Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
Water SA
versión On-line ISSN 1816-7950
versión impresa ISSN 0378-4738
Water SA vol.41 no.3 Pretoria abr. 2015
http://dx.doi.org/10.4314/wsa.v41i3.12
The applicability of nanofiltration for the treatment and reuse of textile reactive dye effluent
MN ChollomI, *; S RathilalI; VL PillayII; Dorcas AlfaI
IFaculty of Engineering and the Built Environment, Department of Chemical Engineering, Durban University of Technology, 4001 Berea, 70 Mansfield Road. Durban, South Africa
IIStellenbosch University, Department of Process Engineering, Private Bag X1 Matieland 7602, South Africa
ABSTRACT
The main aim of the study was to test the feasibility of using nanofiltration (NF) processes for the treatment of reactive dye-bath effluents from the textile industry, in order to recover the water and chemicals (salts) for reuse purposes. The study of the reusability of nanofiltered water for dyeing has been given little or no attention. About 30% of reactive dyes remain unfixed on fibres; the unfixed dyes are responsible for the colouration in effluents. Membrane processes were employed to treat reactive dye-bath effluents to recover the salts and water. Investigations were conducted firstly with ultrafiltration (UF) used as a pre-treatment for NF. Secondly, evaluations were performed for 2 types of NF membranes (SR90 and NF90), in terms of quality of permeate produced and fluxes achieved for 2 different samples of effluent. The effect of cleaning on membrane performance was assessed. A reusability test was carried out on both permeate samples for dyeing light and dark shade recipes. The use of UF as pre-treatment to NF resulted in rejection of colloidal substances > 90% and a 15% flux improvement. Permeate from NF90 had a conductivity of 76 µS/cm and total organic carbon (TOC) of 20 mg/ℓ, as compared to SR90 which had a conductivity of 8.3 mS/cm and a TOC of 58 mg/ℓ. Light shade from NF90 gave satisfactory results on dyeing, with no colour difference. However a variation in colour was noticed when the medium sample was used to dye the light shade. Both NF permeates gave satisfactory results when used to dye the dark shades. Permeate from NF90 was within the accepted range for reuse, while permeate from SR90 had a higher salt recovery. Chemical cleaning resulted in 80% flux recovery. From the reusability test it was concluded that permeate from NF90 met the reuse criteria for feed water to the dye bath.
Keywords: textile effluent, nanofiltration, reactive dyes, water recovery, salt recovery
INTRODUCTION
Many types of dyes are used to treat different fibres in the manufacture of textiles. The dye class used is only suitable for a specific type of fibre; therefore the fixation rate of each class is different. Disperse dyes have a higher percentage of fixation to the fibre as compared to acid and reactive dyes. Most of the fibres dyed in the textile industries are carried out on cotton with about 50% of these dyed using reactive dyes. For every kilogram of cloth to be dyed, the required amount of water, salts and dye is 70-150 ℓ, 0.6 kg NaCl and 40 g, respectively (Allègre et al., 2006). Reactive dyes are commonly used because of their brightness and range of colours, their ease of application and high wet-fastness on the fibres. The main disadvantage of using reactive dyes is that a large fraction (10-50%) of the dye is wasted during the process (Allègre et al., 2006).
Sources of freshwater are expended at a quicker rate than they are replenished owing to the massive rise in population and industrialization. The upturn in industrialization is diminishing freshwater sources. Huge quantities of water are required by manufacturing facilities and the contaminants from manufacturing processes pollute the remaining freshwater sources. Over the years, reclaiming of wastewater has become very important, especially in the textile industry, because of the scarcity of freshwater supply and the increasingly strict government regulations regarding effluent release into water bodies (Singh and Arora, 2011; Zahrim et al., 2011; Dasgupta et al., 2015). Various conventional methods are being used to treat textile effluents, including: biological methods (aerobic and anaerobic), physico-chemical treatment (coagulation and flocculation), adsorption, ion-exchange, and chemical treatment with oxidizing agents (Khouni et al., 2011; Thamaraiselvan and Noel 2015). The limitation of all these methods, however, is that total colour removal is not achieved and chemical by-products are introduced. A sludge management problem is also a limitation arising from the use of chemicals. Thus, the water quality produced does not meet the requirements for textile reuse (Ismail and Dincer, 2003; Bes-Piá et al., 2009; Van der Bruggen, 2013; Thamaraiselvan and Noel, 2015). The methods favour end-of-line treatment in which all of the waste from different processes is collected and treated. The characteristics of the final effluent therefore represent an overall average of the different effluent components which makes it complex and difficult to treat (Erswell et al., 1988; Schoeberl et al., 2004). Membrane-based processes provide appealing possibilities of separating hydrolysed dye stuff and dyeing auxiliaries, thereby reducing colouration and COD content. The choice of a membrane process to separate contaminants is guided by the quality of permeates required for reuse by the textile company. Nanofiltration (NF) is a membrane-based process that retains organic compounds of relatively low molecular weight, and divalent ions or large molecular ions such as hydrolysed reactive dyes of 700-1 000 molecular weight, as well as dyeing auxiliaries. It has the advantage of recovery of valuable materials and is to be preferred to reverse osmosis which has higher maintenance and operational costs (Chakraborty et al., 2003; Koyuncu et al., 2004; Zahrim et al., 2011; Aouni et al., 2013). One disadvantage of using NF, however, is the reduction in permeate flux rate, which occurs due to concentration polarization and fouling. This reduces productivity of the membrane and requires the membranes to be cleaned regularly.
To the best of the authors' knowledge previous studies have focused on making synthetic feed simulating dye-bath effluent (Irena et al., 2007) or using biologically treated wastewater to serve as feed to NF (Fersi et al., 2005). Treated effluents from other conventional methods have also been used as feed to NF (Khouni et al., 2011; Thamaraiselvan and Noel, 2015).
In a study by Chakraborty et al. (2003), the effluents from the dye bath were diluted to meet NF requirements, though the concentration of the feed was not stated. However, the use of these streams does not give a true representation of the effluent from the dye bath and the effects it has on membrane performance in terms of permeate quality and recovery of chemicals for reuse. Typical textile wastewater characteristics have been outlined in the studies of Bisschops and Spanjers (2003); Aouni et al. (2012); Kurt et al. (2012) and Thamaraiselvan and Noel (2015). Little or no attention has previously been given to the importance of investigating the influences of the use of permeate water obtained from NF processes on the quality of the final textile product; this is a crucial aspect that requires investigation, especially in the integration of membranes for the treatment of reactive dye-bath effluent for recycling purposes.
In this paper, NF was used to treat effluent directly from the dye bath for reuse, and a reusability test was carried out on permeate samples. The study was carried out using ultrafiltration (UF) for the first stage of the experiment as a pre-treatment for NF. The second stage was carried out using two NF units (SR 90 and NF 90). The effect of feed pre-treatment was investigated and the performance of both NF membranes was determined. The effect of cleaning on membrane performance was assessed. Finally, permeate samples were recycled and investigations for its reusability for dyeing were then carried out. From the study conducted, we found out that the use of UF as a pre-treatment yielded an increase in productivity of the membrane. High organics removal was achieved by both NF membranes, but with NF90 performing better because the permeates could be used for dyeing even the most critical shades. The quality of the permeate was used as an indicator for reusability. The fouling and cleaning of the membrane was also considered.
MATERIALS AND METHODS
The study was conducted in 2 stages, the first stage being pre-treatment of the feed using UF. During the second stage, NF was applied to the pre-treated permeates from UF.
Sample analysis
Effluent samples were obtained from a local textile industry in KwaZulu-Natal (KZN), South Africa. The company carries out about 80% dyeing on cotton and 20% on polyester. Most of the dyes used are reactive dyes; salts of sodium chloride are used for dye fixation and sodium carbonate is used to raise the dye liquor pH. The quantity of sodium chloride used for dyeing is dependent on the shade of colour required; for light shades, 10 g/ℓ of NaCl is required, medium shades require 50 g/ℓ, while darker shades require 80-100 g/ℓ The fixation rate of the dye is 70-80%. Therefore, the dye-bath effluents consist of 20-30% hydrolysed dyes, salts and other additives that are used during the dyeing process, causing a variation in the degree of pollution. The characteristic of the effluent depends on the dyeing process being carried out at the time of sample collection. Two different dye shades were evaluated: light shade (LS) and medium shade (MS).
Table 3 reveals that the quality of the feed samples was different for the measured parameters. The medium shade had the highest organic load, colour and salinity. This is due to different dyeing recipes that are used in the textile. As a result of this, variation in wastewater quality is experienced.
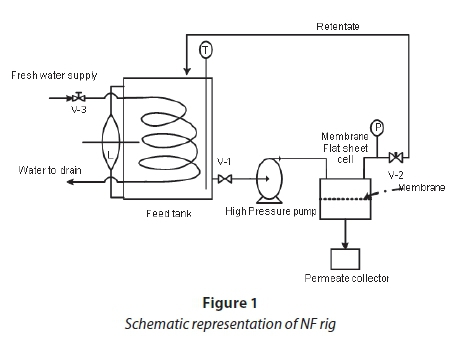
Ultrafiltration (UF) and nanofiltration rigs (NF)
UF and NF rigs were used for the experiments. Figure 1 is a schematic representation of the NF rig used; a similar rig was also used for UF filtration. The selection of nanofiltration membranes was based on their ability to retain dye molecules in the range 700-1 000 g/mol, as well as other dyeing auxiliaries used, thereby allowing the passage of monovalent compounds such as NaCl. The membrane characteristics for UF and NF are shown in Table 1.
Membrane permeability
Water permeabililty tests were carried out using the NF (Fig. 1) membranes with distilled water. The flux values of distilled water at different operating pressures were measured.
Ultrafiltration
The feed was first filtered using hollow-fibre UF membranes supplied by Inge GmbH multi-bore capillary modules. The membranes were operated in a cross-flow mode of filtration. The cross-flow velocity was 1 m/s, at an operating pressure of 0.1 MPa. A similar rig to the one shown in Fig. 1 was used for the pre-treatment. The reject stream was returned back to the feed tank, while permeate was collected separately in a beaker for analysis. The rejection (R) of the species was calculated according to:

where: Cf = feed concentration (g/ℓ); Cp = permeate concentration (g/ℓ)
Nanofiltration process
Two NF flat-sheet membranes, SR 90 and NF90, were used for the experiment. The retentate stream was recycled back to the feed tank, while permeate was collected in a beaker and measured. At the start of each experiment the feed volume was 10 ℓ. The system was operated in a cross-flow mode of filtration and the operating parameters were: cross-flow velocity of 0.03 m/s, pressure of 1 MPa and a temperature of 18°C. These parameters were kept constant for all experiments, with the exception of concentration, which varied according to the feed sample as shown in Table 3. Permeate flux and permeate quality were measured every 30 min for 3 h for each experiment. The efficiency of the removal of different contaminants was determined using Eq. 1.
Analytical equipment
The most important parameters that determine the reuse of water in a textile industry include chemical oxygen demand (COD), total organic carbon (TOC), turbidity, conductivity, pH and colour (Marcucci et al., 2001). These parameters were analysed using the following equipment: HACH turbidity meter to measure turbidity, HACH conductivity meter for conductivity, HACH DR 3900 spectrophotometer for COD, TOC and colour at 435 nm, 425 nm and 465 nm, respectively. A HACH pH meter was used for pH. COD was analysed using the USEPA digestion method. TOC was determined using the direct method, while colour was determined by the standard platinum-cobalt method. The criterion for water reuse in a textile industry varies because of the different dyeing methods. Generally the water is considered acceptable if it has no colours and is free of suspended particles (Zahrim et al., 2011).
Cleanability and recovery of the membrane
Recovery of the fouled membrane was first carried out by flushing and relaxation for NF membranes, while UF membranes were cleaned by backwashing. A pressure of 1.4 MPa with a velocity of 0.03 m/s was used for flushing, while a low pressure of 0.1 MPa was used during chemical cleaning. Flushing was performed with distilled water while chemical cleaning was carried out using appropriate cleaning chemicals as suggested by the manufacturer. Pure water flux was then carried out on the fouled, flushed and chemically cleaned membranes to determine flux recovery and flux loss in the case of the fouled membrane. The chemicals used were 0.1 wt % NaOH, 0.2 wt % HCl and 1 wt % EDTA, and finally 0.025 wt % of sodium lauryl sulphate for both membrane types. The effectiveness of the cleaning protocol was calculated using the water flux recovery ratio:

where: Jc = flux after cleaning of fouled membrane (LMH); Jo = flux of the virgin membrane (LMH).
RESULTS AND DISCUSSION
Filtration with time at constant pressure using NF
Figures 2 and 3 show filtration as a function of time for both feed samples used for the study. Both graphs show the improvement of flux after the pre-treatment of the feed. This improvement was 11-18% for both membranes. The average specific fluxes (flux per net driving force) for SR90 for the feed samples with pre-treatment were in the range of 5-9 LMH/kPa. For NF90 this range was 3-6 LMH/kPa. This shows that the permeate production rate was higher in SR90 as compared to NF90 at the same operating conditions. A decline in flux for both membranes was observed. This was attributed to a gradual deposition of organic material and salts onto the membrane surface. For the first 30 min the decline in flux for SR90 was 11% and for NF90 was 25%. This explained that a significant osmotic pressure effect was experienced by the membrane as a result of high salt concentrations. After 3 h of filtration the flux had further dropped down to 25% for SR90 and 61% for NF90. Greater loss was experienced by NF90. The huge loss in flux by NF90 was not verified in this study.
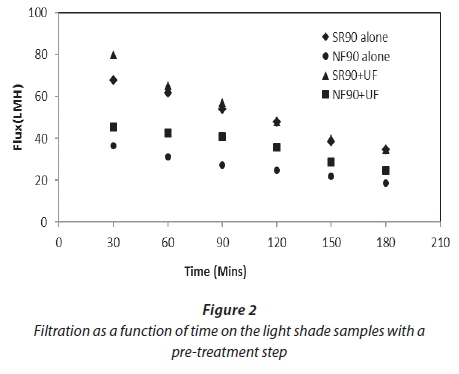
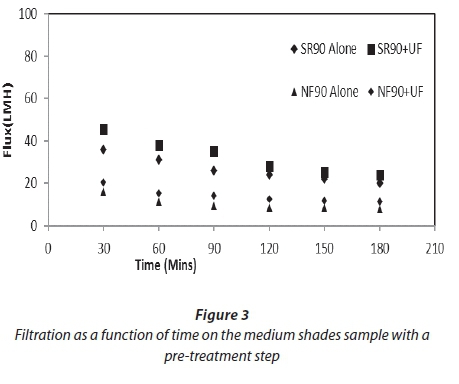
The rate of fouling for the first 30 min indicated an important osmotic pressure effect due to high salt concentrations, especially for the MS samples with higher salt contents. After 3 h of filtration the flux had further dropped; this decline was probably due to concentration polarization.
The severe fouling rate in the NF membranes was assumed to be as a result of the reaction between the dyes and the membranes, since the feed sample had a combination of 1 or 2 dyes. From previous studies, it has been reported that each dye class could cause membrane fouling and that the manner of interaction between the membrane and these dyes differs. Studies by Ismail and Dincer (2003) and (Zahrim et al., (2011) showed that dye molecules adsorbed on the membrane surface affect permeate flux, thereby increasing fouling due to physicochemical interactions, e.g., hydrophobic interactions, polar interactions and charge transfer.
There are various factors that can influence the adsorption of dyes on the membranes. These include: the nature of the membrane material, the type of solute, solute concentration, and pH (Aouni et al., 2012). During all experiments, the membrane surface was observed to be coloured, with the intensity varying according to the feed solution that was filtered.
Another cause of the high rate of fouling, which led to the loss of flux, was attributed to the salinity of the feed sample. The feed samples with the highest salinity had the highest rate of fouling. Jiraratananon et al. (2000) explained that the penetration of reactive dye into the membranes could be enhanced by the use of NaCl during the dyeing process, thereby resulting in the NF membranes being heavily coloured after the experiments. This subsequently led to a flux decline.
Furthermore, the high rate of fouling was due to the high pH of the feeds, signifying that most of the feed samples were alkaline in nature. Ismail and Dincer (2003) stated that under alkaline conditions the formation of a strong and stable dye-salt complex will result in an increase in hydrophobicity and, as such, the adsorption of dye molecules on the membrane surface will increase thereby increasing membrane fouling. Also, the high rate of fouling was probably due to differences in the membrane material and the membrane manufacturing process, thereby resulting in differences in contact angle, surface roughness, and membrane hydrophilicity/hydrophobicity. Comparing the rate of fouling between the NF membranes, NF90 had a higher fouling rate.
The contact angle of a membrane is a semi-quantitative index which is connected to the hydrophilicity/hydrophobicity of a membrane surface. With a low contact angle, the hydrophilicity will increase while the propensity for fouling of the membrane is decreased Aouni et al. (2012). Lau and Ismail (2009) showed that a small contact angle, which corresponds to the hydrophilic surface of a membrane, could reduce the tendency of the membrane to foul, through preferential wetting of the membrane material by water. The greater contact angle in NF90 (54°) compared to SR90 (27°) could possibly be a reason for the high fouling rate shown for NF90.
Percentage removal after UF treatment
The rejection of polluting substances was high on using UF. Turbidity rejection was quite high; more than 90% of the turbidity was reduced for LS while only 78% removal was achieved for MS. The rejection of total suspended solids was high for both feed samples. Low colour rejection was achieved by the UF; the rejections were 7-35% for the feed samples. MS had a higher colour removal; this was achieved partly due to the adsorption of dyes onto the layer of the membranes which further acted as a barrier to the passage of the dyes. The rejection on conductivity was not significant; zero values were recorded for all of the feed samples. This was due to the nature of the membrane, which was expected since UF membranes are unable to retain monovalent and divalent salts.
Percentage removal after NF treatment
COD and TOC
Higher percentages of COD and TOC removal were recorded when filtration was carried out using NF. NF90 gave a better performance in terms of COD and TOC removal for both feed samples. From these results, the LS sample had the lowest COD value in the permeate. Its initial feed sample was 486 mg/ℓ; this was lower than the other sample measured, as shown in Table 3. The COD in the MS permeate was high, this was attributed to the high initial feed concentration of 890 mg/ℓ. This suggested that feed concentration plays a significant role in membrane rejection performance. In a study by Alcaina-Miranda et al. (2009), they considered a 76-83% COD reduction in textile industry wastewater to be a satisfactory result. Sojka-Ledakowicz et al. (2010) found that high reduction of COD (up to 99%) could only be achieved by RO membranes. The remainder of the COD in the permeate was possibly from the solutes and other oxidizable low molecular weight materials that went through the membrane. Both NF membranes had achieved high COD and TOC rejection, but higher rejections were achieved by NF90. On comparing the COD values with those obtained in literature and the reuse criteria, it was concluded that the permeates from NF90 met the reuse criteria while only the light shades and the medium shades from SR90 were considered satisfactory for reuse purposes.
Dye rejection
Colour rejection by NF90 was higher than SR90 as it was observed for all treated samples, even though both membranes achieved a high rate of rejection above 90% for all of the samples. Higher colour removal was achieved for the light shade as compared to the dark shade. This was expected due to the initial dye and salt content of the feed samples, which was higher in the medium shade. This is in agreement with a study by Tang and Chen (2002), who reported that lower dye rejection was observed when the concentration of NaCl was increased. However, contrasting views were reported by Marcucci et al. (2001) and Lopes et al. (2005), who stated that the initial dye concentration in the feed does not significantly affect dye rejection. Rather rejection is affected by the membrane type used, in terms of its molecular weight cut-off.
It has been found that the rejection of dyes by the NF membranes is a result of variation in the salt content of the feed sample and the membrane type. With respect to the reuse criteria, permeate samples from NF90 were considered satisfactory, while only the light shades were considered satisfactory for SR90. However, studies by Lopes et al. (2005) considered dye rejection of 85-90% satisfactory for reuse, especially for some of the washing streams used for rinsing dark colours.
Salt rejection on pure salts
Figure 4 shows pure salt rejection by membranes for NaCl and NaSO4 as feed at different concentrations, as well as the rejection for the feed samples.. Salts of NaCl and Na2SO4 are used to enhance dye fixation for most textile processes. It is therefore important to study the salt removal rate for these pure single salts on the NF membranes. Pure salt solutions were prepared and filtration was carried out on the single salts using both NF membranes, to determine the performance of the NF membranes in terms of the rejection of the salts. This provided information that was used to compare the rejection of salts by the NF membranes with the effluent samples. The rejection was studied by setting the same operating parameters that were used for the filtration of the effluent samples.
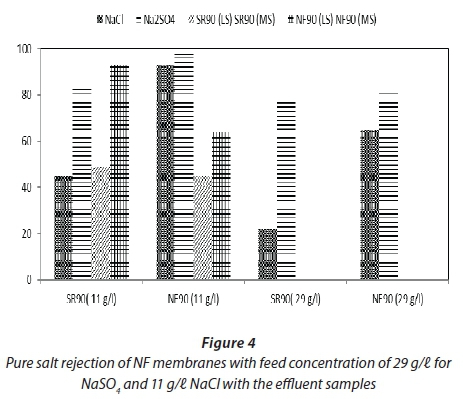
From Fig. 4 it is evident that NF90 showed higher salt rejection for both NaCl and Na2SO4 as compared to SR90, despite its low water permeate fluxes. The higher separation efficiency of NF90 was attributed mainly to its relatively smaller pore structure which restricted the passage of the dissolved salt. The rejection of Na2SO4 for both tested membranes was much higher than NaCl rejection; this was in agreement with a study by Ong et al. (2012) which showed that divalent anions are more strongly rejected by NF membranes, which have a negative surface charge, (SO42-) than monovalent anions. This follows the principle of the Donnan exclusion mechanism.
The rejections were from 65-90% for NF90. SR90 displayed lower rejections of 20-50%. Similarly to other parameters shown in Table 3 there was a variation in sample conductivities. Comparing the performance of NF membranes for the effluent samples and the pure salt filtration, it was observed that, for both cases, at higher salt concentration the rejection of salts by NF decreased. This is in agreement with other published results. Studies by Lau and Ismail (2009) showed that the transport of salt through the membrane is proportional to the salt concentration difference, but independent of the applied pressure.
More so, salt rejection in a membrane depends mainly on membrane type; it is either charged with positive or negative ions or it is neutral. Lau and Ismail (2009) stated that monovalent salts such as NaCl are usually completely ionized into Na+ and Cl- in alkali or pure water; an increase in salt concentration will increase the ion concentration in the solution. Therefore, based on the Donnan principle, the repulsive force from the negatively-charged membrane will decrease upon increasing salt concentration. A lower repulsive force means that more Cl- anions will be allowed to pass through the membrane and thus salt rejection is reduced. In general, the increase in salt concentration usually reduces electrostatic repulsion for NF, causing the salt rejection rate to decrease.
However, for NF90, rejection was between 60 and 90% for the samples irrespective of the feed concentration, implying that salt rejection followed both the sieving and Donnan exclusion principle. For SR90, the tendency of salt rejection decreased; this was largely due to the remarkable decrease in the Donnan exclusion effect as a result of membrane type. Even though NF90 had a higher conductivity rejection than SR90, the performance of SR90 met the objective of the study in terms of salt recovery.
Membrane cleaning
UF cleaning
Permeate flux in UF membrane after physical and chemical cleaning is shown in Fig. 5. During the backwash the flow of permeate is reversed so that the surface foulants on the membrane are dislodged. Flux recovery after backwash was 64%; this was considered low since 34% of the membrane was still fouled. Because of the low flux recovery after the backwash, it was assumed that different types of fouling might have occurred on the membrane (cake formation and adsorption). Therefore, in order to get rid of the adsorbed foulants, a chemical cleaning was then carried out on the membrane. The flux recovery after chemical cleaning was 95%, such that only 5% of the flux was lost.
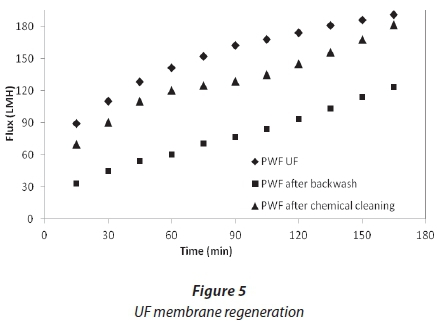
NF cleaning
Cleaning was carried out at the end of each experiment, after a reduction in flux from 30%. Figures 6 and 7 show the effect of flushing on both membranes. Only 51%, for NF90, and 66%, for SR90, of the flux was recovered. This indicated a 49% and 34% loss, respectively. The loss was attributed to the internal membrane fouling, and flushing alone could not recover the flux. Chemical cleaning was then carried out on both membranes. Flux recovered was 76% for NF90 and 82% for SR90. There was a loss in flux for both membranes; however, a higher loss was recorded for the NF90 membranes. The loss in flux was assumed to be due to irreversible fouling and therefore could not be recovered. With respect to membrane regeneration, the results were said to be satisfactory, since over 80% of the flux was recovered. This further suggested that the membranes used can be implemented for similar processes on a large scale. However, the cleaning cycles will have to be carried out intermittently to avoid huge losses in membrane flux. Different authors have reported different values for the recovery of flux in the treatment of textile wastewater, and which range from 80-100% (Jiraratananon et al., 2000).
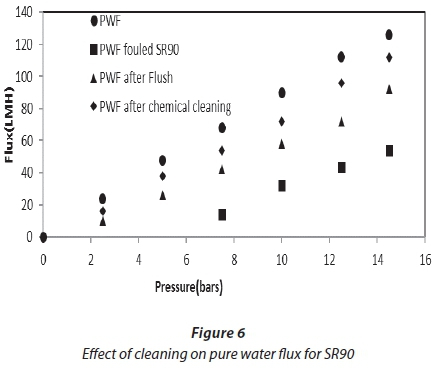
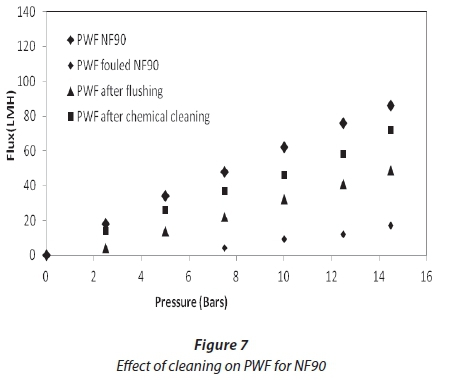
Comparative analysis of treated water and dyeing water
Reclaiming of textile reactive dye-house wastewaters with NF from the reactive dye effluent has some advantages: reducing the consumption of freshwater and the recycling of chemicals. The definition of a generally acceptable standard for water reuse has not been fully adopted in the textile industry. This is so because the water quality requirements for each textile are different. Usually, softened water is needed for scouring, dyeing and for preparation of printing pastes, but is not necessary for all of the washing cycles. The critical conditions that are considered are water turbidity, which should be lower than the turbidity of the ground-water, or 0-15 NTU for reclaimed water. Water hardness can be between 50 and 60 mg/ℓ (Van der Bruggen et al., 2004; Vergili et al., 2012). Furthermore, high amounts of other constituents such as heavy metals are not permitted in the reclaimed water (Capar et al., 2006). Table 4 shows the textile reuse criteria.
The NF permeate quality was compared to the initial process water quality and the reuse criteria as seen in Table 4. COD for SR90 and NF90 were higher in MS as compared to LS. With respect to colour, there were still residual colours sustained in permeates, even though LS appeared colourless. From Table 4, it is observed that the measured parameters in the permeate were slightly above the reuse standards and the process water. MS was still clearly coloured and its specs were above the reuse standards. NF90 permeates were very much closer to the reuse guidelines and the process water characteristics. The COD for LS was below the reuse standard and the process water, while MS was above. With respect to colour, permeate samples appeared to be colourless and LS was below the reuse standard, except for the MS which was 1 point above the reuse standard. However, a slight concentration of dyestuff in solution is usually visually noticeable.
TSS and turbidity were at acceptable values. Conductivity for LS met the reuse values, while for MS it exceeded them, but this was not considered to be a problem affecting the objective of this study which was to recover salts. Even though the quality of permeates from NF showed some variation with the textile freshwater and reuse criteria, the reuse test was still carried out on permeates.
Dyeing was carried out using permeate samples from NF at the laboratory of the textile industry where effluent was collected. The dyeing was carried out on a fibre composed of 80% cotton and 20% polyester. A light shade recipe was chosen. LS from both NF membranes gave satisfactory results on dyeing with no colour difference. This was verified by comparing it with fibre dyed using the process water. However, a variation in colour was noticed when the MS sample was used to dye the light shade. Dyeing of the light shade recipes requires high-quality water unlike the dark shade which does not. Therefore the variation in colour for the MS was due to the organics contained in it. It was then suggested that permeates from the medium shade be used to dye the dark shade only, since the salt concentration differs according to the colour shade intended to be dyed. Permeate samples from NF membranes were then dyed on a dark shade and compared to the process water and good results were obtained without variations. These findings were verified and compared to a study by Vreese and Bruggen (2007) on cotton and polyester dyeing using effluents treated by nanofiltration. They came to the conclusion that the limited presence of a colour in permeate cannot be a hindrance to dyeing; instead the conductivity of the permeate has a higher impact on the possibility of its reuse.
It was concluded that both NF membrane permeates from the light shade samples could be used for dyeing of light shades, while the darker shades could be dyed with dark shade permeates only. Alternatively, if the NaCl required for reuse in the dye bath is lower in the permeate, it can be augmented by the addition of more NaCl to reach the desired concentration. However, if it is higher, fresh water should be added to reduce the concentration.
CONCLUSION
The quality of water used is defined as acceptable as long as the impurities in the water do not affect its functions. The following conclusions can be drawn from this study:
- The use of UF as a pre-treatment is able to reduce the effects of fouling on the NF membranes.
- High rejection in terms of COD, TOC and colour were obtained for both NF membranes, while colour removal by SR90 was not totally achieved but was only partially achieved by NF90. Higher fluxes and salt recovery were recorded for SR90 as compared to NF90.
- The reusability tests carried out showed that permeate recycled from NF90 could be used for any section in the textile production process, including the most critical, such as dyeing on light shades.
In summary, we concluded that membrane-based processes can be integrated into the water circuits of the textile industry to treat even the difficult effluent streams, such as the dye bath, despite its variations in composition.
ACKNOWLEDGEMENT
The authors wish to thank the Durban University of Technology for funding this research and also SANPAD and the Young Water Professionals for organising workshops on article writing.
REFERENCES
ALCAINA-MIRANDA MI, BARREDO-DAMAS S, BES-PIÁ A, IBORRA-CLAR MI, IBORRA-CLAR A and MENDOZA-ROCA JA (2009) Nanofiltration as a final step towards textile wastewater reclamation. Desalination 240 (1-3) 290-297. [ Links ]
ALLÈGRE C, MOULIN P, MAISSEU M and CHARBIT F (2006) Treatment and reuse of reactive dyeing effluents. J. Membr. Sci. 269 (1-2) 15-34. [ Links ]
AOUNI A, FERSI C, CUARTAS-URIBE B, BES-PÍA A, ALCAINA-MIRANDA MI and DHAHBI M (2012) Reactive dyes rejection and textile effluent treatment study using ultrafiltration and nanofiltration processes. Desalination 297 78-96. [ Links ]
AOUNI A, FERSI C and DHAHBI M (2013) Performance evaluation of direct nanofiltration process to fouling by treating rinsing-bath effluents for water reuse. Desalin. Water Treat. 52 (7-9) 1770-1785. [ Links ]
BES-PIÁ A, IBORRA-CLAR A, GARCÍA-FIGUERUELO C, BARREDO-DAMAS S, ALCAINA-MIRANDA MI, MENDOZA-ROCA JA and IBORRA-CLAR MI (2009) Comparison of three NF membranes for the reuse of secondary textile effluents. Desalination 241 (1-3) 1-7. [ Links ]
BISSCHOPS I and SPANJERS H (2003) Literature review on textile wastewater characterisation. Environ. Technol. 24 1399-1411. [ Links ]
CAPAR G, YETIS U, OLCEROGLU AH and YILMAZ L (2006) Effect of color and surfactants on nanofiltration for the recovery of carpet printing wastewaters. Sep. Sci. Technol. 41 (12) 2771-2784. [ Links ]
CHAKRABORTY S, PURKAIT MK, DASGUPTA S, DE S and BASU JK (2003) Nanofiltration of textile plant effluent for color removal and reduction in COD. Sep. Purif. Technol. 31 (2) 141-151. [ Links ]
DASGUPTA J, SIKDER J, CHAKRABORTY S, CURCIO S and DRIOLI E (2015) Remediation of textile effluents by membrane based treatment techniques: A state of the art review. J. Environ. Manag. 147 55-72. [ Links ]
ERSWELL A, BROUCKAERT CJ and BUCKLEY CA (1988) The reuse of reactive dye liquor using charged ultrafiltration membranes technology. Desalination 70 157-167. [ Links ]
FERSI C, GZARA L and DHAHBI M (2005) Treatment of textile effluents by membrane technologies. Desalination 185 399-409. [ Links ]
IRENA P, RAJ ANP, SONJA Š-T and MAJCEN LMA (2007) The removal of reactive dye printing compounds using nanofiltration. Dyes Pigm. 74 (3) 512-518. [ Links ]
ISMAIL K and DINCER T (2003) Effects of operating conditions on the salt rejection of nanofiltration membranes in reactive dye/salt mixtures. Sep. Purif. Technol. 33 (3) 283-294. [ Links ]
JIRARATANANON R, SUNGPET A and LUANGSOWAN P (2000) Performance evaluation of nanofiltration membranes for treatment of effluents containing reactive dye and salt. Desalination 130 177-183. [ Links ]
KHOUNI I, MARROT B and BEN AMAR R (2011) Decolourization of the reconstituted dye bath effluent by commercial laccase treatment: Optimization through response surface methodology. Chem. Eng. J. 156 (1) 121-133. [ Links ]
KOYUNCU I, TOPACIK D and YUKSEL E (2004) Reuse of reactive dyehouse wastewater by nanofiltration: process water quality and economical implications. Sep. Purif. Technol. 36 (1) 77-85. [ Links ]
KURT E, KOSEOGLU-IMER DY, DIZGE N, CHELLAM S and KOYUNCU I (2012) Pilot-scale evaluation of nanofiltration and reverse osmosis for process reuse of segregated textile dyewash wastewater. Desalination 302 24-32. [ Links ]
LAU W-J and ISMAIL AF (2009) Polymeric nanofiltration membranes for textile dye wastewater treatment: Preparation, performance evaluation, transport modeling, and fouling control - a review. Desalination 245 321-348. [ Links ]
LOPES CN, PETRUS JCC and RIELLA HG (2005) Color and COD retention by nanofiltration membranes. Desalination 172 (1) 77-83. [ Links ]
MARCUCCI M, NOSENZO G, CAPANNELLI G, CIABATTI I, CORRIERI D and CIARDELLI G (2001) Membrane separation for wastewater reuse in the textile industry. Desalination 138 75-82. [ Links ]
ONG CS, LAU WJ and ISMAIL AF (2012) Treatment of dyeing solution by NF membrane for decolorization and salt reduction. Desalin. Water Treat. 50 245-253. [ Links ]
SCHOEBERL P, BRIK MR and FUCHS BW (2004) Treatment and recycling of textile wastewater case study and development of a recycling concept. Desalination 171 173-183. [ Links ]
SINGH K and ARORA S (2011) Removal of synthetic textile dyes from wastewaters: A critical review on present treatment technologies. Environ. Sci. Technol. 41 (9) 807-878. [ Links ]
SOJKA-LEDAKOWICZ J, ZYLLA R, MROZINSKA Z, PAZDZIOR K, KLEPACZ-SMOLKA A and LEDAKOWICZ S (2010) Application of membrane processes in closing of water cycle in a textile dye-house. Desalination 250 (2) 634-638. [ Links ]
TANG C and CHEN V (2002) Nanofiltration of textile wastewater for water reuse. Desalination 143 11-20. [ Links ]
THAMARAISELVAN C and NOEL M (2015) Membrane processes for dye wastewater treatment: Recent progress in fouling control. Crit. Rev. Environ. Sci. Technol. 45 (10) 1007-1040. [ Links ]
VAN DER BRUGGEN B (2013) Integrated membrane separation processes for recycling of valuable wastewater streams: Nanofiltration, membrane distillation, and membrane crystallizers revisited. Ind. Eng. Chem. Res. 52 (31) 10335-10341. [ Links ]
VAN DER BRUGGEN B, CURCIO E and DRIOLI E (2004) Process intensification in the textile industry: the role of membrane technology. J. Environ. Manage. 73 (3) 267-274. [ Links ]
VERGILI I, KAYA Y, SEN U, GÖNDER ZB and AYDINER C (2012) Techno-economic analysis of textile dye bath wastewater treatment by integrated membrane processes under the zero liquid discharge approach. Res. Conserv. Recycl. 58 25-35. [ Links ]
VREESE ID and BRUGGEN BVD (2007) Cotton and polyester dyeing using nanofiltered wastewater. Dyes Pigm. 74 313-319. [ Links ]
ZAHRIM AY, TIZAOUI C and HILAL N (2011) Coagulation with polymers for nanofiltration pre-treatment of highly concentrated dyes: A review. Desalination 266 (1-3) 1-16. [ Links ]
Received 30 October 2014
Accepted in revised form 11 March 2015
* To whom all correspondence should be addressed. e-mail: mnchollom@gmail.com














