO OJ, CHIMUKA L, AOYI O and BRIDGET JB (2016) Application of mucilage from Dicerocaryum eriocarpum plant as biosorption medium in the removal of selected heavy metal ions. J. Environ. Manage. 177 (2016) 365-372. https://doi.org/10.1016/j.jenvman.2016.04.011 [ Links ]
BENHURA MAN and MARUME M (1993) The mucilaginous polysaccharide material isolated from ruredzo (Dicerocaryum zanguebarium). Food Chem. 46 (1) 7-11. https://doi.org/10.1016/0308-8146(93)90067-P [ Links ]
BLACK AP and VILARET MR (1969) Effect of particle size on turbidity removal. J. Am. Water Works Assoc. 61 (4) 209-214. [ Links ]
BLACK AP and HANNAH SA (1961) Electrophoretic studies of turbidity removal by coagulation with aluminum sulfate. J. Am. Water Works Assoc. 53 (4) 438-452. [ Links ]
DWAF (Department of Water Affairs and Forestry, South Africa) (1996) South African Water Quality Guidelines for Domestic Water Use (2nd edn). DWAF, Pretoria. [ Links ]
DWAF (Department of Water Affairs and Forestry, South Africa) (DWAF) (1999) Resource Directed Measures for Protection of Water Resources. River Ecosystems. DWAF, Pretoria. [ Links ]
DIAZ A, RINCON N, ESCORIHUELA A, FERNADEZ N, CHACIN E and FORSTER CF (1999) A preliminary evaluation of turbidity removal by natural coagulants indigenous to Venezuela. Process Biochem. 35 (3-4) 391-395. https://doi.org/10.1016/S0032-9592(99)00085-0 [ Links ]
GHEBREMICHAEL KA, GUNARATNA KR, HENRIKSSON H, BRUMER H and DALHAMMAR G (2005) A simple purification and activity assay of the coagulant protein from Moringa oleifera seed. Water Res. 39 (11) 2338-2344. https://doi.org/10.1016/j.watres.2005.04.012 [ Links ]
HERMOND H and FECHNER-LEVY E (2000) Chemical Fate and Transport in the Environment. Academic Press, San Diego. [ Links ]
LIN JL, HUANG CP, PAN JR and WANG DS (2008) Effect of Al(III) speciation on coagulation of highly turbid water. Chemosphere 72 (2008) 189-196. https://doi.org/10.1016/j.chemosphere.2008.01.062 [ Links ]
LIN MY, LINDSAY HM, WEITZ DA, BALL RC, KLEIN R and MEAKIN P (1989) Universality in colloid aggregation. Nature 339 (1989) 360-362. https://doi.org/10.1038/339360a0 [ Links ]
LUSEBA D, ELGORASHI EE, NTLOEDIBE DT and VAN STADEN J (2007). Antibacterial, anti-inflammatory and mutagenic effects of some medicinal plants used in South Africa for the treatment of wounds and retained placenta in livestock. S. Afr. J. Bot. 73 (3) 378-383. https://doi.org/10.1016/j.sajb.2007.03.003 [ Links ]
MILLER SM, FUGATE EJ, CRAVER VO, SMITH JA and ZIMMERMAN JB (2008) Toward understanding the efficacy and mechanism of Opuntia spp. as a natural coagulant for potential application in water treatment. Environ. Sci. Technol. 42 (12) 4274-4279. https://doi.org/10.1021/es7025054 [ Links ]
NDABIGENGESERE A and NARASIAH KS (1998) Quality of water treated by coagulation using Moringa oleifera seeds. Water. Res. 32 (3) 781-791. https://doi.org/10.1016/S0043-1354(97)00295-9 [ Links ]
NDABIGENGESERE A, NARASIAH KS and TALBOT BG (1995) Active agents and mechanism of coagulation of turbid waters using Moringa oleifera. Water Res. 29 (2) 703-710. https://doi.org/10.1016/0043-1354(94)00161-Y [ Links ]
OKUDA TA, BAES AU, NISHIJIMA W and OKADA M (1999) Improvement of extraction method of coagulation active components from Moringa oleifera seed. Water Res. 33 (15) 3373-3378. https://doi.org/10.1016/S0043-1354(99)00046-9 [ Links ]
OKUDA TA, BAES AU, NISHIJIMA W and OKADA M (2001). Isolation and characterization of coagulant extracted from Moringa oleifera seed by salt solution. Water Res. 35 (2) 405-410. [ Links ]
PRICHARD M, CRAVEN T, MKANDAWIRE T, EDMONDSON AS and O'NEILL JG (2010) A study of the parameters affecting the effectiveness of Moringa oleifera in drinking water purification. Phys. Chem. Earth 35 (13-14) 791-797. https://doi.org/10.1016/j.pce.2010.07.020 [ Links ]
PRICHARD M, MKANDAWIRE TM, EDMONDSON AS, O'NEIL JG and KULULANGA G (2009) Potential of using plant extracts for purification of shallow well water in Malawi. Phys. Chem. Earth 34 (13-16) 799-805. https://doi.org/10.1016/j.pce.2009.07.001 [ Links ]
SAENZ C, SEPULVEDA E and MATSUHIRO B (2004) Opuntia spp. mucilage's: a functional component with industrial perspectives. J. Arid Environ. 57 (3) 275-290. https://doi.org/10.1016/S0140-1963(03)00106-X [ Links ]
USEPA (United States Environmental Protection Agency) (2009) An Urgent Call to Action - Report of the State-EPA Nutrient Innovations Task Group 2009. URL: http://www.epa.gov/waterscience/criteria/nutrient/nitgreport.pdf (Accessed 3 September 2012). [ Links ]
VAN DER MERWE D, SWAN DG and BOTHA CJ (2001) Use of ethnoveterinary medicinal plants in cattle by Setswana-speaking people in the Madikwe area of the North West Province in South Africa. J. S. Afr. Vet. Assoc. 72 (4) 189-196. https://doi.org/10.4102/jsava.v72i4.651 [ Links ]
VAN WYK BE and GERICKE N (2000) People's Plants. A Guide to Useful Plants of Southern Africa. Briza Publications, Pretoria. 351 pp. [ Links ]
WHO (WORLD HEALTH ORGANISATION) (2006) Guideline for Drinking Water Quality. Vol. 1, Recommendations (3rd edn.). WHO, Geneva. [ Links ]
YIN C (2010) Emerging usage of plant-based coagulants for water and wastewater treatment. Process Biochem. 45 (9) 1437-1444. https://doi.org/10.1016/j.procbio.2010.05.030 [ Links ]
ZHANG J, ZHANG F, LUO Y and YANG H (2006) A preliminary study on cactus as coagulant in water treatment. Process Biochem. 41 (3) 730-733. https://doi.org/10.1016/j.procbio.2005.08.016 [ Links ]
Received 20 July 2015
Accepted in revised form 9 November 2016
* To whom all correspondence should be addressed. S +27 73 135-1734; e-mail: odojones@gmail.com














