Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Water SA
On-line version ISSN 1816-7950
Print version ISSN 0378-4738
Water SA vol.42 n.1 Pretoria Jan. 2016
http://dx.doi.org/10.4314/wsa.v42i1.09
Fate, behaviour, and implications of ZnO nanoparticles in a simulated wastewater treatment plant
EFC ChaúqueI; JN ZvimbaII; JC NgilaI; N MuseeIII, *
IDepartment of Applied Chemistry, University of Johannesburg, Doornfontein 2028 Johannesburg, South Africa
IIWater Research Commission, Private Bag X03, Gezina, 0031, Pretoria, South Africa
IIIDepartment of Chemical Engineering, University of Pretoria, Private Bag X20, Hatfield 0028, Pretoria, South Africa
ABSTRACT
Increased use of engineered nanoparticles (ENPs) has resulted in their entry into municipal wastewater treatment plants (WWTPs) as their final sinks. However, the adverse impact of ENPs on the bacterial activity in the activated sludge WWTPs is not yet well understood, despite their increased release into such systems. In this study, the impacts on WWTPS associated with the disposal of zinc oxide (ZnO) ENPs was investigated using a simulated WWTP developed as per the prescribed Organization for Economic Co-operation and Development (OECD 303A) specifications. Analyses were done to determine zinc concentrations at various stages of the setup, mainly in the raw wastewater and treated effluent, using inductively coupled plasma optical emission spectrometry (ICP-OES). The results obtained indicated low levels of zinc residue (about 50-200 μg/L) in the treated effluent compared to relatively high concentrations of Zn in the sludge (about 3 000 mg/kg). Results reported herein imply precipitation of ZnO ENPs during wastewater treatment processes and hence its high levels in the sludge. The presence of solid Zn in the sludge was determined using X-ray diffraction spectroscopy (XRD). Overall, no significant impact of ZnO ENPs on the performance of the simulated WWTP was observed, in terms of the removal levels of chemical oxygen demand (COD) during the treatment process
Keywords: wastewater, activated sludge, nanoparticles, zinc oxide, OECD 303A
INTRODUCTION
Biological wastewater treatment processes employ a consortium of heterotrophic and autotrophic bacteria, essentially to degrade organic matter present in wastewater. Generally, the activated sludge process treats biodegradable organic material in domestic sewage as well as effluents from other sources such as pulp and paper mills, food industries, abattoirs, textile mills, edible oils, coal gasification wastes, petrochemical wastes, and oil refinery wastes (Henze et al., 2002; Metcalf and Eddy, 2002). The sorption of pollutants on activated sludge is among the fundamental processes for the removal of toxic substances including metals, synthetic organic chemicals, suspended solids, and pathogens in wastewater (Dobbs et al., 1989; Musee et al., 2007; Sheng et al., 2008). However, the sorption process may be ineffective as the bacteria used for wastewater treatment can be inhibited by toxic substances, e.g., heavy metals (Çeçen et al., 2010), thus adversely impacting the biologically-based treatment processes.
The advent of nanotechnology has resulted in fast production and wide usage of engineered nanoparticles (ENPs) in consumer products and industrial applications. This has led to unintended release of ENPs into environmental systems at different stages of their product life cycles (e.g. manufacturing, use, etc.) (Koehler et al., 2008; Musee, 2011). For instance, zinc oxide (ZnO) ENPs are incorporated in numerous products including sunscreens, paints, cosmetics, dye-synthesized cells, plastic additives, catalysts and electronics (Woodrow Wilson, 2009; BCC Research, 2012; Piccinno et al., 2012); which in turn could lead to their release into natural and technical systems, e.g., wastewater treatment plants (WWTPs).
For example, ENPs released into wastewater from various sources eventually enter WWTPs (Kiser et al., 2009), and currently ENPs are considered among the rapidly emerging contaminants in municipal wastewater systems (Brar et al., 2010; Musee et al., 2011). Therefore, WWTPs are likely potential major point sources of ENP release into the environment, due to broad-scale and increasing usage of nanoproducts (Woodrow Wilson, 2015; BCC Research, 2012; Piccinno et al., 2012). Among the likely pathways of ENP release into the environment from WWTPs are water systems, soils, and air, through treated effluent, bio-solids, and plant-generated aerosols, respectively, (Limbach et al., 2008; Kiser et al., 2010; Musee, 2011; Westerhoff et al., 2011).
The effects of ZnO ENPs on aquatic organisms such as bacteria in different media have been investigated, and the observed toxicity was attributed to either soluble (dissolved) Zn2+ species, ZnO particulates, or both forms (Jiang et al., 2009; Wong et al., 2010; Thwala et al., 2013). Padmavathy and Vijayaraghavan (2008), using the disk diffusion method, and Premanathan et al. (2011), using the resazurin incorporation method, found the minimum inhibitory concentration (MIC) of nano ZnO against Escherichia coli to be 400 and 500 μg/mL, respectively. Moreover, Premanathan et al. (2011) indicated that the inhibitory activity was due to the generation of reactive oxygen species (ROS) which, in turn, induced apoptosis. However, a study by Liu et al. (2011) indicated that ZnO ENP toxicity to the biological populations in activated sludge (i.e. endogenous respiration, BOD biodegradation and nitrification) was solely due to soluble Zn2+ generated upon ZnO ENP dissolution. This finding points to the role of ENP dissolution for the generation of toxic effects on activated sludge bacterial communities. However, Musee and co-workers (2014) reported limited dissolution and impact of ZnO ENPs on bacterial viability under typical wastewater conditions.
In aqueous environments, natural organic matter (NOM) and other biomass can adsorb onto ENP surfaces (Zhang et al., 2009) and, in turn, enhance their stability, even at high ionic strength (Keller et al., 2010; Zhou and Keller, 2010). It has been reported that, for example, typical environmental concentrations of humic acids are sufficiently high to stabilize even high ZnO ENP concentrations, of up to 100 mg/L (Omar et al., 2014). However, the impacts of metal oxide ENPs on the treatment efficiency of WWTPs are largely unknown, and therefore, not adequately quantified (Brar et al., 2010). Moreover, there is evident discord in the published literature regarding the fate and behaviour of ZnO ENPs (Musee et al., 2014) as well as how this influences their toxicity (Liu et al., 2011).
The main goal of this study was to elucidate the fate and behaviour of ZnO ENPs in a simulated WWTP, developed as prescribed by the Organization for Economic Co-operation and Development (OECD) guideline 303A (OECD 303A, 2001). To achieve this goal, three specific objectives were set:
i. To establish if transformation of ZnO ENPs occurred during the wastewater treatment process through characterization of ENP physicochemical parameters - e.g., morphology, formation of mineral phases, and association with organic matter - using XRD, transmission electron microscopy (TEM), and ultraviolet and visible spectroscopy (UV-Vis), before and after ZnO ENPs were dosed into influent wastewater
ii. Quantify the effect of ZnO ENPs on wastewater treatment processes by monitoring COD removal following the introduction of ENPs
iii. Through objectives (i) to (ii), establish the efficiency of the OECD WWTP model in the removal of COD and organic matter
MATERIALS AND METHODS
Collection and characterization of wastewater
Raw wastewater was withdrawn from a WWTP in Johannesburg (Johannesburg Water, Northern Wastewater Treatment Works; Gauteng, South Africa) that mainly collects and treats domestic sewage. The wastewater was stored at 4°C before use. Wastewater physical and chemical characteristics, namely, dissolved oxygen (DO) and chemical oxygen demand (COD) were measured using an oxygen meter (HANNA Instruments, Portugal), and a photometer (HACH, DR 3900, USA), respectively. The pH and conductivity were determined using pH meter and conductivity meters (METTLER TOLEDO Technologies, USA). All wastewater parameters were characterized as per the Standard Methods for the Examination of Water and Wastewater (Eaton et al., 2005). The wastewater physicochemical characteristics were continuously monitored (every 12 h for 77 d) in the influent as well as aeration chamber and effluent, before and after the ZnO ENPs were dosed into the simulated WWTP.
Zinc oxide nanoparticle suspensions
Non-coated ZnO ENPs (#544906) were obtained from Sigma (Johannesburg, South Africa). TEM (JEOL Model JEM -2100F, Japan) and XRD (Philips, X'Pert PRO MPD, mineral powder diffraction) analyses of ZnO ENPs were carried out to establish their morphology and chemical state, respectively. XRD used to analyse ZnO ENP morphology and size in the sludge was equipped with monochromatic Cu Ka radiation (λ = 0.15406 nm). Diffraction patterns were recorded in the 26 angular range from 10-80° with step sizes of 0.02°, at 40 kV and 40 A. Additional solid state analysis of the sludge was done using UV-Vis spectrophotometry (UV-2450 UV-Vis spectrophotometer, Shimadzu, Japan) coupled with UV-Probe and the patterns were collected for the 250 to 700 nm electromagnetic spectrum region using BaSO4 as the reference material.
Three suspensions of ZnO ENPs with concentrations of 5, 10, and 20 mg/L were prepared by adding 0.1250, 0.2500, and 0.5000 g, respectively, of ZnO nanopowder to 1 L deionized water. Using a sonicator (Model 2000U, Ultrasonic Power Corp.) the suspensions were sonicated at 20 KHz for 30 min to break the aggregates before each suspension was added to the wastewater in the holding container. Each set concentration (5, 10, or 20 mg/L) was achieved by making the volume to 25 L using wastewater. Ice was added into the sonicator to minimize the possibility of ZnO ENP dissolution due to heat generated during the sonication process.
Simulated activated sludge wastewater treatment plant setup
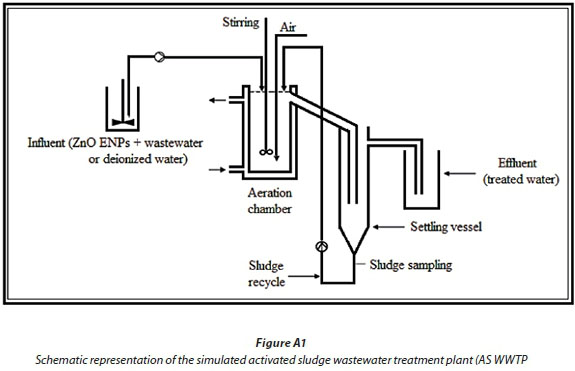
The simulated activated sludge wastewater treatment plant (SAS WWTP) was constructed following the Organization for Economic Co-operation and Development (OECD 303A, 2001) specifications. The tests were conducted in aerobic digesters each designed to hold 3 L of activated sludge. Each model unit comprised of: influent holding container (25 L), a stirred and aerated tank reactor (aeration chamber), and a clarifier (settling vessel) simulating biological treatment using an activated sludge system (see supporting information in Appendix 1: Figs A1 and A2; schematic diagram and photograph of the simulated wastewater treatment plant), respectively.
Aeration chambers
Each aeration chamber (3 L) was continuously stirred using IKA RW 16 basic stirrer to ensure thorough mixing of the substrate, essentially to mimic actual WWTP operational conditions. The aeration chamber was aerated at a flow rate of 0.29 L/min to maintain the dissolved oxygen above 2 mg/L using compressed air through a glass frit. In this study, 2 model units were used as test and control units. Both units (test and control) were fed with wastewater spiked with ZnO ENPs using Watson-Marlow (Falmouth, Cornwall, UK) 120S/DV pumps at 29 r/min. Deionized water was used as the control exposure media to offer a baseline comparison with the complex wastewater, and particularly to account for the ENP removal mechanism(s).
For the test unit, the influent wastewater was spiked with ZnO ENPs (at varied concentrations of: 5, 10, 20 mg/L) in 25 L containers and continuously stirred using IKA RW 20 digital stirrer at 1 800 r/min to keep the ENPs well dispersed in suspension. The use of ZnO ENPs in suspension was an attempt to mimic their actual pathway introduction into the WWTPs, and also to enhance reproducibility of the test conditions. Settling vessels of 1.5 L were used to separate treated effluent from the activated sludge. In accordance to standard practice, a portion of the sludge from the settling vessel was re-introduced as return activated sludge (RAS) into the aeration chamber to replenish the biomass, and to maintain the total suspended solids (TSS) in the range of 2 to 3 g/L of dry sludge. The aeration chamber with hydraulic retention time (HRT) of 6 h was designed to maintain nitrifying conditions, and the influent was introduced at feed flow rate of 0.50 L/h using peristaltic pumps. The study lasted for 77 d to establish the long-term effects of ZnO ENPs on the removal of organic matter from WWTPs, where COD removal was monitored continuously as a surrogate parameter, as previously reported (Musee et al., 2014).
Simulated activated sludge WWTP operation
Using 425 μm stainless steel mesh the raw wastewater was filtered before it was pumped into the operating units to remove big particulates, and to avoid clogging the tubing system. This operation mimicked the screening process of raw wastewater used in large-scale WWTPs. The simulated WWTP was acclimatized, stabilized, and optimized during the first 14 days followed by dosing with ZnO ENPs over 21 days for each concentration (5, 10, or 20 mg/L). Continuous sampling was carried out at the influent holding container, aeration chamber, settling vessel, and waste activated sludge chamber in order to monitor various wastewater physicochemical parameters. The effluent samples from the aeration chamber were filtered using 0.45 μm polyvinylidene difluoride (PVDF) syringe filter before performing COD and zinc analyses. On the other hand, the analysed influent samples were collected directly from the influent container. A similar procedure was followed in operating the control unit and analysing samples for the COD.
Determination of ZnO ENP dissolution
To determine the dissolution of ZnO ENPs in wastewater, the influent and effluent wastewater were continuously collected after every 12 h for 77 d from the set up and acidified with 10 M HNO3 to pH < 2, before being stored at 4°C until analysis. Additionally, the sludge was also continuously withdrawn, filtered (Whatman 41 filter paper), dried in the oven at 103-105°C, ground with mortar and pestle, and then stored in dry powder form before analysis. In this study, the digestion method used followed the procedure of Martin et al. (1994).
Zinc dissolution was evaluated using inductively coupled plasma optical emission spectrometry (ICP-OES) (SPECTRO ARCOS, Analytical Instruments GmbH, Germany) by measuring the concentration of zinc in the raw wastewater. First, the background zinc concentration in the blank solution was determined and subtracted from the concentration of Zn2+ in the wastewater dosed with ZnO ENP at 3 different concentrations. The ICP-OES was operated under forward power of 1 400 W, plasma argon flow rate of 13 L/ min, auxiliary argon flow rate of 2.00 L/min, and nebulizer argon flow rate of 0.95 L/min.
Aliquots from the test and control units were collected periodically to monitor the ZnO ENPs dissolution with time. The influence of ZnO ENP concentration on the wastewater treatment process was investigated as a function of COD removal efficiency.
The organic matter removal in the SAS WWTP was assessed through monitoring of COD removal after dosing ZnO ENP into the test unit containing influent wastewater and the control unit containing deionized water. Four ZnO ENP concentrations values were used in the study: 0, 5, 10, and 20 mg/L.
RESULTS AND DISCUSSION
Simulated activated sludge wastewater treatment plant performance
Physical parameters of commercial ZnO ENPs
The raw wastewater characteristics were monitored over time and yielded the following results: total dissolved solids (TDS) 344 ± 43 mg/L, pH 7 ± 0.4, chemical oxygen demand (COD) 543 ± 159 mg/L, conductivity 715 ± 52 μS/cm, salinity 0.3 ± 0.1 psu, and total suspended solids (TSS) 588 ± 39 mg/L. DO in the raw wastewater was 4 ± 0.4 mg/L, and was maintained above 2 mg/L in the aeration chamber throughout the experiment using compressed air to provide the bacteria with adequate oxygen supply.
Zinc oxide ENP suspensions
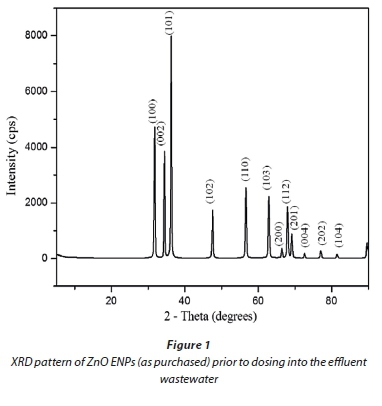
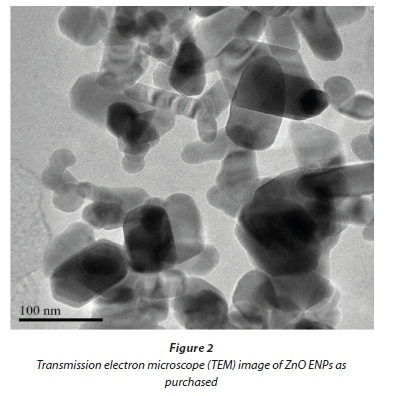
XRD results for commercial ZnO ENPs (Fig. 1) confirmed that they were in pure phase, as evidenced by narrow spectral peaks with high intensity and hexagonal Wurtzite crystal structure. TEM images showed a size distribution for the ENPs in the range 10 to 130 nm (Fig. 2), consisting of a heterogeneous mixture of rods, cubes, regular, and irregular spheres.
Effect of ZnO ENPs on COD removal
As observed in Fig. 3, organic matter removal reached a steady state at about 50 mg/L COD in the effluent, irrespective of its initial concentration in the influent and ZnO ENP dosing concentration. The results in Fig. 3 further indicate efficient organic matter removal with the average COD in the effluent ranging from 60-100 mg/L. Therefore, our results point to efficient removal of ZnO ENPs in the secondary treatment stage of WWTPs (cf. Sonune and Ghate, 2004). The average removal efficiency of COD observed was 91% in the test chamber; hence the simulated WWTP met the validity criteria as outlined in the OECD 303 A guidelines (OECD 303A, 2001). The COD removal results also suggest that dosing the influent with ZnO ENPs had no significant adverse impacts on the overall treatment efficiency of the stimulated WWTP, which is in agreement with earlier studies (Hou et al., 2013; Tan et al., 2015), especially at low dosing concentrations.

In light of our results and currently predicted relevant environmental concentrations of ZnO ENPs of around 24-300 μg/ L, where removal efficiency was assumed to be about 93% (Sun et al., 2014), it is unlikely that ZnO nanoparticles will impact on COD removal in WWTPs. However, as the production and use of ZnO ENPs increases their environmental concentrations will also increase with the possibility of causing potentially adverse effects. For example, Musee et al. (2014) reported negative impacts of ZnO ENPs at higher concentrations (100 mg/L): a 3-L bioreactor (simulated WWTP) with hydraulic residence time (HRT) of 6 h at a dose rate of 0.83 mg/min for 240 h yielded a mean COD removal efficiency of 71 ± 7%, which was much lower than that for the control (80 ± 5%). Similar inhibition of COD removal due to ZnO ENPs has also been reported for a batch membrane bioreactor, especially at high dose concentrations (Huang et al., 2013).
Notably, in the present study, though the simulated WWTP was exposed to high concentrations of ZnO ENPs, of up to 20 mg/L (levels unlikely to be found in actual treatment systems), the COD removal remained high. On the other hand, earlier toxicity studies showed that 10 mg/L of ZnO ENPs could induce significant growth inhibition of up to 90% and 20%, respectively, for Bacillus subtilis and E. coli (Adams et al., 2006). Thus, a number of reasons may account for high COD removal as the activated sludge in WWTPs entails large consortia of bacteria species; therefore, the potential impact of ZnO NPs on activated sludge based on the toxicity of ZnO NPs for pure bacteria is inadequate to account for these results.
Firstly, following ZnO ENP dissolution, cationic species could form complexes with natural organic matter, such as humic acid which is ubiquitous in wastewater and contains functional groups such as carboxylic (-COO-) and phenolic (-ArO-). These functional groups are known for their high complexation capacity with metal ions (Zhang et al., 2009). Thus, humic acid may cause the removal of zinc through processes such as: complexation, precipitation of zinc (e.g. through formation of insoluble Zn(OH)+), and/or sorption of particulate ZnO ENPs (Applerot et al., 2009; Musee et al., 2014). In addition, neither ionic nor pure particulate forms of ZnO ENPs were present at high enough levels in the wastewater to cause adverse impacts by altering the microbial function in COD removal.
Secondly, ZnO ENPs may have agglomerated after being introduced into the influent as the dispersion of ENPs in wastewater is influenced by solution chemistry parameters, such as pH, ionic strength, and dissolved organic carbon. Kang et al. (2009) showed that higher conductivity and divalent cation concentrations (e.g., Mg2+ and Ca2+) in wastewater effluents caused the ENPs be more aggregated than in freshwater systems, thereby influencing their plausible interactions with microbial populations. In this study, high conductivity of wastewater was observed. Agglomeration may have led to a drastic reduction in the bioavailable forms of ENPs (ionic and/ or particulate forms) essential to influence COD removal. For instance, it is likely that once the ENPs underwent agglomeration several processes could result, namely: (i) reduction in surface area essential for biointerfaces with bacteria (as evidenced by the increasing hydrodynamic size), (ii) reduction in the number of particles, and (iii) reduction in interfacial free energy (Oberdörster et al., 2005; Pettibone et al., 2008; Lowry et al., 2012). However, it should be noted that, to date, it is unclear as to the degree to which each of these potential mechanisms (i-iii) accounts for the diminished impact of ZnO ENPs on COD removal. Thus the mechanisms that may explain why the ZnO ENPs had minimal impact on the removal of COD in this study merit further investigation.
ZnO ENP removal and plausible mechanisms during SAS WWTP processes
Residual zinc concentration in the effluent was 50-150 μg/L at 5 mg/L dosing concentration of ZnO ENPs (Fig. 4a). In the deionized water (control unit) residual Zn2+ was found to be approximately 6 times higher (~ 1 mg/L; Fig. 4b) than in the test unit. Dissolution of ENPs is dependent on the media chemistry (e.g. pH, organic matter, ionic strength, etc.) and inherent physicochemical properties (size, coating, surface chemistry, etc.) (Xia et al., 2008; Bian et al., 2011; Peralta-Videa et al., 2011).
Results indicate that the organic matter played an important role in accounting for the significant differences (based on ANOVA statistical test at p = 0.05) in the amounts of zinc released by both the test and control media. As an example, the organic matter may have inhibited the chemical and physical 'speciation' of the ZnO ENPs through complexation processes and, in turn, led to a limited release of ionic species. In addition, the organic matter, through binding processes, may have adsorbed the solubilized metal species, causing a reduction in the quantities of metal ions observed in the effluent relative to the influent. Such scenarios point to different removal pathways of ZnO ENPs from the influent wastewater and the deionized water. For instance, as deionized water is devoid of organic matter, the ENP removal mechanism was possibly governed by other forms of abiotic factors, namely, pH and ionic strength, as adsorption was not feasible.
Effect of concentration on ZnO ENP removal
High levels of ENP removal from the wastewater could not be wholly linked to the influence of pH and ionic strength, although earlier findings have indicated that ZnO ENP stability is dependent on pH and ionic strength of wastewater (Chaúque et al., 2014). Additional influencing factors on ENP stability in aqueous media include natural organic matter (NOM). NOM is composed of humic substances (Becker et al., 2004), highly ubiquitous in wastewater, and offers a large surface area for contaminant adsorption (Metcalf and Eddy, 2002). Furthermore, NOM has been shown to enhance ENP stability in wastewater (Keller et al., 2010; Mu and Chen, 2011), and is therefore a significant factor influencing their removal in wastewater (Kiser et al., 2010).
For instance, NOM is known to stabilize ZnO ENPs (Bian et al., 2011) and, in turn, retard the dissolution rate. For ZnO ENPs in wastewater, it has been suggested that the retardation of dissolution was due to the hydrophobic nature of capric/ caprylic triglyceride dispersion formation (Lombi et al., 2012). Moreover, Liu et al. (2011) observed low zinc releases from wastewater due to the adsorption of Zn2+ ions onto the activated sludge immediately after their release from ZnO ENPs. To date, it is unclear which of the two mechanisms dominantly influences the fate and behaviour of ENPs as contaminants in wastewater, and/or whether they act concurrently in an antagonistic or synergistic fashion.
In this study, considering that the activated sludge pH was at 7 ± 0.4, the biomass was kept in the range of 2 000-3 000 mg/L of TSS - which is within the limits of typical WWTPs with biomass concentrations of 1 000-5 000 mg/L of TSS (Metcalf and Eddy, 2002) - and an average sludge retention time (SRT) of 6 d, provided favourable conditions for enhanced stabilization of ZnO ENPs by the NOM. The most plausible explanation is that the NOM played a dominant role in removing zinc ions from the influent wastewater. This is because humic acid is known to stabilize ZnO ENPs even at high concentrations (Omar et al., 2014), in addition to its ability to counteract the influence of high ionic strength, expressed as salinity (0.3 ± 0.1 psu) for the wastewater investigated in this study.
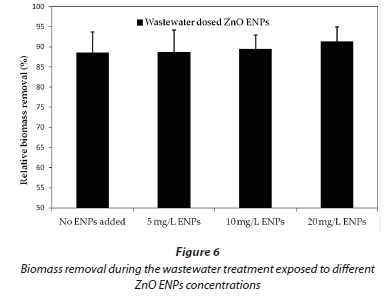
Low zinc concentrations (less than 3%) in the effluent, as shown in Fig. 4a, yielded similar observations to those of other researchers; e.g. Bolyard et al. (2013) and Mu and Chen (2011). These workers reported zinc release into the treated effluent as less than 2%, which accounted for the large percentages of zinc found in the activated sludge. Our findings suggest that the digestion process in the test chamber was responsible for the high percentage removal of ZnO ENPs, as confirmed by the observed low concentration of Zn2+ in the effluent. Furthermore, our results indicate that the release of zinc ions into the treated effluent exhibited a linear increase as the dosing ZnO ENPs concentration increased. Nevertheless, the Zn2+ released into the effluent was not high enough to adversely impact the organic matter removal, as ZnO ENP concentration was increased from 5 mg/L to 20 mg/L (Figs 5 and 6).

Spiking the influent with increasing concentrations of ZnO ENPs showed a substantial increase in the released Zn2+. In this study, lower dosing concentrations exhibited higher ZnO NP dissolution compared to higher concentrations. The average concentrations of the released zinc ions were, respectively, 0.105 (2.1% of nominal ZnO), 0.150 (1.52%), and 0.270 (1.35%) mg/L at ZnO NPs concentrations of 5, 10, and 20 mg/L (Fig. 5). Our results were similar to those reported elsewhere for ZnO ENPs, e.g. Mu and Chen (2011), Xiong et al. (2011), and Thwala et al. (2013), whereas the dosing concentration increase led to enhanced aggregation (Maximova and Dahl, 2006), which is expected because of a higher probability of particle collisions - which ultimately leads to a decline in the dissolution rate (Xiong et al., 2011; Thwala et al., 2013).
Figure 6 shows biomass removal during wastewater treatment at different ZnO ENP dosing concentrations. The change in percentage biomass removal as the concentration of ZnO ENPs increased was found to be minimal. We had postulated that the ZnO ENP adsorption to the activated sludge would largely account for the removal mechanism. However, this does not appear to entirely account for these results. Our findings are, in some cases, in close agreement with that reported in the literature by various workers, while in other cases they differ. A few examples of studies related to biomass removal and release of Zn2+ ions into the treated effluent are discussed here to illustrate this observation:
Kiser et al. (2009) reported the sorption of titanium from TiO2 ENPs by biomass during wastewater treatment; however, 10-100 μg/L Ti remained in the effluent. Limbach and colleagues (2008) observed the presence of 2-5 mg/L of cerium oxide in the effluent following exposure to 100 mg/L of CeO2 ENPs in a model wastewater treatment process. Mu and Chen (2011) illustrated that a large amount of ZnO ENPs were adsorbed on the surface of activated sludge after a long-term exposure. Moreover, Liu and colleagues (2011) indicated that both the zinc ions and ZnO ENPs were adsorbed on activated sludge.
Studies by Klaine et al. (2008), Keller et al., (2010) and Bian et al. (2011) also showed that the removal of ENPs from aqueous media was dictated by chemical composition of the testing media chemistry (e.g. pH, organic matter, and ionic strength). Keller and co-workers (2010) reported enhanced stability of metal-oxides such as ZnO, TiO2 and CeO2 ENPs with organic matter adsorption to the particle surface providing a form of barrier to aggregation.
Analysis of ZnO ENPs in the sludge
In the previous section, results indicated that a large amount of the ZnO ENPs spiked into the influent were adsorbed onto the activated sludge, and removed from the effluent (Figs 4a and 5). Therefore, only a small fraction of the ENPs was likely to be released into the treated effluent. However, the second route for the transport of the ZnO ENPs into the environment is via the sludge. The characteristics of ENPs in the sludge were therefore analysed using XRD (Fig. 7), and UV-Vis spectrophotometer coupled with UV-Probe (Fig. 8).
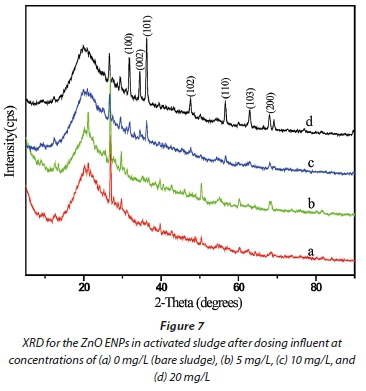
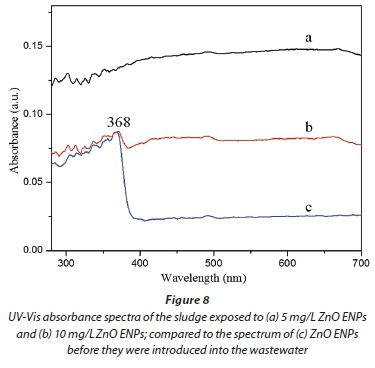
From the XRD spectra (Fig. 7, line b), no signal was detected in the sludge for the ZnO ENPs at low concentration of 5 mg/L, and this observation was confirmed by UV-Vis results (Fig. 8, line a). However, the XRD pattern showed marginal peaks after the dosage was increased to 10 mg/L and was characterized by low intensities (Fig. 7, line c), collaborated by UV-Vis results (Fig. 8, line b). For the UV-Vis, at 10 mg/L ZnO ENPs, an excitonic absorption peak at 368 nm was observed (Fig. 8, line b), though not as distinctive as in the case of ENPs before they were dosed into the influent (Fig. 1). Clear XRD patterns were observed at a dosage of 20 mg/L, and the peaks corresponded to characteristic polycrystalline hexagonal Wurtzite structure - signifying that the ZnO ENPs were in pure phase. The size distribution of ZnO ENPs in the sludge was computed based on XRD analysis and modified Scherrer's formula (Monshi et al., 2012):

where: L represents mean size of the particle distribution (nm), K is geometric factor (generally K = 0.9), λ is the wavelength of Cu Ka radiation (λ = 0.15406 nm), θ is the Bragg angle of diffraction, and ß is the full width at half maximum (FWHM) of the diffraction main peak at 26. The computed mean crystallite size distribution of sludge, determined for the influent dosed with 10 and 20 mg/L, was approximately 29.22 ± 2.47 nm.
In earlier work (Musee et al., 2014), where a high ZnO ENP concentration of 100 mg/L was used, sharper peaks were observed including the 002 diffraction peak - which signified one-dimensional nano-rod formation of ZnO ENPs. In the current study similar observation of sharp peaks was made at a relatively lower dosage of 20 mg/L ZnO ENPs.
Two key aspects merit highlighting. First, from our study, it was found to not be possible to detect very low concentrations of ZnO ENPs using the current analytical techniques, in this case ICP-OES, for determining zinc ions (Fig. 4) in complex matrixes such as wastewater, or XRD for determining zinc phases in the sludge (Fig. 7). Secondly, a comparison of XRD pattern results for ZnO ENPs (summarized in Figs 1 and 7, respectively), before being introduced into influent and after dosed in the influent, shows that their morphology did not change despite possible adsorption of the ENPs onto the sludge matrix.
Moreover, we observed a high efficiency of removal of ZnO ENPs (> 96%) from effluent wastewater. Therefore, the low concentrations of ZnO ENPs detected in the treated effluent indicated that it is unlikely that there is significant release and dispersion of ENPs into aquatic systems resulting from WWTPs as point sources. Furthermore, the low quantities of ENPs likely to be released through treated effluent into the environment can be further reduced by incorporating additional treatment steps such as the use of membranes.
Thirdly, it is important to take caution when generalizing findings about the fate and behaviour of ENPs, for example, based on studies on the same or other types of nanoparticles in in WWTPs. This is because increasing reports have shown that WWTPs exhibit non-uniformity of removal efficiencies for diverse types and forms of ENPs: e.g., 94% of CeO2 (Limbach et al., 2008); 75-85% of TiO2 (Kiser et al., 2009); 94% of CeO2 (Gómez-Rivera et al., 2012); 97% of TiO2; 95% of nC60; and 88% of Ag (Wang et al., 2012); >95% of TiO2 (Gartiser et al., 2013). These differences can be linked to the ENP type (e.g. surface properties and chemistry), and the nature of wastewater physical and chemical properties - where the latter varies widely due to the constituent components.
Due to elevated concentrations of ZnO ENPs in the sludge caution should be taken in utilizing such sludge for agricultural purposes or during disposal phase. As such, this calls for adoption of additional treatment steps to mitigate against possible dispersion of ENPs from various disposal mechanisms such as landfilling or incineration, as well as in agricultural applications. This has significant relevance in a country like South Africa, for instance, where over 80% to 97% of sludge from WWTPs (Musee, 2011) is used for agricultural purposes, and the rest disposed of through landfilling. Recent studies (Lombi et al., 2012; Wang et al., 2012) have also highlighted the likelihood of increased transfer of ENPs into the environment from WWTPs via different pathways, for instance, leaching from landfills - and therefore call for the adoption of mitigative approaches to guard against unknown long-term adverse effects of ENPs in aquatic systems.
Nitrification is an important process in numerous WWTPs and, therefore, likely to be sensitive to toxic effects of ENMs. Thus, to ensure the simulated wastewater treatment system results provide representative findings, both nitrification and denitrification processes will be considered in future work.
CONCLUSIONS
In this study, we carried out experiments to: (i) determine the fate and behaviour of ENPs in a simulated WWTP system following the OECD 303 A guideline, (ii) investigate plausible transformation of ZnO ENPs after entry into wastewater up to the point of discharge of the treated effluent, and (iii) examine the effect of increasing concentration of ZnO ENPs on the efficiency of organic matter removal from wastewater. The following observations were made and inferences have been drawn:
• ZnO ENPs were efficiently removed from wastewater with an insignificant portion being released into the environment through the treated effluent. A large percentage of the ENPs settled out into the sludge. However, the fraction of unreacted ENPs in the effluent may potentially be released into the environment. In order to mitigate any possible release of nanoparticles into the environment, additional removal methods may need to be put in place. Further investigations are necessary to collect more data on these processes.
• Under wastewater conditions most ENPs aggregated and were attached to the biomass and, therefore, were removed from influent through adsorption processes, for all of the concentration levels investigated.
• The currently available techniques for detection and quantification of metal-based ENPs, such as XRD and UV-Vis, were found to be inadequate since they could not detect ZnO ENPs in the sludge even after the influent was dosed with concentrations as high as 5 mg/L and above. Yet, a concentration of 5 mg/L ZnO ENPs is many times above the expected actual concentrations of metal-based nanoparticles in the environment (Musee, 2011; Gottschalk et al., 2013).
ACKNOWLEDGEMENTS
The study was supported by the Water Research Commission (WRC) ( Project No. K5/2122) (EFCC, JNZ, JCN, NM) and Parliamentary Grant #EINI002 (NM, JNZ) from the CSIR. We would like to thank Siyasanga Mpelane (MINTEK) and Mathokone Jonas Shai (University of Johannesburg) for their assistance in providing the TEM and XRD services, respectively. We also acknowledge useful and insightful comments of anonymous reviewers of the manuscript.
REFERENCES
ADAMS LK, LYON DY and ALVAREZ PJJ (2006) Comparative ecotoxicity of nanoscale TiO2, SiO2, and ZnO water suspensions. Water Res. 40 3527-3532. http://dx.doi.org/10.1016/jwatres.2006.08.004 [ Links ]
APPLEROT G, LIPOVSKY A, DROR R, PERKAS N, NITZAN Y, LUBART R and GEDANKEN A (2009) Enhanced antibacterial activity of nanocrystalline ZnO due to increased ROS-mediated cell injury. Adv. Funct. Mater. 19 842-852. http://dx.doi.org/10.1002/adfm.200801081 [ Links ]
BCC RESEARCH (2012) Nanotechnology: a realistic market research. NANO 31E, Wllesley, USA. [ Links ]
BECKER WC, O'MELIA CR, AU KK and YOUNG JS (eds) (2004) Using oxidants to enhance filter performance. American Water Works Association, Denver. [ Links ]
BIAN SW, MUDUNKOTUWA IM, RUPASINGHE T and GRASSIAN VHC (2011) Aggregation and dissolution of 4 nm ZnO nanoparticles in aqueous environments: Influence of pH, ionic strength, size, and adsorption of humic acid. Langmuir 27 6059-6068. http://dx.doi.org/10.1021/la200570n [ Links ]
BOLYARD SC, REINHART DR and SANTRA S (2013) Behaviour of engineered nanoparticles in landfill leachate. Environ. Sci. Technol. 47 8114-8122. [ Links ]
BRAR SK, VERMA M, TYAGI RD and SURAMPALLI RY (2010) Engineered nanoparticles in wastewater and wastewater sludge -Evidence and impacts. Waste Manage. 30 504-520. http://dx.doi.org/10.1016/j.wasman.2009.10.012 [ Links ]
ÇEÇEN F, SEMERCI N and GEYIK AG (2010) Inhibition of respiration and distribution of Cd, Pb, Hg, Ag, and Cr species in a nitrifying sludge. J. Hazardous Mater. 178 619-627. http://dx.doi.org/10.1016/j.jhazmat.2010.01.130 [ Links ]
CHAÚQUE EFC, ZVIMBA JN, NGILA JC and MUSEE N (2014) Stability studies of commercial ZnO engineered nanoparticles in domestic wastewater. Phys. Chem. Earth 67-69 140-144. http://dx.doi.org/10.1016/j.pce.2013.09.011 [ Links ]
DOBBS RA, WANG L and GOVIND R (1989) Sorption of toxic organic compounds on wastewater solids: correlation with fundamental properties. Environ. Sci. Technol. 23 1092-1097. http://dx.doi.org/10.1021/es00067a004 [ Links ]
EATON AD, CLESCERI LS, GREENBERG AE and FRANSON MAH (2005) Standard Methods for the Examination of Water and Wastewater. American Public Health Association, Washington, DC. [ Links ]
FRANKLIN N, ROGER NJ, APTE S, BATLEY G, GADD G and CASEY P (2007) Comparative toxicity of nanoparticulate ZnO, Bulk ZnO, and ZnCl2 to a freshwater microalga (Pseudokirchneriella subcapitata): The importance of particle solubility. Environ. Sci. Technol. 41 8484-8490. http://dx.doi.org/10.1021/es071445r [ Links ]
GARTISER S, FLACH F, NICKEL C, STINTZ M, DAMME S, SCHAEFFER A, ERDINGER L and KUHLBUSCH TAJ (2014) Behaviour of nanoscale titanium dioxide in laboratory wastewater treatment plants according to OECD 303 A. Chemosphere 104 197-204. http://dx.doi.org/10.1016/jxhemosphere.2013.1L015 [ Links ]
GÓMEZ-RIVERA F, FIELD JA, BROWN D and SIERRA-ALVAREZ R (2012) Fate of cerium dioxide (CeO2) nanoparticles in municipal wastewater during activated sludge treatment. Bioresour. Technol. 108 300-304. http://dx.doi.org/10.1016/j.biortech.2011.12.113 [ Links ]
GOTTSCHALK F, SUN TY and NOWACK B (2013) Environmental concentrations of engineered nanomaterials: Review of modeling and analytical studies. Environ. Pollut. 181 287-300. http://dx.doi.org/10.1016/j.envpol.2013.06.003 [ Links ]
HENZE M, HARREMOES P, JANSEN J, LA C and ARVIN E (2002) Wastewater Treatment: Biological and Chemical Processes (3rd edn). Springer-Verlag, Berlin. ISBN: 3-540-42228-5. http://dx.doi.org/10.1007/978-3-662-04806-1 [ Links ]
HOU L, XIA J, LI K, CHEN J, WU X and LI X (2013) Removal of ZnO nanoparticles in simulated wastewater treatment processes and its effects on COD and NH4-N reduction. Water Sci. Technol. 67 254-260. http://dx.doi.org/10.2166/wst.2012.530 [ Links ]
HUANG F, WANG Z, MEI X and WU Z (2013) Effects of short-term presence of nanoparticles on MBR sludge properties and membrane fouling. Technol. Water Treat. 8 14-26. [ Links ]
JIANG W, MASHAYEKHI H and XING B (2009) Bacterial toxicity comparison between nano- and micro-scaled oxide particles. Environ. Pollut. 157 1619-1625. http://dx.doi.org/10.1016/j.envpol.2008.12.025 [ Links ]
KANG M, MAUTER S and ELIMELECH M (2009) Microbial cyto-toxicity of carbon-based nanomaterials: implications for river water and wastewater effluent. Environ. Sci. Technol. 43 2648-2653. http://dx.doi.org/10.1021/es8031506 [ Links ]
KELLER AA, WANG H, ZHOU D, LENIHAN HS, CHERR G, CARDINALE BJ, MILLER R and JI Z (2010) Stability and aggregation of metal oxide nanoparticles in natural aqueous matrices. Environ. Sci. Technol. 44 1962-1967. http://dx.doi.org/10.1021/es902987d [ Links ]
KISER MA, RYU H, JANG H, HRISTOVSKI K and WESTERHOFF P (2010) Biosorption of nanoparticles to heterotrophic wastewater biomass. Water Res. 44 4105-4114. http://dx.doi.org/10.1016/j.watres.2010.05.036 [ Links ]
KISER MA, WESTERHOFF P, BENN T, WANG Y, PÉREZ-RIVERA J and HRISTOVSKI K (2009) Titanium nanomaterial removal and release from wastewater treatment plants. Environ. Sci. Technol. 43 6757-6763. http://dx.doi.org/10.1021/es901102n [ Links ]
KOEHLER A, SOM C, HELLAND A and GOTTSCHALK F (2008) Studying the potential release of carbon nanotubes throughout the application life cycle. J. Clean Prod. 16 927-937. http://dx.doi.org/10.1016/j.jclepro.2007.04.007 [ Links ]
KLAINE JS, ALVEREZ PJJ, BATLEY GE, FERNANDES TF, HANDY RD, LYON DY, MAHENDRA S, MCLAUGHLIN MJ and LEAD JR (2008) Nanomaterials in the environment: Behavior, fate, bioavail-ability and effects. Environ. Toxicol. Chem. 27 (9) 1825-1851. http://dx.doi.org/10.1897/08-090.1 [ Links ]
LAU BLT and HSU-KIM H (2008) Precipitation and growth of zinc sulfide nanoparticles in the presence of thiol-containing natural organic ligands. Environ. Sci. Technol. 42 (19) 7236-7241. http://dx.doi.org/10.1021/es801360b [ Links ]
LIMBACH LK, BEREITER R, MULLER E, KREBS R, GALLI R and STARK WJ (2008) Removal of oxide nanoparticles in a model wastewater treatment plant: Influence of agglomeration and surfactants on clearing efficiency. Environ. Sci. Technol. 42 (15) 5828-5833. http://dx.doi.org/10.1021/es800091f [ Links ]
LIU G, WANG D, WANG J and MENDOZA C (2011) Effect of ZnO particles on activated sludge: Role of particle dissolution. Sci. Total Environ. 409 2852-2857. http://dx.doi.org/10.1016/j.scitotenv.2011.03.022 [ Links ]
LOMBI E, DONNER E, TAVAKKOLI E, TURNEY TW, NAIDU R, MILLER BW and SCHECKEL KG (2012) Fate of zinc oxide nanoparticles during anaerobic digestion of wastewater and post-treatment processing of sewage sludge. Environ. Sci. Technol. 46 (16) 9089-9096. http://dx.doi.org/10.1021/es300839e [ Links ]
LOWRY GV, GREGORY KB, APTE SC and LEAD JR (2012) Transformations of nanomaterials in the environment. Environ. Sci. Technol. 46 (13) 6893-6899. http://dx.doi.org/10.1021/es300839e [ Links ]
MARTIN TD, CREED JT and BROCKHOFF CA (1994) Sample preparation procedure for spectrochemical determination of total recoverable elements. Revision 2.8, Method 200.2. United States Environmental Protection Agency Environmental Monitoring Systems Laboratory -Office of Research and Development, Cincinnati. [ Links ]
MAXIMOVA N and DAHL O (2006) Environmental implications of aggregation phenomena: current understanding. Curr. Opin. Colloid. Interface Sci. 11 246-266. http://dx.doi.org/10.1016/j.cocis.2006.06.001 [ Links ]
METCALF and EDDY (2002) Wastewater Engineering: Treatment and Reuse (4th. edn). McGraw Hill, New York. [ Links ]
MONSHI A, FOROUGHI MR and MONSHI MR (2012) Modified Scherrer equation to estimate more accurately nanocrystallite size using XRD. World J. Nano Sci. Eng. 2 154-160. http://dx.doi.org/10.4236/wjnse.2012.23020 [ Links ]
MU H and CHEN Y (2011) Long-term effect of ZnO nanoparticles on waste activated sludge anaerobic digestion. Water Res. 45 5612-5620. http://dx.doi.org/10.1016/j.watres.2011.08.022 [ Links ]
MU H, ZHENG X, CHEN Y, CHEN H and LIU K (2012) Response of anaerobic granular sludge to a shock load of zinc oxide nanoparticles during biological wastewater treatment. Environ. Sci. Technol. 46 (11) 5997-6003. http://dx.doi.org/10.1021/es300616a [ Links ]
MUSEE N, TRERISE MA and LORENZEN L (2007) Post-treatment of distillery wastewater after UASB using aerobic techniques. S. Afr. J. Enol. Vitic. 28 (1) 50-55. [ Links ]
MUSEE N (2011) Simulated environmental risk estimation of engineered nanomaterials: a case of cosmetics in Johannesburg City. Hum. Exp. Toxicol. 30 (9) 1181-1195. http://dx.doi.org/10.1177/0960327110391387 [ Links ]
MUSEE N, THWALA M and NOTA N (2011) The antibacterial effects of engineered nanomaterials: implications for wastewater treatment plants. Environ. Monit. 13 (5) 1164-1183. http://dx.doi.org/10.1039/c1em10023h [ Links ]
MUSEE N, ZVIMBA JN, SCHAEFER LM, NOTA N, SIKHWIVHILU LM and THWALA M (2014) Fate and behaviour of ZnO- and Ag-engineered nanoparticles and a bacterial viability assessment in a simulated wastewater treatment plant. J. Environ. Sci. Health A 49 (1) 59-66. http://dx.doi.org/10.1080/10934529.2013.824302 [ Links ]
OBERDÖRSTER G, OBERDÖRSTER E and OBERDÖRSTER J (2005) Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ. Health Perspect. 113 (7) 823-839. http://dx.doi.org/10.1289/ehp.7339 [ Links ]
OECD 303A (2001) Aerobic sewage treatment - activated sludge units. Guidelines for the testing of chemicals. OECD, Paris. [ Links ]
OMAR FM, AZIZ HA and STOLL S (2014) Aggregation and disaggregation of ZnO nanoparticles: Influence of pH and adsorption of Suwannee River humic acid. Sci. Total Environ. 468-469 195-201. http://dx.doi.org/10.1016/j.scitotenv.2013.08.044 [ Links ]
PADMAVATHY N and VIJAYARAGHAVAN R (2008) Enhanced bioactivity of ZnO nanoparticles-an antimicrobial study. Sci. Technol. Adv. Mater. 9 035004. http://dx.doi.org/10.1088/1468-6996/9/3/035004 [ Links ]
PERALTA-VIDEA JR, ZHAO L, LOPEZ-MORENO ML, DE LA ROSA G, HONG J and GARDEA-TORRESDEY JL (2011) Nanomaterials and the environment: A review for the biennium 2008-2010. J. Hazardous Mater. 186 1-15. http://dx.doi.org/10.1016/).jhazmat.2010.11.020 [ Links ]
PETTIBONE JM, CWIERTNY DM, SCHERER M and GRASSIAN VH (2008) Adsorption of organic acids on TiO2 nanoparticles: Effects of pH, nanoparticle size, and nanoparticle aggregation. Langmuir 24 6659-6667. http://dx.doi.org/10.1021/la7039916 [ Links ]
PICCINNO F, GOTTSCHALK F, SEEGER S and NOWACK B (2012) Industrial production quantities and uses of ten engineered nanomaterials in Europe and the world. J. Nanopart. Res. 14 1109-1119. http://dx.doi.org/10.1007/s11051-012-1109-9 [ Links ]
PREMANATHAN M, KARTHIKENYAN K, JEYASUBRAMANIAN K and MANIVANNAN G (2011) Selective toxicity of ZnO nanoparticles toward Gram-positive bacteria and cancer cells by apoptosis through lipid peroxidation. Nanomed. Nanotechnol. Biol. Med. 7184-192. http://dx.doi.org/10.1016Ai.nano.2010.10.001 [ Links ]
SHENG GP, ZHANG ML and YU HQ (2008) Characterization of adsorption properties of extracellular polymeric substances (EPS) extracted from sludge. Colloid Surf. B Biointerfaces 62 83-90. http://dx.doi.org/10.1016/jxolsurfb.2007.09.024 [ Links ]
SONUNE A and GHATE R (2004) Developments in wastewater treatment methods. Desalination 167 55-63. http://dx.doi.org/10.1016/).desal.2004.06.113 [ Links ]
SUN T, GOTTSCHALK F, HUNGERBUHLER K and NOWACK B (2014) Comprehensive probabilistic modelling of environmental emissions of engineered nanomaterials. Environ. Pollut. 185 69-76. http://dx.doi.org/10.1016/j.envpol.2013.10.004 [ Links ]
TAN M, QIU G and TING Y-P (2015) Effects of ZnO nanoparticles on wastewater treatment and their removal behavior in a membrane bioreactor. Bioresour. Technol. 185 125-133. http://dx.doi.org/10.1016/j.biortech.2015.02.094 [ Links ]
THWALA M, MUSEE N, SIKHWIVHILU L and WEPENER V (2013) The oxidative toxicity of Ag and ZnO nanoparticles towards the aquatic plant Spirodela punctuta and the role of testing media parameters. Environ. Sci.: Process. Impacts 151830-1843. http://dx.doi.org/10.1039/c3em00235g [ Links ]
WANG Y, WESTERHOFF P and HRISTOVSKI KD (2012) Fate and biological effects of silver, titanium oxide, and C60 (fullerene) nanomaterials during simulated wastewater treatment processes. J. Hazardous Mater. 201-202 16-22. http://dx.doi.org/10.1016/).jhazmat.2011.10.086 [ Links ]
WESTERHOFF P, SONG G, HRISTOVSKI K and MEHLIKA AK (2011) Occurrence and removal of titanium at full scale wastewater treatment plants: implications for TiO2 nanomaterials. J. Environ. Monit. 131195-1203. http://dx.doi.org/10.1039/c1em10017c [ Links ]
WONG SWY, LEUNG PTY, DJURIŠIČ AB and LEUNG KMY (2010) Toxicities of nano zinc oxide to five marine organisms: influences of aggregate size and ion solubility. Anal. Bioanal. Chem. 396 609-618. http://dx.doi.org/10.1007/s00216-009-3249-z [ Links ]
WOODROW WILSON INTERNATIONAL CENTRE FOR SCHOLARS (2015) A nanotechnology consumer products inventory. Project on Emerging Nanotechnologies. URL: www.nanotech-project.org (Accessed 31 March 2015). [ Links ]
XIA T, KOVOCHICH M, LIONG M, MADLER L, GILBERT B, SHI H, YEH JI, ZINK JI and NEL AE (2008) Comparison of the mechanism of toxicity of zinc oxide and cerium oxide nanoparticles based on dissolution and oxidative stress properties. Am. Chem. Soc. 2 (10) 2121-2134. http://dx.doi.org/10.1021/nn800511k [ Links ]
XIONG D, FANG T, YU T, SIMA X and ZHU W (2011) Effects of nano-scale TiO2, ZnO and their bulk counterparts on zebrafish: Acute toxicity, oxidative stress and oxidative damage. Sci. Total Environ. 409 1444-1452. http://dx.doi.org/10.1016/j.scitotenv.2011.01.015 [ Links ]
ZHANG Y, CHEN Y, WESTERHOFF P and CRITTENDEN J (2009) Impact of natural organic matter and divalent cations on the stability of aqueous nanoparticles. Water Res. 43 4249-4257. http://dx.doi.org/10.1016/j.watres.2009.06.005 [ Links ]
ZHOU D and KELLER AA (2010) Role of morphology in the aggregation kinetics of ZnO nanoparticles. Water Res. 44 2948-2956. http://dx.doi.org/10.1016/j.watres.2010.02.025 [ Links ]
Received: 1 July 2014
Accepted in revised form 18 November 2015
* To whom all correspondence should be addressed. Fax: +27 12 420 5048; e-mail: ndeke.musee@up.ac.za; museen2012@gmail.com