Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
Water SA
versión On-line ISSN 1816-7950
versión impresa ISSN 0378-4738
Water SA vol.42 no.1 Pretoria ene. 2016
http://dx.doi.org/10.4314/wsa.v42i1.12
The major and trace element chemistry of fish and lake water within major South African catchments
LJ JordaanI, IV, *; V WepenerII; JM HuizengaII, III
ICouncil for Geoscience, Private Bag X112, Pretoria, 0001, South Africa
IIUnit for Environmental Sciences and Management, North West University, Private Bag X6001, Potchefstroom, 2520, South Africa
IIISchool of Earth and Environmental Sciences, James Cook University, Townsville, Queensland, 4811, Australia
IVDepartment of Zoology, University of Johannesburg, P.O. Box 524, Auckland Park, 2006, South Africa
ABSTRACT
Chemical elements in lake water are incorporated into fish tissues through bioconcentration and biomagnification. Lake water and fish tissue samples from 23 lakes, located within 4 major South African catchments, were analysed to investigate the link between element concentrations in lake water and otolith, fin spine, muscle, liver and gill tissues. The comparison is complicated by the seasonal variation in water chemistry as well as the large natural variation between individual fish within a lake. Comparisons between fish from different lakes can also only be done within the same species, which may not occur within all the lakes within the project area. This may be further complicated by erratic anthropogenic contamination. It is therefore more successful to use inter-element ratios for comparison than absolute element concentrations. Using the Sr/Ca elemental ratio, a species-specific correlation was identified between lake water, otolith, fin spine and gill tissue samples. The best discrimination between fish species was achieved using a Na/Ca versus Mg/Ca elemental ratio diagram of gill tissues. The best discrimination between fish from different lakes was achieved using a Ba/Mg versus Sr/Mg elemental ratio diagram for fin spine tissue.
Keywords: trace elements, water chemistry, fish tissue, elemental ratios, lake water, bioaccumulation
INTRODUCTION
According to Gibbs (1970), atmospheric precipitation, rock dominance and an evaporation-crystallization process are the three major mechanisms controlling surface water chemistry. The waters of rock-dominated systems are in partial equilibrium with the materials in their catchments and their position within this group is dependent on the relief, chemical composition and climate of the catchment. Gorham (1961) stated that Ca, Mg, Na and K account for most of the ionic composition of the world's freshwater systems. Freshwater bodies should therefore have characteristic chemistries related to the underlying geology. Fish living in these water bodies should thus also have characteristic chemistries related to these water bodies.
Dallas and Day (2004) define bioaccumulation as the ability of an organism to accumulate and concentrate substances directly from the surrounding water (bioconcentration) or indirectly via the food chain (biomagnification). Bioaccumulation of metals by fish is influenced by a number of factors. The physico-chemical water quality conditions, e.g. pH, influence bioaccumulation indirectly by changing the solubility of metal compounds, or directly by damage to epithelia, making them more permeable. Hardness and salinity also reduce metal uptake through competition for binding sites (Jezierska and Witeska, 2006). The hydrological period is known to influence metal bioaccumulation mostly due to a concentration of salts in the water during the dry season. Robinson and Avenant-Oldewage (1997) noted significant differences in metal content of fish tissues between wet and dry seasons. Kotze et al. (1999) noted significant differences in metal content of tissues during different surveys at Lake Loskop and Mamba Weir in the Olifants River.
Coetzee et al. (2002) reported that Zn, Pb, Cr and Ni showed significant positive correlations with fish size. Nussey et al. (2000) found that the smaller the fish the higher the bodyload of metals due to various bioaccumulation processes. Coetzee et al. (2002) found few significant differences in the metal content of fish tissues between males and females. Different species may accumulate different amounts of metals due to different living and feeding habits (Jezierska and Witeska, 2006). Kotze et al. (1999) noted species differences in metal content of tissues from fish in the Olifants River. Robinson and Avenant-Oldewage (1997), Kotze et al. (1999) and Coetzee et al. (2002) noted significant differences in metal content of fish tissue samples from distinct localities in the Olifants River.
Traditionally, bioaccumulation monitoring is used as an indicator of pollutant exposure by comparing the concentrations of toxicologically important metals (e.g. Cd, Pb) in an indicator species and tissue type between sites or surveys (Wepener et al., 2011). However, for the purposes of this paper, the relationship between the ratios of selected metals in fish tissue types and the water, rather than individual metal concentrations, were used to determine if the fish tissue reflected the major geological features of the catchment. Seasonal variations in the metal concentrations of lake water due to dilution by relatively clean rainwater are largely negated by the use of elemental ratios rather than absolute concentrations. Different fish tissues incorporate and retain chemical elements from the environment for varying time periods. Spine tissue bioaccumulates metals from the environment over a longer period than blood and muscle tissue. The seasonal variation in water chemistry is therefore more likely to be represented in the blood and muscle tissue than in the spine tissue. A multi-year sampling campaign may therefore produce better correlation than a single sampling exercise.
Otoliths are acellular and metabolically inert structures that permanently retain any chemical elements that are accreted onto their growing surfaces (Campana, 1999). Secor et al. (1995) and Bath et al. (2000) noted that some otolith element ratios (Sr/Ca and Ba/Ca) are proportional to their ratios in the surrounding water. Whitledge (2008) could distinguish fish from 4 American rivers based on their otolith Sr/Ca and Ba/Ca ratios. Wells et al. (2003) investigated westslope cutthroat trout (Oncorhyncus clarki lewisi) from the Coeur d'Alene River in Idaho and found that otolith and scale chemistries were linearly related to the Sr/Ca and Ba/Ca ratios in the water. Walther and Thorrold (2006) calculated that water contributed 83% of Sr and 98% of Ba in otoliths formed in spiked seawater, implying that the chemical signatures recorded in otoliths of marine fishes should reflect the ambient water composition of these elements at the time of deposition.
Otoliths are made of calcium carbonate. Sr2+, Ba2+ and Mg2+ have very similar ionic radii to Ca2+ and can thus substitute for Ca2+. In addition, elements like Sr, Ba, Mn, Fe, Pb, Li, Mg, Cu and Ni are metabolically inert and are not resorbed from otoliths (Sako et al., 2005). Limburg (1995) noted that the use of Sr/Ca ratios in freshwater systems is limited relative to seawater systems, due to lower element concentrations (up to 10 times). Recent studies by Krause and Secor (2004) have however indicated that the range of Sr in freshwater systems may approach that of seawater. Campana (1999) observed that the concentrations of the most common elements (Ca, Na, K, Mg and Cl) differ substantially between fresh and salt water, yet do not appear to be reflected in the otoliths. Trace elements like Sr, Zn, Pb, Mn, Ba and Fe in fresh and seawater are however consistent with an environmental effect.
Gillanders (2005) indicated that it is plausible that the effect of ambient Sr outweighs that of salinity. Secor et al. (1995) and Kawakami et al. (1998) positively linked the Sr/Ca ratio in sagittal otoliths to salinity. Martin et al. (2004) found significantly elevated Sr/Ca ratios in otoliths of marine larval spot (Leiostomus xanthurus) at a salinity of 25%o vs. 15%o.
Campana (1999) noted that existing literature does not support an overall relationship between otolith Sr/Ca ratio and temperature for either fresh or seawater fish. Townsend et al. (1992) suggested that temperature-dependent Sr/Ca fractionation only happens at low water temperatures (< 10°C). Martin et al. (2004) found a significant linear relationship between temperature and Sr/Ca ratios in otoliths of marine larval spot (Leiostomus xanthurus).Elsdon and Gillanders (2002, 2004) investigated juvenile black bream (Acanthopagrus butcheri)and found that water temperature significantly influenced the Sr/Ca and Ba/Ca ratios in otoliths while salinity alone did not influence these ratios. Bath et al. (2000) found that temperature significantly influenced Sr incorporation but not Ba incorporation into otoliths of marine fish.
The primary aim of this paper is therefore to investigate whether a simplified analytical technique will be able to relate fish tissue chemistry to water chemistry as well as to distinguish between fish from different lakes. The secondary aim is to determine whether any deviation from the expected fish tissue chemistry could be linked to either pollution events or the sampling of fish introduced from another catchment. The study was undertaken to develop a scientific method to minimize illegal entries at major South African freshwater fishing tournaments (Jordaan, 2015).
MATERIALS AND METHODS
Description of the project area
The project area consisted of selected lakes within the Vaal, Mgeni, Crocodile (West) and Olifants River catchments (Fig. 1). The catchments were selected due to different sizes, different sources of pollution and different underlying geological composition.
Collection and preparation of samples
Samples included: water taken from the surface of lakes, and fish samples taken mainly by bank and boat angling as well as gill netting (Table 1). Water samples were collected in 2 ℓhigh-density polyethylene (HDPE) containers, not acidified, cooled and sent to the laboratory for analyses within 24 h.
Four major species of fish were targeted, i.e., common carp (Cyprinus carpio),sharptooth catfish, (Clarias gariepinus),largemouth bass, (Micropterus salmoides),and Mozambique tilapia (Oreochromis mossambicus).Some minor species were also included in the analysis. Fish samples were collected in plastic containers, packed in ice and brought to the laboratory, where they were frozen to -5°C. Fish muscle samples were removed from the fillets of each fish. Fish spine samples were collected from the dorsal, ventral or pectoral fins of each fish. Otoliths and gills were extracted by dissecting the fish skulls from the ventral side. Liver samples were collected by ventral dissection. Samples were oven-dried at approx. 80°C for 14 days. All soft tissues were removed from spine samples, whereafter they were pulverized in a swing mill. Gill filaments were manually removed from dried gill samples before crushing.
Chemical analyses
Trace element concentration analyses of water samples consisted of first filtering samples through 0.45 μm cellulose nitrate filters. Water samples were then diluted 5 times to add the internal standards (In and Ir) and to reduce total dissolved solids. The samples were made up in 2 mℓ/100 mℓ HNO3 to keep analyte elements in solution. Analytical grade HNO3 and ultra-pure water were used in all preparations. Samples were analysed on a Perkin Elmer SCIEX ELAN DRCII ICP-MS with AS 93 plus auto-sampler (Jordaan and Maritz, 2010).
Trace element concentration analyses of fish samples consisted of crushing the dried material with an agate mortar and pestle, or a swing mill for spine samples. Preparation of tissue samples for analyses followed a simplified version of the method described by Wepener and Vermeulen (2005). A 0.3 g portion of sample was digested in HNO3 and HClO4 for 2 h at 85°C in an aluminium heating block. Samples were diluted to add the internal standards (In and Ir), and analysed on a Perkin Elmer SCIEX ELAN DRC II ICP-MS with AS 93 plus auto-sampler (Jordaan and Maritz, 2010).
Quality assurance
To evaluate the water method, liquid samples from the SABS Water-Check (group 1) inter-laboratory proficiency test (South African Bureau of Standards, 2010a, 2010b, 2010c) were regularly analysed. Elements analysed included Al, Ba, Be, B, Cd, Cr, Co, Cu, Fe, Pb, Mn, Hg, Mo, Ni, Si, Sr, V, Zn, As and Se.
Average Z-scores obtained were as follows: April 2010, 0.73; June 2010, 0.73 and October 2010, 0.69. All Z-scores between -2 and 2 are considered satisfactory.
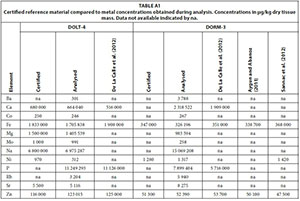
The certified reference materials DOLT-4 (Dogfish Liver) and DORM-3 (Fish Protein) (National Research Council Canada, 2007, 2008) were analysed in quadruplet to evaluate the performance of the analytical method compared to analyses by Aygun and Abanoz (2011), De La Calle et al. (2012) and Sannac et al. (2012) of the same reference material. Dilution factors were chosen to include both major and trace elements in the same analyses, which implies that the method was not sensitive enough to analyse some elements at ultra-trace levels. Only those elements that were mostly above the detection limit (more than 93% of analyses in total dataset) were considered for scientific interpretation (Appendix 1: Table A1). The Rb concentration in otolith samples and Ba and U in gill samples were also included even though between 10 and 20% of the data were below the detection limit. The Merck VI certified calibration standard, with the addition of certified Merck single element standards, was used to calibrate both water and fish analysis methods.
Statistical analysis
Summary statistics (mean, standard deviation) of metal concentrations were determined using Microsoft Excel. Scatter plots of elemental ratios were manually developed to visually establish the best parameters to maximize separation of dissimilarity and minimize the separation of similarity.
RESULTS AND DISCUSSION
Sample identification
In Table 1 the number of species sampled and tissues collected from each lake are represented. The codes provided for the different lakes and fish species are used in the figures and tables throughout the paper. The Olifants River catchment was sampled during both the wet and dry season while most of the lakes in the other catchments were sampled during only one of the seasons for water analyses. For the purpose of this project August-November is considered the dry season and December-July is considered the wet season.
Otolith tissue
From the literature it is evident that no single model has been presented that generally explains the link between metal concentrations in otoliths and water chemistry for all species across the freshwater and marine salinity and temperature range. This is however possible for specific examples. The dissolved metal concentrations in water samples and the concentrations of metals in the otolith tissue of carp from the project area are presented in Table 2. Discrimination between lakes is only possible if there are measurable differences in water chemistry between these lakes, usually as a result of different catchment geology of anthropogenic input. Figure 2 is a diagram of the Sr/Ca elemental ratio of lake water and fish otoliths from the project area, as used by Elsdon and Gillanders (2003) and Bath et al. (2000). There are large variations in Sr/Ca ratios of the lake water (all freshwater lakes) and overlaps between lakes from the four catchments. When considering the Olifants River catchment, the Highveld lakes have lower Sr/Ca ratios that combine to form an average for Lake Loskop. Downstream the Olifants River mixes with water from the Elands River with a much higher Sr/Ca ratio to produce an intermediate ratio at Lake Arabie. The Vaal River catchment shows an increase in Sr/Ca ratio along the length of the project area. In the Crocodile River catchment trends are opposite for the Lake Rietvlei and Lake Roodeplaat systems.
The largest variation in Sr/Ca ratios of the otoliths is related to the specific fish species, as indicated by the linear regressions of the data from the project area (Fig. 2). Even though data were collected over several years during both the wet and dry seasons, which implies that there may be significant variation in the data from a single lake, the data are still relatively constant and separated mainly according to species. According to the literature, water (i.e. environmental exposure) has the major influence on otolith chemistry and food the least (Walther and Thorrold, 2006). Fish behaviour may thus not play a significant role in otolith chemistry. Salinity should not play a major role in this study since all lakes are freshwater lakes even though some are moderately polluted. A clear trend from lakes in colder areas to lakes in warmer areas was also not observed. The major extrinsic contributing factor thus appears to be the chemistry of the lake water while an intrinsic factor may be due to physiological mechanisms of otolith formation that possibly differs between fish species (Campana, 1999). Fish must however be in equilibrium with lake water and must not be able to migrate between different lakes or catchments.
Together with the modified alkali-lime index [Na2O+K2O-CaO] and the aluminium saturation index [Al/(Ca-1.67P+Na+K)], the Fe/Mg ratio is often used as a means of rock classification in igneous geology (Frost et al., 2001). This primary variation in the Fe/Mg ratio may induce variation in weathered/transported materials and the water that contributes to these processes. Lake sediments and the otoliths of fish within these lakes may thus show variation in their Fe/Mg ratio, in part due to the chemistry and weathering processes in the upper catchment. Figure 3 is a diagram of Fe versus Mg of fish otoliths from the project area normalized to Ca, used as a discrimination diagram. Solid symbols indicate carp otoliths and are divided according to the lakes from where they were collected. Open symbols indicate the rest of the otoliths and are only divided according to species. The Mg/Ca parameter distinguishes between 3 groups of species (carp; labeo; tilapia, bass and catfish). The Fe/Ca parameter distinguishes mostly between different lakes even though much overlap exists. A similar distinction between lakes is seen in all species investigated. Fe and Mg are thus not incorporated into otoliths in exactly the same manner for all species while the chemistry of the environment is roughly reflected. A clear distinction can also not be made between otoliths from different catchments.
Cobalt and Ni can sometimes be used as indicators of genetic processes (Herd et al., 2009) and ore-forming processes (Bralia et al., 1979) in geology. According to the Goldschmidt classification (Battey, 1981) both Co and Ni are siderophile elements and are thus normalized to Fe. Figure 4 represents the Co/Fe elemental ratio versus the Ni/Fe elemental ratio of fish otoliths from the project area. A positive correlation is observed between these two components. The diagram does not distinguish between different fish species. Only carp (large solid symbols), bass, tilapia and catfish otoliths are indicated on the diagram, but all samples obtained from the project area follow the same trend. Distinction between otoliths from different lakes can broadly be made although large overlaps do occur. Co and Ni are thus incorporated into otoliths in a fashion indicating environmental chemistry rather than differences between species.
The bass populations from Lake Middelburg and Lake Witbank contain one individual each that has extremely elevated Ni/Fe otolith ratios (Fig. 4). These lakes are directly downstream from a severely polluted coal-mining area. The area also contains coal-fired power stations and steel mills utilizing iron ore from the Bushveld Igneous Complex, providing an ample Ni source.
Plotting the different elements detected in the fish otoliths from the project area in a similar fashion as in Figs 3 and 4 allow distinction between 2 groups of elements: Sr, Ba, Na, K, P and Mg, which best distinguish between different fish species and to a lesser degree between different lakes, and Fe, Co, Ni, Mn, Ga, V and Ca which only broadly distinguish between lakes. It was not possible to use La, Pr and Sm to distinguish between lakes.
Spine tissue
Biomineralization of otoliths, discussed above, differs from vertebrate bone in that otolith epithelium is not in direct contact with the region of calcification (Campana, 1999). However, in an experiment where juvenile snapper (Pagrus auratus)were exposed to water with elevated Sr concentrations (10 x ambient) for 5 days it was shown by Pollard et al. (1999) that Sr is absorbed from the water and deposited in the dorsal spines where it was persistent for at least 36 days and showed no sign of decay during the experiment.
The concentrations of metals in the spine tissue of the four target fish species from the different lakes are presented in Table 3. Using a plot of the Sr/Ca elemental ratio of lake water versus fish otoliths, as used by Bath et al. (2000) and Elsdon and Gillanders (2003) for plotting spine samples from the project area, provides similar results as obtained for otoliths (Fig. 5). The regression of the carp spine data however exhibits a steeper slope while the data spread is greater. Carp spine samples from Lake Inanda, Lake Vaalkop and Lake Roodeplaat are more displaced from the regression line than the other carp spine samples (Lake Vaalkop and Lake Roodeplaat carp samples are overlain by catfish samples). The distinction between different species is not as clear as when using the otolith Sr/Ca ratio.
Using Fe as a discriminator between spine samples from the project area, as was done for the otolith samples (Fig. 3), did not produce the expected results. A diagram of Na versus Mg normalized to Ca did however distinguish better between fish species as well as individual lakes (Fig. 6).
Following the identification of elements from otoliths that are more suitable to discriminating between species (Sr, Ba, Na, K, P and Mg), a diagram of Ba versus Sr normalized to Mg produced some separation between fish spine samples from different lakes and to a lesser extent from different species (Fig. 7). Barium versus Sr normalized to Ca gives similar results, but with less separation of the tilapia spine samples. Figures 8, 9 and 10 show catfish, bass and tilapia spine samples, respectively, plotted per lake for comparison with Fig. 7 where carp samples are plotted per lake. In all three species the samples from a specific lake cluster together and are separated from samples from other lakes, indicating that the Ba/Mg versus Sr/Mg plot can successfully distinguish between fish from different lakes for at least 4 species.
Muscle tissue
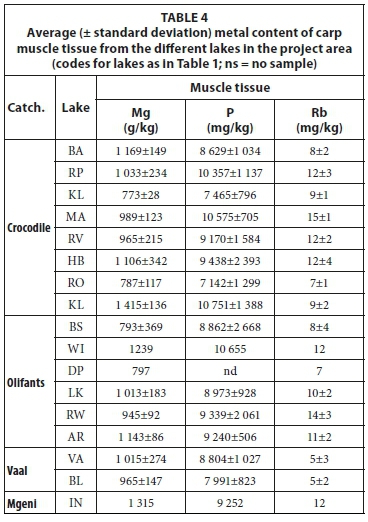
Metal concentrations in the muscle tissue of carp from the different lakes are presented in Table 4. Rb is the only element that shows some differentiation between fish muscle from the different lakes. When plotted against P and normalized to Mg (Fig. 11), a weak separation between lakes can be seen in the Rb/Mg ratio and a broad distinction between species in the P/Mg ratio. The Rb/Mg ratio distinguishes better between carp muscle tissue samples from lakes in the Crocodile catchment than in the Olifants catchment.
Liver tissue
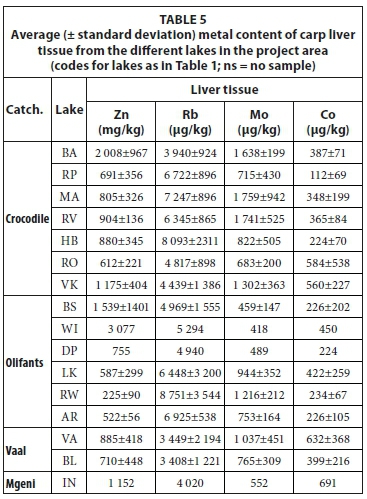
Liver tissue contained higher concentrations of trace elements than other tissues, which made additional comparison of samples at trace element level possible. The concentrations of metals in the liver tissue of carp from the different lakes are presented in Table 5. Major elements did not distinguish between different species or between liver samples from different lakes. Figure 12 is a diagram of the Co/Mo elemental ratio versus the Zn/Rb elemental ratio of liver samples from the project area. These ratios were empirically chosen purely to give the best distinction between species as well as between samples from the same species collected from different lakes. Figure 12 shows some distinction between tilapia, bass, carp, labeo and catfish. Among the carp samples, it also made some distinction between different lakes. In the Olifants River catchment 3 groups can be identified: (i) Lake Rust de Winter, (ii) Lake Witbank and (iii) Lakes Bronkhorstspruit, Loskop and Arabie. If a connection between water quality and fish tissue elemental concentrations is assumed than the separation may be explained as Lake Rust de Winter is in the Elands River, a tributary of the Olifants River and Lake Witbank is subjected to pollution from adjacent coal-mining activity. In the Crocodile River 4 groups can be identified: (i) Lake Roodekopjes, (ii) Lake Bon Accord, (iii) Lake Vaalkop and (iv) Lakes Roodeplaat, Hartbeespoort, Marais and Rietvlei. Except for Lake Roodeplaat the division is based on different tributaries within the catchment. A clear distinction is not found for the two lakes in the Vaal River catchment. If the liver is considered a transient location of elements (higher throughput rate) then it may capture shorter-term chemical variations in the environment better than bone or muscle tissue.
Gill tissue
Gill metal loadings have good potential for correlation with metals dissolved in lake waters as gills are in direct contact with the lake water. This is also the basic assumption behind the biotic ligand model used to predict toxicity due to dissolved metals (Niogi and Wood, 2004). The concentrations of metals in the gills of carp from the different lakes are presented in Table 6. Using a plot of the Sr/Ca elemental ratio of lake water versus fish otoliths as used by Bath et al. (2000) and Elsdon and Gillanders (2003) for plotting gill samples from the project area, again exhibits similar results to otoliths (Fig. 13). A clear correlation is observed between lake water and fish gills with good separation between the different species. Labeos correspond with either carp or tilapia while bass and catfish greatly overlap.
Figure 14 represents the Mg/Ca elemental ratios versus the Na/Ca elemental ratios of fish gill samples from the project area. It completely separates carp, bass, tilapia and catfish, as well as most of the labeo species. Both ratios span a much wider range than for the spine samples (Fig. 6) and thus produce a better separation between species. Within species the Mg/Ca and Na/Ca ratios can however not separate between samples from individual lakes.
Figure 15 is a diagram of the Co/Ni elemental ratio versus the Sr/Ca elemental ratio of fish gill samples from the project area. In this case a clear separation is observed between different species as well as between fish from specific lakes as demonstrated by the carp samples. The Co/Ni ratio is the main factor separating the bass, tilapia and catfish samples while the Sr/Ca ratio is the prominent ratio in defining the carp as a group as well as separating carp samples from individual lakes. This diagram produces the best discrimination between Lakes Vaal and Lake Bloemhof. Distinction between carp samples from the Olifants River catchment is not clear while carp samples from the Crocodile River catchment show much less overlap.
CONCLUSIONS
When comparing lake water chemistry to fish tissue chemistry there are several important factors to consider. Firstly there must be a measurable difference in water chemistry between the different lakes within the project area, which is predominantly controlled by the underlying geology and the anthropogenic activity within the catchment. Secondly the fish must be in equilibrium with the lake water and must not be able to migrate between different lakes or catchments.
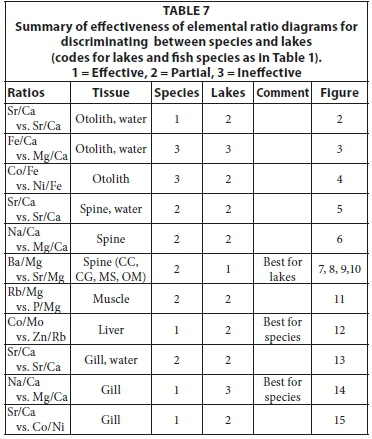
The elemental ratios that can be used for comparison depend on the ability to accurately analyse these elements in water or fish samples as well as on the specific fish tissue type. The Sr/Ca has traditionally been used to compare fish with lake water. In the project area, the Sr/Ca ratio also correlated very well between individual fish species and water from specific lakes. A summary of the effectiveness of elemental ratio diagrams in discriminating between fish species and lakes is given in Table 7. Using other elemental ratios was less successful, mainly due to the low abundance of these elements in lake water.
Considering only fish tissue samples, the best separation between species was achieved using a Na/Ca versus Mg/Ca elemental ratio diagram for gill tissue, followed by a Co/Mo versus Zn/Rb elemental ratio diagram for liver tissue. The best discrimination between fish from different lakes was achieved using a using a Ba/Mg versus Sr/Mg elemental ratio diagram for spine tissue from carp, catfish, bass and tilapia.
ACKNOWLEDGEMENTS
The authors would like to acknowledge Bella Honeybourne, the organizing committee of the 'Three Species Bonanza', EcoCare Trust, Waterlab and the various nature conservation organizations for their support during fieldwork as well as the Council for Geoscience and the University of Johannesburg for financial and technical support.
REFERENCES
AYGUN SF and ABANOZ FG (2011) Determination of heavy metal in anchovy (Engraulis encrasicolus L. 1758) and whiting (Merlangius merlangus euxinus Nordman, 1840) fish in the middle Black Sea. Kafkas Univ. Vet. Fak. Derg 17(Suppl A) S145-152. [ Links ]
BATH GE, THORROLD, SR, JONES CM, CAMPANA SE, MCLAREN JW and LAM WH (2000) Strontium and barium uptake in aragonite otoliths of marine fish. Geochim. Cosmochim. Acta. 641705-1714. [ Links ]
BATTEY MH (1981) Mineralogy for Students (2nd edn). Longman, London and New York. 355 pp. [ Links ]
BRALIA A, SABATINI G and TROYA F (1979) A revaluation of the Co/Ni ratio in pyrite as geochemical tool in ore genesis. Miner. Deposita 14(3) 353-374. http://dx.doi.org/10.1007/BF00206365 [ Links ]
CAMPANA SE (1999) Chemistry and composition of fish otoliths: Pathways, mechanisms and applications. Mar. Ecol. Prog. Ser. 188236-297. http://dx.doi.org/10.3354/meps188263 [ Links ]
COETZEE L, DU PREEZ HH and VAN VUREN JHJ (2002) Metal concentrations in Clarias gariepinus and Labeo umbratus from the Olifants and Klein Olifants River, Mpumalanga, South Africa: Zinc, copper, manganese, lead, chromium, nickel, aluminium and iron. Water SA 28 (4) 433-448. http://dx.doi.org/10.4314/wsa.v28i4.4917 [ Links ]
DALLAS HF and DAY JA (2004) The effect of water quality variables on aquatic ecosystems: A review. WRC Report No. TT 224/04. Water Research Commission, Pretoria. 222 pp. [ Links ]
DE LA CALLE I, COSTAS M, CABALEIRO N, LAVILLA I and BENDICHO C (2012) Use of high-intensity sonication for pre-treatment of biological tissues prior to multi-elemental analysis by total reflection X-ray fluorescence spectrometry. Spectrochim. Acta Part B 67 43-49. http://dx.doi.org/10.1016/j.sab.2011.12.007 [ Links ]
ELSDON TS and GILLANDERS BM (2002) Interactive effects of temperature and salinity on otolith chemistry: challenges for determining environmental histories of fish. Can. J. Fish. Aquat. Sci. 591796-1808. http://dx.doi.org/10.1139/f02-154 [ Links ]
ELSDON TS and GILLANDERS BM (2003) Relationship between water and otoliths elemental concentrations in juvenile black bream Acanthopagrus butcheri. Mar. Ecol. Prog. Ser. 260 263-272. http://dx.doi.org/10.3354/meps260263 [ Links ]
ELSDON TS and GILLANDERS BM (2004) Fish otolith chemistry influenced by exposure to multiple environmental variables. J. Exp. Mar. Biol. Ecol. 313 269-284. http://dx.doi.org/10.1016/j.jembe.2004.08.010 [ Links ]
FROST BR, BARNES CG, COLLINS WJ, ARCULUS RJ, ELLIS DJ and FROST CD (2001) A geochemical classification for granitic rocks. J. Petrol. 42 (11) 2033-2048. http://dx.doi.org/10.1093/petrology/42.11.2033 [ Links ]
GIBBS RJ (1970) Mechanisms controlling world water chemistry. Science 1701088-1090. http://dx.doi.org/10.1126/science.170.3962.1088 [ Links ]
GILLANDERS BM (2005) Otolith chemistry to determine movements of diadromous and freshwater fish. Aquat. Living Resour. 18 291-300. http://dx.doi.org/10.1051/alr:2005033 [ Links ]
GORHAM E (1961) Factors influencing supply of major ions to inland waters, with special reference to the atmosphere. Geol. Soc. Am. Bull. 72 795-840. http://dx.doi.org/10.1130/0016-7606(1961)72[795:FISOMI]2.0.CO;2 [ Links ]
HERD CDK, DWARZSKI RE and SHEARER C (2009) The behaviour of Co and Ni in olivine in planetary basalts: An experimental investigation. Am. Mineral. 94 244-255. http://dx.doi.org/10.2138/am.2009.2768 [ Links ]
JEZIERSKA B and WITESKA M (2006) The metal uptake and accumulation in fish living in polluted waters. In: Twardowska I, Allen HE, Hagblom MM and Stefaniak S (eds) Soil and Water Pollution Monitoring, Protection and Remediation. Springer, Krakow, Poland. 107-114. http://dx.doi.org/10.1007/978-1-4020-4728-2_6 [ Links ]
JORDAAN LJ (2015) Determining the role of catchment geochemistry on the chemistry of water, sediment and fish from impoundments within selected large catchments in South Africa. PhD thesis, Department of Zoology, University of Johannesburg. URL: http://hdl.handle.net/10210/13873. 281 pp. [ Links ]
JORDAAN LJ and MARITZ H (2010) Analytical methods 2009. Council for Geoscience analytical Chemistry Laboratory. Confidential report 2010-0088. Council for Geoscience, Pretoria, South Africa. [ Links ]
KAWAKAMI Y, MOCHIOKA N, MORISHITA K, TAJIMA T, NAKAGAWA H, TOH H and NAKAZONO A (1998) Factors influencing otolith strontium/calcium ratios in Anguilla japonica elvers. Environ. Biol. Fishes 52 (1-3) 299-303. http://dx.doi.org/10.1023/A:1007415420540 [ Links ]
KOTZE P, DU PREEZ HH and VAN VUREN JHJ (1999) Bioaccumulation of copper and zinc in Oreochromis mossambicus and Clarias gariepinus, from the Olifants River, Mpumalanga, South Africa. Water SA 25 (1) 99-110. [ Links ]
KRAUSE RT and SECOR DH (2004) Incorporation of strontium into otoliths of an estuarine fish. J. Exp. Mar. Biol. Ecol. 30285-106. http://dx.doi.org/10.1016/j.jembe.2003.10.004 [ Links ]
LIMBURG KE (1995) Otolith strontium trace environmental history of subyearling American shad Aloas sapidissima. Mar. Ecol. Prog. Ser. 119 25-35. http://dx.doi.org/10.3354/meps119025 [ Links ]
MARTIN GB, THORROLD SR and JONES CM (2004) Temperature and salinity effects on strontium incorporation in otoliths of larval spot (Leiostomus xanthurus). Can. J. Fish. Aquat. Sci. 61 34-42. http://dx.doi.org/10.1139/f03-143 [ Links ]
NATIONAL RESEARCH COUNCIL CANADA (2007) DORM-3 Fish protein certified reference material for trace metals. Institute for National Measurement Standards, National Research Council, Canada. 4 pp. [ Links ]
NATIONAL RESEARCH COUNCIL CANADA (2008) DOLT-4. Dogfish liver certified reference material for trace metals. Institute for National Measurement Standards, National Research Council, Canada. 4 pp. [ Links ]
NIOGI S and WOOD CM (2004) Biotic ligand model, a flexible tool for developing site-specific water quality guidelines for metals. Envron. Sci. Technol. 38 (23) 6177-6192. http://dx.doi.org/10.1021/es0496524 [ Links ]
NUSSEY G, VAN VUREN JHJ and DU PREEZ HH (2000) Bioaccumulation of chromium, manganese, nickel and lead in the tissues of the moggel, Labeo umbratus (Cyprinidae), from Witbank Dam, Mpumalanga. Water SA 26 (2) 269-284. [ Links ]
POLLARD MJ, KINGSFORD MJ and BATTAGLENE SC (1999) Chemical marking of juvenile snapper, Pagrus auratus (Paridae), by incorporation of strontium into dorsal spines. Fish. Bull. 97(1) 118-131. [ Links ]
ROBINSON J and AVENANT-OLDEWAGE A (1997) Chromium, copper, iron and manganese bioaccumulation in some organs and tissues of Oreochromis mossambicus from the lower Olifants River, inside the Kruger National Park. Water SA 23 (4) 387-403. [ Links ]
SAKO A, O'REILLY CM, HANNIGAN R, BICKFORD N and JOHNSON RL (2005) Variations in otolith elemental compositions of two clupeid species, Stolothrissa tanganicae and Limnothrissa miodon in Lake Tanganyika. Geochem. Explor. Environ. Anal. 5 91-97. http://dx.doi.org/10.1144/1467-7873/03-039 [ Links ]
SANNAC S, LENER JP and DARROUZER J (2012) Enhancing the productivity of food sample analysis with the Agilent 7700x ICP-MS. Application note, Food testing. Agilent Technologies Inc. Paris, France. [ Links ]
SECOR DH, HENDERSON-ARZAPALO A and PICCOLI PM (1995) Can otolith microchemistry chart patterns of migration and habitat utilization in anadromous fishes? J. Exp. Mar. Biol. Ecol. 195 15-33. http://dx.doi.org/10.1016/0022-0981(95)00054-U [ Links ]
SOUTH AFRICAN BUREAU OF STANDARDS (2010a) SABS Water-Check Group 1, April 2010, Final Report. SABS, Pretoria. 14 pp. [ Links ]
SOUTH AFRICAN BUREAU OF STANDARDS (2010b) SABS Water-Check Group 1, June 2010, Final Report. SABS, Pretoria. 14 pp. [ Links ]
SOUTH AFRICAN BUREAU OF STANDARDS (2010c) SABS Water-Check Group 1, October 2010, Final Report. SABS, Pretoria. 14 pp. [ Links ]
TOWNSEND DW, RADTKE RL, CORWIN S and LIBBY DA (1992) Strontium:calcium ratios in juvenile Atlantic herring Clupea harengus otoliths as a function of water temperature. J. Exp. Mar. Biol. Ecol. 160131-140. http://dx.doi.org/10.1016/0022-0981(92)90115-Q [ Links ]
WALTHER BD and THORROLD SR (2006) Water not food, contributes the majority of strontium and barium deposited in the otoliths of marine fish. Mar. Ecol. Prog. Ser. 311 125-130. http://dx.doi.org/10.3354/meps311125 [ Links ]
WELLS BK, RIEMAN BE, CLAYTON JL, HORAN DL and JONES CM (2003) Relationships between water, otolith, and scale chemistries of Westslope cutthroat trout from the Coeur d'Alene River, Idaho: The potential application of hard-part chemistry to describe movements in freshwater. Tran. Am. Fish. Soc. 132409-424. http://dx.doi.org/10.1577/1548-8659(2003)132<0409:rbwoas>2.0.co;2 [ Links ]
WEPENER V and VERMEULEN LA (2005) A note on the concentrations and bioavailability of selected metals in sediments of Richards Bay Harbour, South Africa. Water SA 34 (4) 589-595. [ Links ]
WEPENER V, VAN DYK C, BERVOETS L, O'BRIEN G, COVACI A and CLOETE Y (2011) An assessment of the influence of multiple stressors on the Vaal River, South Africa. Phys. Chem. Earth 36(14-15) 949-962. http://dx.doi.org/10.1016/j.pce.2011.07.075 [ Links ]
WHITLEDGE G (2008) Assessment of otolith chemistry as an indicator of fish movement or transfer between the Illinois River system and Lake Michigan. Reports. Paper 6. Southern Illinois University, Carbondale. URL: http://opensiuc.lib.siu.edu/fiaq_reports/6. [ Links ]
Received: 17 December 2014
Accepted in revised form 20 November 2015
* To whom all correspondence should be addressed.e-mail: wikusj@geoscience.org.za