Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Water SA
On-line version ISSN 1816-7950
Print version ISSN 0378-4738
Water SA vol.40 n.3 Pretoria Jun. 2014
http://dx.doi.org/10.4314/wsa.v40i3.12
Treatment of landfill leachate: removal of ammonia by struvite formation
CC Camargo; JR Guimaráes; AL Tonetti*
School of Civil Engineering, Architecture and Urbanism, FEC/UNICAMP, Avenida Albert Einstein, 951, Cidade Universitária 'Zeferino Vaz, PO Box 6021, 13083-852, Campinas, SP, Brazil
ABSTRACT
This paper presents a study of ammoniacal nitrogen removal by chemical precipitation resulting in the formation of ammonium and magnesium phosphat (MgNH4PO4∙6H2O), known as struvite, from the leachate in the Delta A landfill, located in the city of Campinas, Sáo Paulo, Brazil. After the addition of a magnesium source (MgCl2∙6H2O), and phosphorus (Na2HPO4∙12H2O), ammoniacal nitrogen was precipitated as highly insoluble salt. The removal of ammoniacal nitrogen from the leachate exceeded 85% when the reaction was performed at an initial pH of 10.0. The highest efficiencies were achieved when the molar ratio between the ions involved in the reaction, i.e., Mg2+:PO43-:NH4+, was 1.2:1.0:1.0, respectively.
Keywords: landfill, leachate, ammonia, nitrogen removal
INTRODUCTION
Brazil has a daily production of about 260 000 tons of municipal solid waste (IBGE, 2008). Of this total, approximately 65% is disposed in landfills, considered to be the most appropriate form of disposal within the Brazilian context (Povinelli and Além Sobrinho, 2009). However, from an environmental point of view, there are problems that should be addressed inside the landfill, such as the emission of greenhouse gases (GHG), and the generation of leachate from rainwater and residue decomposition.
The leachate has unique characteristics, such as a high concentration of ammoniacal nitrogen (NH3 and NH4+), high chemical oxygen demand, low potential for biological degradation, and the presence of metals and other organic and inorganic substances that confer a high toxicity to this type of waste, and hinder its treatment. Studies by Clement and Merlin (1995) indicate that ammoniacal nitrogen in its non-ionized form (NH3) is the major contributor to the toxicity of the leachate. Li and Zhao (1999) argue that its concentration in leachate must be reduced to at least 100 mg∙l-1 prior to its entry into biological reactors for activated sludge.
Chemical precipitation in the form of the double salt of magnesium and ammonium phosphate, also known as struvite, is one of several options for removing ammoniacal nitrogen from this matrix. Struvite is an inorganic mineral in the form of a white crystal, with a chemical formula of MgNH4PO4∙6H2O. Struvite is highly soluble in acid, and insoluble in a basic medium; the pH of minimum solubility is around 9.0 (Munch and Barr, 2001). Moreover, as a slow release source of phosphorus, magnesium, and nitrogen, struvite is a potentially valuable fertiliser (Iaconi et al., 2010).
Based on these facts, the aim of this work was to reduce the ammoniacal nitrogen concentration of a landfill leachate by chemical precipitation in the form of struvite, by evaluating the efficiency of the treatment, the pH of the medium, and the ratio between the reagents.
MATERIALS AND METHODS
The leachate used for the experiments originated in the main landfill of the city of Campinas, in the state of São Paulo, Brazil. This landfill occupies an area of 400 000 m2, and responded to the completion of this work by receiving 100% of household waste generated in the city, averaging 1 000 t∙day-1. Operations began in 1992, and in 2011 the production of leachate was approximately 10.4 m3∙h-1.
The raw leachate samples were collected and brought to the Laboratory of the School of Civil Engineering, Architecture, and Urbanism (FEC), at the University of Campinas (Unicamp).
The reagents used in the precipitation test were chosen based on the work of Li et al. (1999). According to these authors, the combination of MgCl2∙6H2O with Na2HPO4∙12H2O is the most efficient in removing ammoniacal nitrogen.
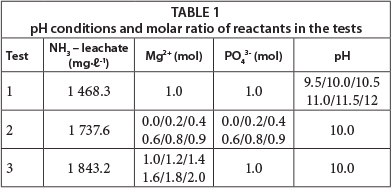
Before conducting the tests, a sample of the raw leachate was analysed to determine the ammoniacal nitrogen (NH4+ + NH3) concentrations. From the obtained values, the amounts of reagents (MgCl2.6H2O and Na2HPO4.12H2O) to be used in each test were calculated, according to the desired molar ratio of Mg2+:NH4+:PO43-. Table 1 shows the ammoniacal nitrogen concentrations found, in addition to the molar ratios and pH values evaluated in the various experiments.
The experimental part was carried out according to the flowchart shown in Fig. 1. The leachate was placed in 6 jars and pH was adjusted with solutions of NaOH 15 mol∙ℓ-1 and H2SO4 6 mol∙ℓ-1, followed by the addition of the reagents MgCl2∙6H2O and Na2HPO4∙12H2O.
The tests were conducted in a jar test apparatus, consisting of 6 vessels with the following dimensions: 20.0 x 11.5 x 11.5 cm. Each vessel had a capacity of 2ℓ, which resulted in a water depth of 15 cm and surface area of about 132 cm2.
In each test the samples were subjected to 30 min of reaction, with stirring at 100 r-min-1, the velocity gradient equivalent to 64 s-1, and 30 min of decantation, followed by the collection of 500 mℓ samples from the hole at the centre of the jar. The samples were subjected to the following analyses: pH, conductivity (cond), ammoniacal nitrogen (NH4+ + NH3), total Kjeldahl nitrogen (TKN), phosphorus (P), chemical oxygen demand (COD), and magnesium (Mg). All techniques were performed according to the guidelines established in the Standard Methods for the Examination of Water and Wastewater (APHA et al., 2012).
Tests without addition of reagents
To assess the possible volatilisation loss of the ammonia gas, as well as the precipitation of phosphorus in a different form than struvite, a test was conducted without the addition of the two principal reagents. In this case, only the pH was adjusted, and the next steps of the procedure were maintained, with the 30 min reaction and decantation time.
RESULTS AND DISCUSSION
Table 2 shows that the leachate had low BOD, low BOD/COD ratio, a pH around 8.0, and high concentrations of ammoniacal nitrogen.
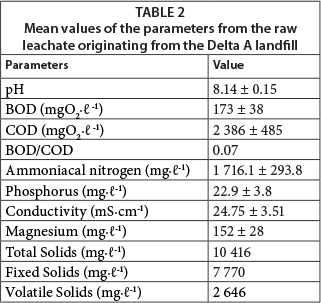
Table 3 shows the results corresponding to Test 1, wherein the molar ratio Mg2+:NH4+:PO43- was 1.0:1.0:1.0, respectively, and the pH varied between 9.5 and 12.0 (Table 1). Under these conditions the ammoniacal nitrogen removal efficiency was at least 77.8 %, reaching 89.1 % when the pH was adjusted to 10.0; the final concentration was 159.4 mg∙ℓ-1.
There was a decrease in pH at the end of the reaction, which, according to Li et al. (1999) can be explained by the analysis of Eq. (1). The reaction between magnesium cations and phosphate anions releases acid H3O+ ions into the solution, causing a decrease in pH values. There was an increase in the conductivity of the solution, from 22.64 to 39.40 mS∙cm-1, as a result of the increase in H3O+ ions, which have a high ionic conductivity. The solution also displayed increased concentrations of Na+ and Cl-, as a result of the reagents used.
The magnesium and phosphorus concentrations decreased, along with the final ammonia concentration; a result consistent with struvite formation. However, the phosphorus values were still very high, always exceeding 30 mg∙ℓ-1.
According to the results from Test 1, the pH of the solutions , in the subsequent stages, was adjusted to 10.0. In Test 2 (Table 4) the molar ratio of magnesium and phosphorus varied from 0.0 to 0.9 (Table 1), aiming at a reduction in the final concentration of phosphorus. A value of 1 mg∙ℓ-1 for phosphorus is stipulated by the state of Rio Grande do Sul (Brazil), as the upper limit for release into water bodies (CONSEMA 128, 2006).
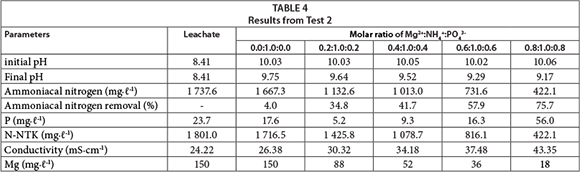
Table 4 shows that with an increasing molar ratio the final concentration of phosphorus increased in the treated leachate as well. Even for the smallest ratios, phosphorus still exceeded 1 mg∙ℓ-1. The ammoniacal nitrogen removal was more effective for a molar ratio of Mg2+:NH4+:PO43 of 0.8:1.0:0.8 (75.7%). Taking into account the data presented in Table 3, it follows that for a molar ratio of 1.0:1.0:1.0 removal reached 89.1%. These results demonstrate that a decrease in the molar ratio of the reactants is not advised when the primary purpose is ammoniacal nitrogen removal.
In Test 3 (Table 5) higher levels of magnesium, in relation to other compounds, were added in an attempt to decrease the residual concentration of phosphorus without compromising the ammonia removal. The procedure was effective, and a molar ratio of 2.0:1.0:1.0 resulted in a final concentration of phosphorus equivalent to 3.6 mg∙ℓ-1. It is noteworthy that, besides the phosphorus present in the raw leachate, more is added as part of one of the reagents.
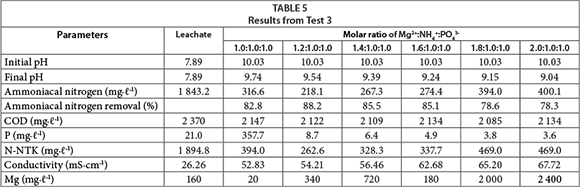
However, the increase in the molar ratios of magnesium led to a decrease in ammonia removal. This phenomenon can be explained by a greater interaction of magnesium with phosphorus; thus, forming compounds other than struvite. Michalowsky and Pietrzyk (2006) report that there are 4 species of magnesium phosphate, which can crystallize from solutions containing ammonia, magnesium, and phosphate: ammonium phosphate and hexahydrate magnesium (struvite, MgNH4PO4∙6 H2O), magnesium hydrogen phosphate (newberyite, MgHPO4∙3H2O), and trimagnesium phosphate in two hydration states: Mg3(PO4)2∙22H2O and Mg3(PO4)2∙8H2O (boberyite). Thus, the best result for the removal of ammonia-cal nitrogen was obtained using a molar ratio of 1.2:1.0:1.0.
Test without addition of reagents
In Test 4 (Table 6), only the pH was adjusted and no reagents were added. This procedure was performed to evaluate the potential loss of ammonia by evaporation, and the phosphorus precipitation caused by the pH of the medium.
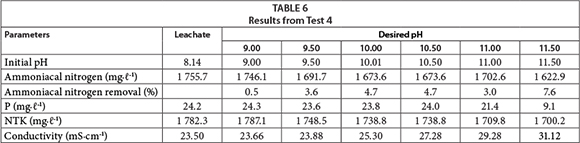
It was observed that by increasing the pH there was a higher rate of ammonia loss by volatilisation. With pH 10.0, the ammoniacal nitrogen decreased by 4.7%. It is important to note that in the Standard Conditions of Pressure and Temperature (CNPT), the equilibrium constant of ammonia is 1.8 x 10-5; therefore, the pKb is 4.74; i.e., at pH 9.26 half of the ammoniacal nitrogen from the medium is in the ammonia form. This value increases exponentially as the solution becomes more basic, forming ammonia gas that can escape into the atmosphere. There is also a reduction in phosphorus concentration, probably caused by precipitation. According to Diamadopoulos and Benedek (1984), this precipitation can be attributed to the interaction between the calcium and phosphates, forming a stable compound (hydroxyapatite Ca10(PO4)6(OH)2).
CONCLUSIONS
Chemical precipitation by struvite formation was effective in removing ammoniacal nitrogen from the leachate; reaching removal levels greater than 85% when the initial pH was equal to 10.0. The most suitable molar ratio for Mg2+:PO43-:NH4+ was 1.2:1.0:1.0 for the removal of ammoniacal nitrogen.
REFERENCES
APHA, AWWA, WEF (2012) Standard Methods for Examination of Water and Wastewater (20th edn.) United Book Press, Baltimore. [ Links ]
CLÉMENT B, MERLIN G (1995) The contribution of ammonia and alkalinity to landfill leachate toxicity to duckweed. Science Total Environ. 170 71-79. [ Links ] CONAMA (2011) Conselho Nacional do Meio Ambiente - Resolucções CONAMA n° 357, 17 de marfo de 2005. Ministério do Meio Ambiente, Distrito Federal. [ Links ]
CONSEMA (CONSELHO ESTADUAL DO MEIO AMBIENTE) (2006) Resolucão n° 128. Dispões sobre a fixação de padrões de emissáo de efluentes líquidos para fontes de emissáo que lancem seus efluentes em águas superficiais no Estado do Rio Grande do Sul. Secretaria do Meio Ambiente, Rio Grande do Sul. [ Links ]
DIAMADOPOULOS E and BENEDEK A (1984) The precipitation of phosphorus from wastewater through pH variation in the presence and absence of coagulants. Water Res. 18 (9) 1175-1179. [ Links ]
IACONI C, PAGANO M, RAMADORI R and LOPEZ A (2010) Nitrogen recovery from a stabilized municipal landfill leachate. Bioresour. Technol. 101 1732-1736. [ Links ]
IBGE (2011) Pesquisa Nacional de Saneamento Básico. Instituto Brasileiro de Geografia e Estatística, Distrito Federal. [ Links ]
LI XZ, ZHAO QL and HAO XD (1999) Ammonium removal from landfill leachate by chemical precipitation. Waste Manag. 19 409-415. [ Links ]
MICHALOWSKY T and PIETRZYK A (2006) A thermodynamic study of struvite + water system. Talanta 68 594-601. [ Links ]
MUNCH E and BARR K (2001) Controlled struvite crystallization for removing phosphorus from anaerobic digester sidestreams. Water Res. 35 (1) 151-159. [ Links ]
POVINELLI J and ALÉM SOBRINHO P (2009) Introdução. In: GomeLP (ed.) PROSAB. Resíduos Sólidos - Estudos de Caracterização e Tratabilidade de Lixiviados de Aterros Sanitários para as Condições Brasileiras. ABES, Rio de Janeiro. [ Links ]
Received 27 September 2013
Accepted in revised form 30 June 2014.
* To whom all correspondence should be addressed.+55 19 3521-2369; e-mail: adriano@fec.unicamp.br














