Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Water SA
On-line version ISSN 1816-7950
Print version ISSN 0378-4738
Water SA vol.40 n.3 Pretoria Jun. 2014
http://dx.doi.org/10.4314/wsa.v40i3.16
Acid mine water neutralisation with ammonium hydroxide and desalination with barium hydroxide
MD MailaI; JP MareeII; LM CeleI
IDepartment of Chemistry, Tshwane University of Technology, Private Bag X680, Pretoria, 0001, South Africa
IIDepartment of Environmental, Water and Earth Sciences, Tshwane University of Technology, Private Bag X680, Pretoria, 0001, South Africa
ABSTRACT
In South Africa, acid mine drainage is polluting increasingly scarce ground- and surface water. The ammonium-barium (NB) process described in this paper consists of neutralisation and metal removal with NH4OH, sulphate removal with Ba(OH)2 and Ca removal with CO2. Laboratory studies showed that metals are removed to low levels. This includes Fe(II), the predominant metal in mine water. It is first oxidised to Fe(III), whereafter it precipitates as Fe(OH)3. Sulphate is removed to low concentrations as BaSO4. During CO2 dosing, CaCO3 is precipitated to its saturation level. The simulation predictions followed the same pattern as the experimental results obtained. This study showed that NH4OH can be used for treatment of acid mine drainage rich in sulphates and NH4OH can be recycled in the process. Hydrated lime treatment resulted in removal of the remaining ammonia using a rotary evaporator.
Keywords: acid mine water, ammonium hydroxide, barium hydroxide, sulphate removal
INTRODUCTION
Acidic mine waters are continuously discharged from certain mines to the environment, with little treatment. Acid mine water contains high levels of SO4 in addition to Fe, Al, Mn and other metals. Coal mining and fertiliser manufacturing are examples of industrial operations that give rise to severe acid pollution (Maree et al., 2004). Clean water is essential for agriculture, domestic and industrial use, and increases in population have led to an increase in the water demand. South Africa (SA) is an arid country, which has exacerbated the problem. It has been predicted that the country's freshwater resources will be fully utilised within the next 20 to 30 years if the current growth in water demand and use (or abuse) is not altered (Van Niekerk and Maree, 2001). In the Western Basin of the Witwatersrand, Gauteng Province, mine water started to decant in 2002. In the Eastern Basin, a single pump station at Grootvlei Mine pumped out between 75 and 108 Mℓ/day of mine-water. The pH can be as low as 2 (Jiménez et al., 2009) and poses a problem because the majority of natural life is adapted to survive at around pH 7. About 540 Mℓ/d of acid mine water is produced in the Gauteng region alone (Hlabela, 2009).
AMD is formed when pyrite in contact with atmospheric oxygen becomes oxidised to soluble iron and sulphuric acid, frequently catalysed by sulphur-oxidising bacteria (Jennings et al., 2008).

Mine-water treatment requires pre-treatment for neutralisation and metal removal, followed by desalination for removal of dissolved salts. The integrated limestone and lime process was developed for neutralisation and partial SO4 removal from AMD (Maree, 2003). Limestone and lime are used to increase the pH and, together with aeration, Fe(II) is oxidised and precipitates as Fe(OH)3. Limestone is used for initial AMD treatment as it is less costly than lime. It is moreover safe to handle and its dissolution occurs at pH below 7, obviating the need for pH control. In the second stage, lime is introduced to precipitate the remaining metals such as Mn and Al. Unfortunately, its successful application is limited as it only lowers SO4 concentration to around 1 200 mg/ℓ (INAP, 2000). Other treatment techniques have been developed that utilise limestone, which can neutralise acid but does not raise the pH sufficiently to remove metals (Ziemkiewicz et al., 1997). Several other processes can be considered for sulphate removal, e.g., biological sulphate removal, SAVMIN (by ettringite formation), and membrane processes. Barium sulphate is highly insoluble which makes Ba dosing suitable for removal of SO4.
BaCO3 can be used for SO4 removal according to the following reaction:

Trusler et al. (1988) developed a BaCO3 method for SO4 removal by using a two-stage fluidised-bed reactor system to overcome the other problems identified by Kun (1972), i.e. long retention times and the high Ba concentrations in the treated water. BaCO3 and lime would be added to the effluent to soften the water and produce a precipitate. The disadvantage of Reaction (2) is that BaSO4 and CaCO3 co-precipitate. Maree et al. (1989) noted a problem in separating co-precipitated BaSO4 and CaCO3. However, the BaCO3 became inactive when coated with precipitated metal hydroxide, which made it unsuitable for most mine waters. Alternatively, Ba(OH)2 can be used in place of BaCO3 and offers the benefits of rapid reaction times and precipitation of only BaSO4.

The purpose of this investigation was to demonstrate that NH4OH in combination with Ba(OH)2 and lime treatment offers an attractive solution for treatment of acid mine-water rich in Fe(II). In this approach, Fe(II) is oxidised and precipitated in the presence of added NH4OH as Fe(OH)3, allowing the precipitated Fe(OH)3 to be separated. In the following stage, SO4 is precipitated with Ba(OH)2, as BaSO4, and separated as a sludge. NH3 is partially stripped off because of the increased pH when Ba(OH)2 is dosed. Next, lime is added to increase the pH to above 12.4, to allow stripping off of the remaining NH3. Calcium is recovered as CaCO3 by introducing CO2 into the solution.
The overall objectives of the study were the following:
- Identify optimum conditions for Fe(II) oxidation when using NH4OH as alkali
- Determine optimum conditions for removal of SO4 when Ba(OH)2 is added for precipitation of BaSO4 (barite)
- Demonstrate that limestone can be used for free acid removal
- Identify optimum conditions for recovery of NH4OH
- Identify optimum conditions for removal of calcium by introduction of CO2
- Compare the behaviour of simulated and real acid mine water
- Compare actual measured water quality with that predicted by Visual MINTEQ software
EXPERIMENTAL
Feedstock
Mine water from the decanting site in Randfontein was used as feed water containing Fe(II) (670 mg/ℓ) and SO4 (2 090 mg/ℓ). Simulated AMD was prepared from an aqueous mixture of FeSO4 (Rochelle Chemicals, Pretoria) and H2SO4 (SMM Instruments, Johannesburg). Simulated mine-water was prepared as follows: FeSO4-7H2O (1.50 g) and concentrated H2SO4 (11.0 mℓ) were each dissolved together in distilled water and made up to 500 ml solution containing 600 mg/ℓof Fe(II) and 2 063 mg/ℓ of SO4.
Reagents
Aqueous NH3 (SMM Instruments, Johannesburg) was used for neutralisation. Compressed air (Afrox, Pretoria) was bubbled into the reactor at a controlled flow-rate to provide the oxygen for iron oxidation. Ba(OH)2 (Merck, SA) was used for SO4 removal. Lime (Rochelle Chemicals, Pretoria) was used for removing the remaining NH3 in the solution and CO2 (Afrox, Pretoria) was used for the precipitation of Ca as CaCO3. CaCO3 (Rochelle Chemicals, Pretoria) was used to remove free acid in the alternative method.oxygen for iron oxidation. Ba(OH)2 (Merck, SA) was used for SO4 removal. Lime (Rochelle Chemicals, Pretoria) was used for removing the remaining NH3 in the solution and CO2 (Afrox, Pretoria) was used for the precipitation of Ca as CaCO3. CaCO3 (Rochelle Chemicals, Pretoria) was used to remove free acid in the alternative method.
Equipment
Neutralisation of acidic mine water with NH4 OH and SO4 removal with Ba(OH)2 was studied using arrays of stirred beakers. NH3 stripping studies were done using a desorption column and a rotary evaporator. The desorption column was a cylinder (diameter: 223 mm; height: 2 000 mm), packed with plastic rings that served to increase the surface area, and fitted with a pump that circulated the sample. A spray nozzle on top of the column distributed the sample evenly across the cross section of the column. In this column NH3(aq) diffuses as NH3 gas. A rotary evaporator was used as an alternative method for removal of NH3.
Experimental procedures
Batch studies were carried out using 5 ℓ and 500 mℓ stirred, glass beakers. Compressed air was passed through the solutions using sintered glass diffusers at different flow-rates. Simulated mine water and NH OH were mixed at Time Zero. NH OH was added slowly to the solution at 10 mℓ intervals and 10 min was allowed at each interval for equilibration. Compressed air was passed through the reaction mixture. Samples were taken at regular intervals and assayed for pH and Fe(II) concentration using redox titration.
SO4 was removed as precipitated BaSO4 by addition of Ba(OH)2. NH3 was removed in 2 stages: Ba(OH)2 was used in the first stage for partial removal and in the second stage lime was added into the solution, fed into the column by a pump and recycled under room temperature for 3 h. Compressed air removed NH3 as NH3 gas. The remaining sample was taken at intervals and assayed for pH and NH3 concentration. NH3 gas was not adsorbed to any surface; hence further experiments to investigate adsorbtion to an acidic solution are planned.
The previous method needs hydrated lime to remove the remaining NH3 from the solution. The costs can be minimised by addition of limestone in the first stage of the process. In this experiment, CaCO3 was added to remove free acid so that NH4OH only removes metal hydroxides. In this process, addition of hydrated lime for NH3 removal was eliminated.
Experimental programme
The effects of the following parameters on the Fe (II) oxidation were determined:
- Air flow-rates (3.1, 5, 5.6, 7.9 ℓ/min)
- NH3/acidity (mol ratios of 0.2, 0.4, 0.6, 0.8, 1.0, 1.2, 1.4, 1.6)
- Temperature (25, 35, 45, 65°C)
Batch studies were carried out to demonstrate the removal of SO4 in the solution with addition of Ba(OH)2 by varying the SO4 removed/Ba dosed mol ratio (mol/mol: 0.5, 1, 1.5).
The effects of the following parameters on the NH3 stripping were determined:
- Air bubbled through to the desorption column (119, 145, 168 ℓ/min)
- Amount of packing material in the desorption column (empty, half-full and fully- packed)
- Temperature in the rotary evaporator for removal of NH3 as a gas in the solution (25, 35, 45, 55°C)
ANALYTICAL
Water samples were collected at various stages, filtered (Whatman 0.45 µm filter paper) and assayed for Fe(II), pH, NH3, Ca, SO4 and alkalinity concentrations using standard procedures (APHA, 2012; Vogel, 1989). NH3 analyses were done using BUCHI Distillation Unit B-324. Metals were assayed using atomic absorption spectroscopy.
Fe(II) concentrations were determined by adding filtered sample (10 mℓ), 1N H2SO4 (10 ml) and Zimmerman-Reinhard reagent (10 mℓ) to an Erlenmeyer flask (100 mℓ) and titrating the solution with 0.1 N KMnO4 until pale pink (Vogel, 1989). NH3 was determined by distillation, where 100 mℓ of sample was placed in a distillation flask and adjusted to pH 12.4 with addition of Ba(OH)2∙8H2O. Indicator boric acid solution was used as absorbent for the NH3 distillate (APHA, 2012). Calcium was determined as total hardness because magnesium was not present. Filtered sample (5 mℓ), water (45 mℓ), dilute NH and Eriochrome Black T indicator were added to an Erlenmeyer flask (100 mℓ). The solution was titrated with 0.02 M EDTA to colour change (APHA, 2012). SO4 was determined by adding filtered sample (2 mℓ) and conditioning reagent (2 mℓ) to a volumetric flask (50 mℓ) made up to volume with distilled water. A 20 ml aliquot of the solution was mixed with 0.15 g of BaCl2 in the cuvette and the turbidity was measured using a turbidity meter (Vogel, 1989).
Alkalinity was determined by titration of sample (5 mℓ) to pH 4.3 using 0.1 N HCl (APHA, 2012). Ca, Ni, Co, Zn, K and Mn were assayed using atomic absorption spectrophotometry (APHA, 2012).
RESULTS AND DISCUSSION
Water quality
Comparison between real and simulated acid mine water
Tables 1 and 2 show the chemical compositions of the water after various treatment stages for simulated and real acid mine water, respectively. Fe(II) concentrations were lowered to <10 mg/ℓ in both cases, after addition of NH4OH. SO4 concentrations after Ba(OH)2 addition were < 400 mg/ℓ. In real acid mine-water, the initial pH was 4.2 and less free acid was present. Ba(OH)2 was added to raise the pH to 11.9. This made addition of lime unnecessary for removal of NH3.


Chemical reactions for simulated acid mine water
In the ammonium-barium (NB) process, a variation of the MBO (magnesium barium oxide) process (Bologo et al., 2011), Mg(OH)2 was replaced with NH4OH. During NH4OH treatment, acid and the metals assayed for were reduced to below the maximum permissible limit according to the general standard. The lowering of the metals in the case of NH4OH treatment was mainly due to the oxidation of Fe2+ to Fe3+ (Reaction (3)) and precipitation as Fe(OH)3 (Reaction (4)). This was owing to the low solubility-product for Fe(OH)3 (K = 2.64 x 1039). NH4OH neutralises the acid produced and forms NH4+ ions (Reaction (5)). NH3 concentration was lowered from 1 020 mg/ℓ to 425 mg/ℓ by dosing Ba(OH)2∙8H2O (Reaction (6)) and simultaneously lowering SO4 concentration from 1 786 mg/ℓ to 350 mg/ℓ (Reaction (6)). Lime was added to lower the remaining NH3 concentration to 8.5 mg/ℓ by stripping using rotary evaporator (Reaction (7)). Calcium was recovered by dosing CO2 (Reaction (8)). NH3 was partially stripped from the solution as NH3 gas after addition of Ba(OH)2 (pH 10.6), as NH3 becomes sufficiently volatile only at pH > 12.4; hence the need for lime addition to increase the pH. Excess Ba(OH)2 was not added to increase the pH because excess Ba in the treated water must be avoided on account of its toxicity to humans and animals.
Fe(II)-oxidation in the presence of NH4OH for neutralisation
The rate at which Fe(II) is oxidised depends on variable parameters, including pH, concentration of dissolved O2 and temperature (Werner and Lee, 1961). Figure 2 shows that the presence of NH4OH in the acidic solution affects the oxidation of Fe(II). The mol ratio of NH3 to acidity was determined to establish how much NH3 was required to remove the free acid so as to remove Fe by raising the pH.
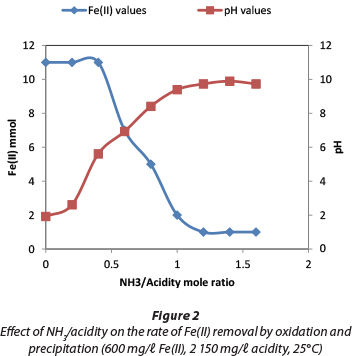
The optimum ΝH3/acidity mol ratio was 1.2, because at that mol ratio the pH of the solution was above 9 and the Fe(II) concentration was <10 mg/ℓ. Figure 3 shows the effect of aeration rate on the oxidation rate of Fe (II). It was noted that the higher the flow-rate of aeration the faster the oxidation rate, although the effect was marginal. Due to the high solubility of (NH4)2SO4 (74.4 g/100 mℓ), co-precipitation of sulphates and metal hydroxides was avoided.

Figure 4 shows the effects of temperature and the graphs showed, as expected, that an increment in temperature resulted in slightly faster Fe(II) oxidation. A problem was that the pH dropped due to NH3 stripping. Therefore, for optimum Fe(II) oxidation, it was carried out at room temperature.

Sulphate and partial ammonia removal using barium hydroxide
Ba(OH)2 was used for SO4 removal instead of lime because of the low solubility product of barite (Ksp = 1.08 x 1010) compared to gypsum (K sp = 4.93 x 10-5). Figure 5 shows experimental, theoretical and simulated results for optimum Ba/SO4 mol ratio dosed. The pattern for the removal of SO4 was similar.

Ammonia removal using hydrated lime
The rate of NH3 desorption depends on the temperature, the height of the packed column and air flow rate (Orvos et al., 2010). Figure 6 shows the effect of air flow rate. It was noted that at the air flow rate of 168 ℓ/min, NH3 concentration was reduced from 525 mg/ℓ to 11.9 mg/ℓ and the pH was lowered from 13.4 to 9.0 due to NH3 gas being stripped out.
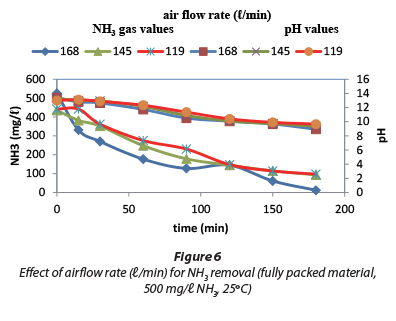
Figure 7 shows the effect of amounts of column packing material on NH3 removal. It was noted that the stripping of NH3 with a fully packed column proceeded at the fastest rate.

The effect of temperature on NH3 stripping using a rotary evaporator was determined. Figure 8 showed that, as expected, the higher the temperature the more NH3 becomes stripped out of the solution. The optimum temperature was 45°C and further increases in temperature, resulted in insignificant effects.

Removal of free acid using limestone
Should the acid in mine-water first be neutralised with CaCO3, it is expected that no addition of lime will be required after Ba dosing for NH3 removal. Table 3 shows the chemical composition of simulated AMD after adding limestone in the first stage, followed by various treatment stages. Limestone was added to pH 5.5 to remove the acid. The addition οf NH4OH resulted in the removal of metals as hydroxides and increases the pH to 10.5, which was higher than that when limestone was omitted. Limestone was essential because addition of Ba(OH)2 was sufficient to allow all of the NH3 to be removed from the solution. The chemical reactions below show how limestone addition assisted in the elimination of hydrated lime for NH3 removal.

Comparison of the experimental results with Visual Minteq model predictions
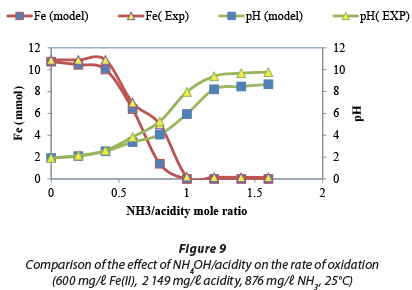
Visual Minteq software is used to predict experimental results at equilibrium (Visual MINTEQ, 2010). Figure 9 showed that the pΗ predicted by the model and that established experimentally followed the same pattern. The model predicted optimum Fe(II) oxidation to occur at a NH3/acidity mol ratio of unity. Experimentally, the optimum Fe(II) oxidation was found to occur at a mol ratio of 1.2.
CONCLUSIONS
It was demonstrated using the NB process at laboratory scale, that:
- By using NH4OH instead of Ca(OH)2, gypsum scaling can be avoided in the full-scale process.
- NH4OH precipitated Fe as the hydroxide in the simulated and real mine-water.
- Ba(OH)2 precipitated SO4 in the mine water as BaSO4 which resulted in partial NH3 removal on account of the raised pH, and the NH4+ compound did not precipitate because of the solubility of NH4SO4
- Hydrated lime treatment resulted in removal of the remaining NH3 by using a rotary evaporator.
- In the treated mine-water, CO2 treatment precipitated Ca as CaCO3.
- The method was able to remove metal in real and simulated acid mine-water.
- Addition of CaCO3 eliminated the need for addition of hydrated lime and limestone is cheaper than hydrated lime.
- The simulation predictions were similar to the experimental results obtained.
ACKNOWLEDGEMENTS
Thanks are due to the following organisations: the National Research Foundation, for funding TUT projects on mine-water neutralisation and SO4 removal. Rand Water, for funding the activities of the Rand Water Chair at TUT, and Tshwane University of Technology, which provides substantial financial support for the research programme on mine-water. The inputs of the following people are acknowledged: Professor Fritz Carlsson for editing the document and Mr Luke Gwatidzo (PhD student) for assistance with the laboratory work.
REFERENCES
APHA (2012) Standard Methods for the Examination of Water and Wastewater (22nd edn.). American Public Health Assoc, New York. [ Links ]
BOLOGO V, MAREE JP and CARLSSON F (2012) Application of magnesium hydroxide and barium hydroxide for the removal of metals and sulphate from mine water (MBO). Water SA 38 (1) 23-28. [ Links ]
HLABELA P (2009) The integrated barium carbonate process for sulphate removal from acid mine water. Ph.D. thesis, North West University. [ Links ]
INAP (INTERNATIONAL NETWORK FOR ACID PREVENTION) (2003) Treatment of sulphates in mine effluents. URL: http://www.inap.com.au/publicdownloads/ResearchProjects/TreatmentofsulphateinMineffluentsLoraxReport.pdf> (Accessed 12 April 2012). [ Links ]
JENNINGS SR, NEUMAN DR and BLICKER PS (2008) Acid Mine Drainage and Effects on Fish Health and Ecology: A Review. Reclamation Research Group Publication, Bozeman, MT. [ Links ]
JIMENEZ A, AROBA J, DE LA TORRE ML, ANDUJAR JM and GRANDE JA ( 2009) Model of behaviour of conductivity versus pH in acid mine drainage water, based on fuzzy logic and data mining techniques. J. Hydroinf. 11 (2) 147-153. [ Links ]
KUN LE (1972) A report on the reduction of the sulphate content of acid mine drainage by precipitation with barium carbonate. Internal report, Anglo American Research Laboratories, Project D/3/W/1. [ Links ]
MAREE JP (2003) Integral Chemical/Biological Process, Republic of South Africa (Patent No. 2003/1362), Australia (Patent No. 2001279996), Canada (Patent No. 2, 418, 472) United States of America (Patent No. 6,863,819), China (Patent 018162053), Great Britain (Patent No. 1,313,668), France (Patent No. 1,313,668), German (Patent No. 1,313,668). [ Links ] MAREE JP, BOSMAN DJ and JENKINS GR (1989) Chemical removal of sulphate, calcium and heavy metals from mining and power station effluents. Proc. 1st Biennial Conf. of the Water Inst. of Southern Africa, 29-30 March 1989, Cape Town. [ Links ]
MAREE JP, DE BEER M, STRYDOM WF, CHRISTIE ADM and WAANDERS FB (2004) Neutralizing Coal Mine effluent with limestone to decrease metals and sulphate concentrations. Mine Water Environ. 23 81-86. [ Links ]
MINTEQ Visual (2000) URL: http://www2.lwr.kth.se/English/OurSoftware/vminteq/Visual%20MINTEQtutorial.pdf (Accessed 24 November 2011). [ Links ]
ORVOS M, BALALZ T and BOTH KF (2010) Waste water treatment with ammonia recovery system. J. Civ. Environ. Eng. 2 (2) 113-117. [ Links ]
TRUSLER GE, EDWARDS R I, BROUCKAERT C J and BUCKLEY CA (1988) The chemical removal of sulphates. Proc. 5th National Meeting of the South african Institution of Chemical Engineers, (15-16 August 1988) Pretoria. W3-0-W3-11. [ Links ]
VAN NIEKERK AM and MAREE JP (2001) Coaltech 2010 Colloquium, 2 March 2001, Witbank Civic Theatre, Witbank SVEHLA G (2011) Vogel's Quantitative Inorganic Analysis (7th edn. [ Links ]). John Wiley and Sons, New York. 368 pp. [ Links ] VOLMAN R (1984) The use of barium sulphide to remove sulphate from industrial effluents. M.Sc. thesis (Chem. Eng.), University of Stellenbosch. [ Links ]
WERNER S and LEE GF (1961) Oxygenation of ferrous iron. Ind. Eng. Chem. 53 (2) 143-146. [ Links ]
ZIEMKIEWICZ PF, SKOUSEN JG, BRANT DL, STERNER PL and LOVETT RJ (1997) Acid mine drainage treatment with armored limestone in an open limestone channel. J. Environ. Qual. 26 560-569. [ Links ]
Received 30 May 2013
Accepted in revised form 20 June 2014
* To whom all correspondence should be addressed.+27 12 382 6315; fax: +27 86 642 5592 ; e-mail: mavis.dineo@gmail.com














