Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
Water SA
versão On-line ISSN 1816-7950
versão impressa ISSN 0378-4738
Water SA vol.40 no.1 Pretoria Jan. 2014
ARTICLES
The decomposition of estuarine macrophytes under different temperature regimes
DA Lemley; GC Snow; LRD Human
Department of Botany, Nelson Mandela Metropolitan University, PO Box 77000, Port Elizabeth, 6031, South Africa
ABSTRACT
The Great Brak Estuary is a temporarily open/closed system situated along the warm temperate coast of the Western Cape, South Africa. The estuary is subject to a variety of anthropogenic impacts (e.g. freshwater abstraction and sewage discharge) that increases its susceptibility to prolonged periods of mouth closure, eutrophication, and ultimately the formation of macroalgal blooms. The aim of this study was to determine the decomposition characteristics of the most dominant submerged macrophyte and macroalgal species in the Great Brak Estuary. Laboratory experiments were conducted to determine the effect of different temperature regimes on the rate of decomposition of 3 macrophyte species and the extent of inorganic nutrients released. The results demonstrated that anaerobic decomposition of Zostera capensis, Ruppia cirrhosa, and Cladophora glomerata resulted in high levels of inorganic nutrient release over the 28-day study period. Ammonium (NH4+) was the dominant form of dissolved inorganic nitrogen (DIN) released during the decomposition process for all three species. The low levels of total oxidised nitrogen (nitrate and nitrite) released during decomposition were attributed to the inhibition of nitrification by heterotrophic bacteria under anoxic conditions. The relative levels of dissolved inorganic phosphorus (DIP) released were lower than that observed for DIN, and peaked early on in the experimental period (ca. 7 days), thereafter stabilising or decreasing. The DIP levels were explained, in part, by the varying nutrient requirements and limitations of each species (e.g. nitrogen-limited). The release of inorganic nutrients was greatest at higher temperatures (i.e. 25°C and 30°C), due to the reduced bacterial activity experienced at lower temperatures (i.e. 15°C). Ultimately, estuarine health deteriorates during macroalgal blooms, and therefore it is important to implement mitigation measures, such as artificial mouth breaching and plant harvesting, in order to minimise or reverse the effects of eutrophication.
Keywords: Great Brak Estuary, decomposition, temperature, inorganic nutrient release, Cladophora glomerata, Zostera capensis, Ruppia cirrhosa
INTRODUCTION
The rate and extent of anthropogenic pressures on estuaries have increased greatly over recent times due to the demands of an ever-increasing global population. Some of the most prominent consequences of anthropogenic activities for the ecological integrity of estuaries include eutrophication, altered freshwater inflow regimes, accelerated rates of sedimentation, habitat destruction and over-exploitation of resources (Snow and Adams, 2007). It is critical to manage and, where possible, prevent these changes from occurring due to the fact that they influence aspects such as salinity, nutrient loading, flushing time and turbidity maxima, which are responsible, to varying degrees, for determining the spatial distribution and composition of biotic communities (e.g. macrophytes) (Snow and Adams, 2007).
In estuarine systems, free-floating macroalgae and submerged macrophytes play an important role in nutrient cycling, in that they act as both nutrient sources and sinks (Hanisak, 1993). Presence or absence of the various floral communities within estuaries is controlled by factors such as estuarine morphometry, nutrient and light availability, herbivory, exposure to waves and currents, and substratum type (Pedersen and Borum, 1996). In recent history, the incidence of fast-growing ephemeral macroalgae proliferations has increased globally due to eutrophication caused by excessive nutrient inputs (mainly nitrogen and phosphorus) into coastal ecosystems (Paalme et al., 2002; Lomstein et al., 2006; Berezina and Golubkov, 2008; Gubelit and Berezina, 2010). Under these conditions macroalgae, either attached or free-floating, accumulate in very high biomass (approx. 40 to 400 kg wet weight m-2) in estuaries (Paalme et al., 2002; Lomstein et al., 2006). The consequences of such macroalgal blooms are numerous, and include oxygen depletion (anoxia), altered biogeochemical cycling in the water column and sediments, changes to the composition of higher trophic levels, shading of slow-growing, submerged macrophytes (such as seagrasses), and an overall reduction in biodiversity and recreational value (Hanisak, 1993; Pedersen and Borum, 1996; Paalme et al., 2002; Lomstein et al., 2006; Gubelit and Berezina, 2010).
Management of blooms can be improved by having better knowledge of the decomposition rates of algal species in order to validate their role in the nutrient dynamics of estuaries (Paalme et al., 2002). Moreover, understanding the decomposition of aquatic plants allows for the determination of their role in providing organic matter to detrital food chains, or alternatively being a source of regenerated inorganic nutrients for autotrophic assimilation (Twilley et al., 1986). Studies such as these are important because under macroalgal bloom conditions, the timing and magnitude of nutrient releases during decomposition are likely to have significant ecological impacts in estuaries (Hanisak, 1993).
The Great Brak Estuary is situated along the warm temperate coast of the Western Cape Province of South Africa, and is classified as a temporarily open/closed estuary in fair condition (Whitfield, 1992). Since the construction of the Wolwedans Dam in 1989 the estuary has experienced significant freshwater inflow reductions (55.8% less than natural). This in turn has led to a greater probability of eutrophication within the estuary, due to an increased frequency of prolonged periods of mouth closure. During periods of extended mouth closure in the Great Brak Estuary, numerous filamentous green algae proliferations have been observed. These 'blooms' have the potential to cause significant alterations to the natural structure and functioning of the estuary and as such need to be monitored and managed. One such way in which this is achieved at the Great Brak Estuary is through mouth management, aimed at preventing flooding of low-lying areas and also the accumulation of high macroalgal biomass within the system (Whitfield, 1992).
The purpose of this study was to quantify the concentration of inorganic nutrients released by estuarine macrophytes, with the broader objective of assessing their contribution to nutrient recycling under anoxic, low-flow conditions. To do this the decomposition characteristics of the dominant submerged macrophyte and macroalgal species within the Great Brak Estuary was studied under different temperature regimes. The Great Brak Estuary provides a good study site in that it is subjected to a variety of anthropogenic impacts (e.g. freshwater abstraction and sewage discharge) that increase its susceptibility to prolonged periods of mouth closure, eutrophication, and ultimately the formation of macroalgal blooms. The species assessed during the study were: Cladophora glomerata (Linnaeus) Kützing, Zostera capensis Setchell, and Ruppia cirrhosa (Pentagna) Grande. Laboratory experiments were conducted on 3 estuarine macrophyte species in order to determine the effect of temperature on decomposition rates and the subsequent release of inorganic forms of nitrogen and phosphorus.
METHODS
Study site
The Great Brak Estuary (34°03'26"S; 22°14'21''E) is classified as a temporarily open/closed estuary (TOCE) located on the south coast of South Africa (Van Niekerk and Turpie, 2012). The estuary is approximately 6.2 km in length with an estimated total surface area of 105.1 ha (Van Niekerk and Turpie, 2012). The estuary drains a forested, semiarid catchment area which is relatively small (192 km2). The catchment receives more or less equal amounts of rain throughout the year with slight peaks in spring and autumn. However, the area is subjected to droughts and occasional flooding and the recorded annual run-off varies from 4.3 x 106 m3 to 44.5 x 106 m3 (DWAF, 2009).
The Wolwedans Dam is located 3 km upstream of the estuary and has a storage capacity of 23 x 106 m3 (DWAF, 2009). The freshwater inflow to the estuary has been considerably reduced during the last decades by afforestation, direct abstraction and damming from 36.8 x 106 m3 under natural condition to 16.25 x 106 m3 at present (DWAF, 2009). The estuary generally exhibits a tidal prism of 0.3 x 106 m3 (DWAF, 2009). The mouth of the estuary is bounded by a low rocky headland on the east and a sand spit on the west. Immediately inland of the mouth, the estuary widens into a lagoon basin containing a permanent island with an area of 0.1 km2. The lower estuary is relative shallow (0.5 to 1.2 m deep) with some deeper areas in scouring zones near the rocky cliffs and bridges. The middle and upper reaches of the estuary are generally less than 2 m deep, with some deeper areas, between 2 and 4 km from the mouth, varying between 3 and 4 m deep. The mouth of the Great Brak Estuary generally closes when high waves coincide with periods of low river flow. Artificial breaching is practised at the Great Brak Estuary to prevent flooding of low-lying properties.
The Great Brak Ecological Water Requirement study recently completed for the Department of Water Affairs (DWA) concluded that pressures currently contributing to the degraded health of the Great Brak Estuary include: river flow reduction; artificial breaching; deteriorating water quality; structures in the intertidal area; development on, and disturbance of, the salt marshes; over-exploitation of fish and bait organisms; disturbance of birds; obstruction of the estuary and river by causeways, weirs and the Wolwedans Dam.
Experimental design
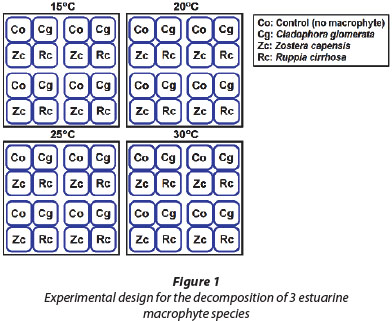
Four 125 ℓ plastic containers where set up in the laboratory, each representing a specific temperature treatment, i.e., 15°C, 20°C, 25°C and 30°C. The temperature variation within each of the treatments over the 28 d period was shown to be negligible (SE < 0.3°C). Temperatures were selected based on existing data pertaining to the range of water temperatures measured in the estuary for the period September 2010 to July 2012 (n = 437), i.e. 11.9°C to 29.4°C, with average 20°C. The containers simulated environmental dark anoxic conditions in that they prevented any light penetration. The water within each of the treatments was regulated at a specific temperature using glass heaters. Water was circulated using an air stone within each of the containers in order to ensure equal temperature distribution.
Within each of the treatments, there were 4 replicates for each species (i.e. Cladophora glomerata, Zostera capensis, and Ruppia cirrhosa) as well as the control. For each of the species samples, 50 g of wet macrophyte material was weighed and placed into plastic Ziploc© bags containing 1 ℓ of estuarine water (approx. 35 PSU). Next, thin silicon tubing was placed into each of the sample bags to act as a sample port for water extraction during the experiment, and subsequently cable-tied shut in order to prevent oxygen ingress. The control samples were constructed in the same way as the species samples; however, no macrophyte material was placed in the 1 ℓ of estuarine water. Figure 1 provides an illustration of the experimental design.
Once the experiment was set up, water samples (50 mℓ) were collected at set time intervals over a 28 d period. The sampling time intervals for the experiment were: initial (0 d), 3 d, 7 d, 21 d, and 28 d. In order to compensate for the reduction in water volume of the incubation bags over the course of the experiment, a correction factor was determined to account for overestimation of nutrient concentrations. This was achieved by multiplying the observed concentrations by the respective volumes from which they were removed (i.e. 1 ℓ, 0.95 ℓ, 0.90 ℓ, 0.85 ℓ and 0.80 £), and subsequently determining the error between the observed and corrected values. Concentrations were reduced by the following percentages at the respective 'points' during the study period: 3 d (5.26 %), 7 d (11.11 %), 21 d (17.65 %) and 28 d (25 %). The initial concentrations were not corrected as these were representative of the starting volume (i.e. 1 ℓ). Lastly, temperature for each of the treatments was recorded simultaneously with water sample collection using a mercury thermometer.
Tissue nutrient content of macrophytes and macroalgae
Above- and below-ground tissue contents of nitrogen and phosphorus were determined on newly collected specimens for the various species (i.e. C. glomerata, Z. capensis, and R. cirrhosa). Whole plants (leaves, rhizomes and roots) or macroalgae (thalli) were harvested and cleaned of sediment in distilled water for 2 to 3 min. The tissue was dried at 60°C for 24 h, finely ground, and content measured using the persulphate digestion method for the simultaneous detection of total nitrogen and total phosphorus (Koroleff, 1983).
Inorganic nutrients
Water samples taken from each of the sample bags in each treatment were gravity-filtered through glass-fibre filters (Whatman© GF/C) and then filtered through hydrophilic polyvinylidene difluoride (PVDF) 0.47 µm pore-size syringe filters. Next, the filtrates were stored in 150 mℓ bottles and frozen until analyses could commence.
The samples were analysed for total oxidised nitrogen (TOxN; nitrate + nitrite) using the reduced copper cadmium method as described by Bate and Heelas (1975). Ammonium (NH4+) and soluble reactive phosphorus (SRP) were analysed using standard spectrophotometric methods described by Parsons et al. (1984). The analyses were done within a 2-week period after sampling.
Data analysis
Data were analysed using Statistica© Version 10 (StatSoft Inc., 2010). The data were tested for normality using the Shapiro-Wilks test. Differences in tissue nutrient content (TN and TP) between the macrophyte species were tested using the paired t-test (i.e. parametric) and Wilcoxon paired sample test (i.e. non-parametric). Relationships between nutrient concentrations and time, for each species, were analysed using the non-parametric Spearman's rank correlation. For differences between nutrient concentrations with temperature, and subsequently between species, a Kruskal-Wallis non-parametric analysis of variance was used. Furthermore, the aforementioned analyses were done in 2 parts; first for the entire data set (i.e. 0 to 28 d), and secondly for the latter part of the decomposition experiment (i.e. 7 to 28 d). All analyses were done at α = 0.05.
RESULTS
Tissue nutrient content
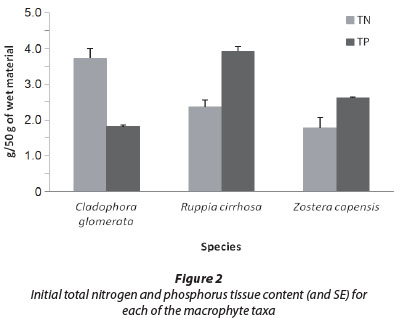
The average total nitrogen (TN) tissue content for each of the species, per 50 g of wet material, was (Fig. 2): Cladophora glomerata = 3.72 g (7.44%), Ruppia cirrhosa = 2.37 g (4.74%) and Zostera capensis = 1.78 g (3.56%). Furthermore, C. glomerata exhibited a significantly higher tissue TN concentration than both R. cirrhosa (t = 3.94; p < 0.05; df = 10) and Z. capensis (t = 4.71; p < 0.05; df = 10), whilst there was no significant difference between the latter two species (p > 0.05). When assessing the average total phosphorus (TP) tissue content, per 50 g of wet material, the following was found (Fig. 3): Cladophora glomerata = 1.81 g (3.62%), R. cirrhosa = 3.92 g (7.84%) and Z. capensis = 2.62 g (5.24%). When comparing the tissue TP concentrations, R. cirrhosa was significantly higher than both C. glomerata (T = 0; Z = 2.20; p < 0.05) and Z. capensis (T = 0; Z = 2.20; p < 0.05). Furthermore, the tissue TP content for Z. capensis was significantly greater than that observed for C. glomerata (T = 0; Z = 2.20; p < 0.05).
Decomposition: Inorganic nutrients
Zostera capensis
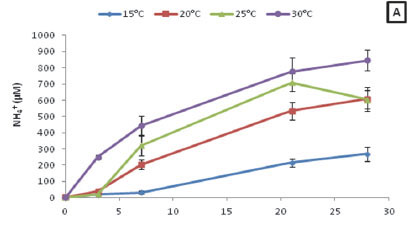
The overall NH4+ concentrations of ambient water in the sample bags for Z. capensis at 25°C and 30°C (H = 23.56; p < 0.05) were significantly higher than that observed for the 15°C treatment (Fig. 3A). Furthermore, a strong positive correlation existed between the NH4+ concentrations for all four temperature treatments and time (R15 = 0.92; R20 = 0.95; R25 = 0.90; R30 = 0.95; p < 0.05). When assessing the NH4+ released during decomposition for each treatment over the course of the experiment, it was found that the NH4+ concentrations ranged from 0.25 to 847.05 µM.
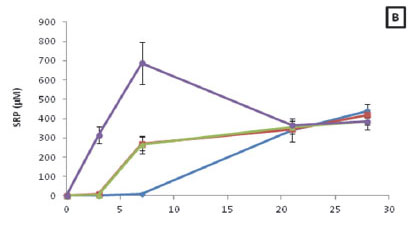
When comparing the SRP concentrations for the four different temperatures it was found that there was no significant difference (p > 0.05) between treatments (Fig. 3B). In addition to this, it was found that there was a strong positive correlation between SRP concentrations for all four treatments and time (R15 = 0.95; R20 = 0.97; R25 = 0.89; R30 = 0.53; p < 0.05). Moreover, when assessing the SRP released during decomposition for each treatment over the course of the experiment, it was found the SRP concentrations ranged from 0.12 to 687.11 µM.
Lastly, the overall TOxN concentrations at 30°C were significantly greater (H = 10.36; p < 0.05) than observed for the 20°C treatment (Fig. 3C). The TOxN concentrations observed for the 15°C treatment were the only ones that illustrated a positive correlation with time (R = 0.48; p < 0.05). When assessing the TOxN released during decomposition for each treatment over the course of the experiment, it was found the TOxN concentrations ranged from 0.1 to 31.67 µM.
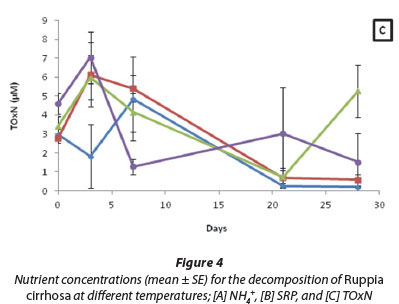
Ruppia cirrhosa
The overall NH4+ concentrations for R. cirrhosa exhibited no significant differences when comparing the four temperature treatments (p > 0.05) (Fig. 4A). However, a strong positive correlation existed between the NH4+ concentrations for all four temperature treatments and time (R15 = 0.88; R20 = 0.75; R25 = 0.85; R30 = 0.58; p < 0.05). When assessing the NH4+ released during decomposition for each treatment over the course of the experiment, it was found the NH4+ concentrations ranged from 8.86 to 766.85 µM.
When comparing the SRP concentrations for the four different temperatures it was found that there was no significant difference (p > 0.05) between treatments (Fig. 4B). Additionally, it was found that a strong positive correlation exists between SRP concentrations for all four treatments and time (R15 = 0.85; R20 = 0.92; R25 = 0.80; R30 = 0.72; p < 0.05). Moreover, when assessing the SRP released during decomposition for each treatment, it was found the SRP concentrations ranged from 0.68 to 520.26 µM.
The overall TOxN concentrations exhibited no significant differences when comparing the four temperature treatments (p > 0.05) (Fig. 4C). In addition to this, a negative correlation existed between TOxN concentrations and time for the 20°C (R = -0.59; p < 0.05) and 30°C (R = -0.54; p < 0.05) treatments. When assessing the TOxN released during decomposition for each treatment over the course of the experiment, it was found the TOxN concentrations ranged from 0.19 to 7.04 µM.
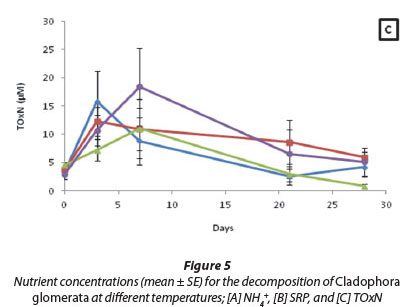
Cladophora glomerata
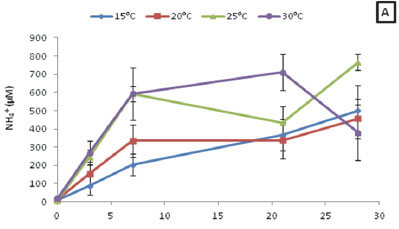
The overall NH4+ concentrations for C. glomerata at 25°C were significantly higher than observed for the 15°C treatment (H = 10.25; p < 0.05) (Fig. 5A). Furthermore, a strong positive correlation generally existed between the NH4+ concentrations for all four temperature treatments and time (R15 = 0.49; R20 = 0.88; R25 = 0.92; R30 = 0.79; p < 0.05). When assessing the NH4+ released during decomposition for each treatment over the 28 d period, it was found the NH4+ concentrations ranged from 17.07 to 1015.56 µM.
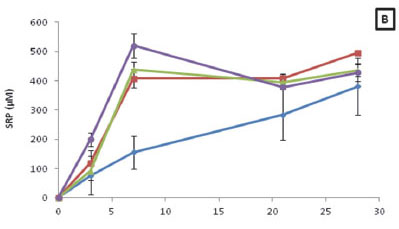
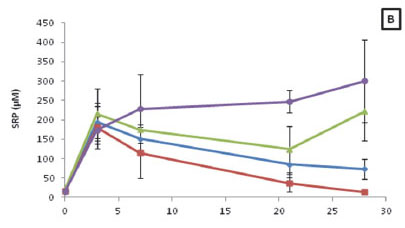
The overall SRP concentrations at 25°C and 30°C were significantly higher (H = 17.20; p < 0.05) than that observed for the 20°C treatment (Fig. 5B). Furthermore, a positive correlation was shown to exist between SRP concentrations and time for the 25°C (R = 0.48; p < 0.05) and 30°C (R = 0.64; p < 0.05) treatments. Moreover, when assessing the SRP released during decomposition for each treatment, it was found the SRP concentrations ranged from 15.31 to 300.24 µM.
The overall TOxN concentrations exhibited no significant differences when comparing the four temperature treatments (p > 0.05) (Fig. 5C). Moreover, a negative correlation existed between TOxN concentrations and time for the 25°C (R = -0.58; p < 0.05) treatment. When assessing the TOxN released during decomposition for each treatment over the course of the experiment, it was found the TOxN concentrations ranged from 0.83 to 18.41 µM.
Interspecies comparison
The NH4+ concentrations at 15°C for R. cirrhosa were significantly greater (H = 10.41; p < 0.05) than that for C. glomerata. Furthermore, no other significant differences (p > 0.05) were observed between species when comparing the NH4+ concentrations for the other temperature treatments.
When comparing the SRP concentrations of each species at different temperatures, it was found that both R. cirrhosa and Z. capensis released significantly higher SRP at 20°C than C. glomerata (H = 25.98; p < 0.05). Additionally, at 25°C the SRP concentrations for R. cirrhosa were significantly higher (H = 18.54; p < 0.05) than both Z. capensis and C. glomerata. Lastly, for the 30°C treatment, both R. cirrhosa and Z. capensis demonstrated significantly elevated SRP concentrations in comparison to C. glomerata (H = 12.48; p < 0.05).
When comparing TOxN concentrations between the species, it was found that C. glomerata exhibited significantly higher levels (H = 9.24; p < 0.05) of TOxN compared to R. cirrhosa at 15°C. For the 20°C treatment, C. glomerata released significantly higher levels of TOxN (H = 12.64; p < 0.05) than both of the other species. Finally, for both the 25°C and 30°C treatments, it was found that the TOxN concentrations for Z. capensis were significantly higher (H25 = 7.69; H30 = 10.57; p < 0.05) than that observed for R. cirrhosa.
Control treatments
The overall ammonium (NH4+), soluble reactive phosphorus (SRP) and total oxidised nitrogen (TOxN) concentrations for the control treatments were shown to be significantly lower (p < 0.001) at all temperatures throughout the study period compared to the Z. capensis, R. cirrhosa and C. glomerata treatments. Furthermore, the control concentration ranges for NH4+ (0 to 4.58 µM), SRP (0.13 to 1.13 µM) and TOxN (0 to 2.81 µM) were greatly reduced in comparison to that observed in the macrophyte treatments.
DISCUSSION
The experiment demonstrated that anaerobic decomposition of Zostera capensis, Ruppia cirrhosa, and Cladophora glom-erata resulted in high levels of inorganic nutrient release over the 28-day study period. Similar results were illustrated in a study by Bourguès et al. (1996) on the Arcachon Bay Lagoon (France), whereby the inorganic nutrient release during anaerobic decomposition of the seagrass Zostera noltii and the mac-roalgae Monostroma obscurum were shown to be comparable. The inorganic nutrient concentrations observed within the 'water column' (i.e. in sample bags) represent the net result of macrophyte nutrient release minus the uptake due to bacterial processes (Bourguès et al., 1996).
Ammonium (NH4+) was the dominant form of dissolved inorganic nitrogen (DIN) released during the decomposition process for all three study species. Although not studied here, it is known that the presence and activity (e.g. mineralisation) of various heterotrophic bacteria and fungi is required for the complete decomposition of plant matter (Anesio et al., 2003; Romani et al., 2006). Additionally, these microorganisms provide a key link between plant detritus and higher trophic levels, through facilitating the degradation of detrital particu-late and dissolved organic matter (Anesio et al., 2003; Romani et al., 2006). During anoxic conditions, however, it is known that nitrification by heterotrophic bacteria is inhibited, and this in turn prevents the transformation of NH4+ into TOxN (i.e. nitrates and nitrites) (Bourguès et al., 1996). This explains the comparatively low levels of TOxN (less than 35 µM) observed for all species and temperature treatments throughout the duration of the experiment. Further support for these findings comes from studies by Hanisak (1993) and Bourguès et al. (1996) which came to the same conclusions. Additionally, under anaerobic conditions that can lead to slow decay rates, the probability of nutrient immobilisation at various stages of macrophyte decomposition increases due to microbial production and adsorption processes (Twilley et al., 1986; Buchsbaum et al., 1991; Paalme et al., 2002). Under such conditions, mac-rophytes and their associated microbial communities act as 'nutrient sinks' due to the fact that inorganic nutrients are stored and/or utilised and not released to the surrounding environment. Therefore, the immobilisation of DIN under anaerobic conditions provides a possible explanation for the reduction in NH4+ released during the latter stages (ca. 21 d) of the experimental period for the higher temperature treatments (i.e. 25°C and 30°C).
Under aerobic conditions, decomposition processes influence the rate of nitrogen turnover in detritus, its availability to detritivores, and the types of nitrogen compounds present (Buchsbaum et al., 1991). After the initial leaching of labile compounds, nitrogen is simultaneously lost by mineralisation from detritus and incorporated by detritus from the surrounding environment (Buchsbaum et al., 1991). The rate of nitrogen mineralisation is affected by availability of nitrogen, due to the extent of nitrogen binding into refractory compounds, and lability of detrital carbon to microbial enzymes (Buchsbaum et al., 1991). Alternatively, the incorporation of nitrogen compounds into detritus is promoted by microbial uptake and the protein-binding capacity of various detrital cell wall constituents such as lignin and phenolic compounds (Buchsbaum et al., 1991). The decomposition of algae therefore provides a potentially important supply of organic and inorganic compounds to the water column where they can be recycled rapidly (Gabrielson et al., 1983; Twilley et al., 1986; Paalme et al., 2002). For example, inorganic nutrients released during decomposition can, through assimilation by phytoplankton and macrophytes, lead to an increase in primary productivity (Paalme et al., 2002). Alternatively, the onset of anoxic conditions and increased toxicity, for example by hydrogen sulphide production, during periods of extensive decomposition can lead to the mortality of benthic invertebrates and submerged macrophytes (Paalme et al., 2002).
The dissolved inorganic phosphorus (DIP) released during the decomposition process was shown to be fairly high for each species and temperature treatment. However, the magnitude of DIP released seemed to reach a maximum early on in the experimental period (approx. 7 days), and subsequently stabilise or decrease thereafter. This may be attributed to the fact that phosphorus is present in a labile form within the macrophyte tissue, and has been shown to have an initial high leaching efficiency with the onset of decomposition (Bourguès et al., 1996). This high leaching efficiency suggests that the inorganic form of phosphorus is dominant relative to the organic portion of tissue TP present in the macrophytes (Twilley et al., 1986). Furthermore, it is expected that TP tissue content of macro-phytes would be lower than TN content (N:P Redfield ratio, approx.. 16:1), due to the fact that most species are nitrogen-limited and therefore assimilation of DIP is restricted under periods of nitrogen depletion (Pedersen and Borum, 1996). The presence of fine-grain sediments in the water column can act as sites of adsorption, and therefore influence the amount of DIP detected in the water column during decomposition. The sediments thus act as a 'buffer' (Twilley et al., 1986). The above explains the prominence of DIN over DIP during the experimental period.
When comparing the decomposition characteristics of each macrophyte species, it is important to realise that the rate and extent of decomposition is largely dependent on the biochemical composition of each specific macrophyte (Buchsbaum et al., 1991; Hanisak, 1993; Bourguès et al., 1996). In this study it was found that the TN tissue content for C. glomerata (7.44%) was almost double that observed for both Z. capensis (3.56%) and R. cirrhosa (4.74%). Similar observations have been made in other studies whereby it has been shown that macroalgal tissues (C. glomerata) generally possess higher tissue nitrogen concentrations compared to vascular plants (Z. capensis and R. cirrhosa) (Duarte, 1992; Bourguès et al., 1996). These differences may be related to phylogenetic differences in storage and structural carbohydrates and pigment systems, as well as morphological differences regarding thallus complexity and age (Twilley et al., 1986; Hanisak, 1993).
The difference in nutrient dependence between plants can be attributed to 2 factors: (i) interspecies differences in nutrient requirements, and (ii) differences in efficiency of nutrient acquisition (Pedersen and Borum, 1996). Due to their low growth rates and low tissue nutrient content, large, long-living vascular plants such as Z. capensis and R. cirrhosa exhibit low nutrient requirements per unit biomass and time (Pedersen and Borum, 1996). This in turn allows slow-growing species to lower the critical tissue nutrient content required to sustain maximum growth compared to ephemeral macroalgae (Pedersen and Borum, 1996).
Under periods of high nutrient availability and low light, it is possible for slow-growing species to build up larger nutrient reserves than fast-growing species, due to their lower critical nutrient concentrations (Pedersen and Borum, 1996). This enables slow-growing species to sustain maximum growth for longer periods, and obtain a competitive advantage in areas where nutrient availability fluctuates (Pedersen and Borum, 1996). Therefore, the potential for vascular plants to accumulate greater nutrient reserves under certain environmental conditions (e.g. lower nutrient availability) in comparison to ephemeral macroalgae provides an explanation for the elevated levels of TP tissue content observed for Z. capensis (5.24%) and R. cirrhosa (7.84%) relative to C. glomerata (3.62%). Moreover, this also supports the generally increased levels of DIP released during the decomposition of the submerged macrophytes (i.e. at 20°C, 25°C and 30°C) in comparison to C. glomerata.
Temperature is regarded as one of the key factors responsible for controlling decomposition processes (Paalme et al., 2002). It is expected that lower temperatures (e.g. 15°C) strongly inhibit the decomposition processes of all macrophyte species (Paalme et al., 2002). However, as the temperature increases towards 30°C there tends to be rapid degradation of algal populations with regards to biomass loss (Hanisak, 1993; Paalme et al., 2002). The decomposition process is initiated when enzymatic breakdown of cellular constituents, such as cell walls, occurs, which ultimately increases its susceptibility to bacterial activity (Gabrielson et al., 1983). Therefore, as was the case in this study, it is important to use living algal material, ones with intact cell walls, when performing decomposition experiments as this ensures greater accuracy when determining the 'actual' extent of inorganic nutrient release (Gabrielson et al., 1983; Paalme et al., 2002). Moreover, the activity of associated faunal and microbial (i.e. bacteria and fungi) communities that are responsible for promoting algal degradation are hindered at lower temperatures (Paalme et al., 2002).
The release of inorganic nutrients observed for each species in this study followed the expected pattern, whereby decomposition was greatest at higher temperatures (i.e. 25°C and 30°C) and lowest under cold conditions (i.e. 15°C). The apparent slower decomposition rate of C. glomerata, initiated after ca. 7 d, during the study period is due to living Cladophora being very resistant to decay (Gabrielson et al., 1983; Paalme et al., 2002). The reason for this resistance and possible long-term viability of Cladophora species during unfavourable conditions has been attributed to the formation of akinetes and/or extensive heterotrophic capabilities (Gabrielson et al., 1983). Akinetes are thick-walled dormant cells that possess dense cytoplasmic contents; characteristics that enable them to act as 'survival structures' (i.e. prevent death under unfavourable conditions) in filamentous green algae (Gabrielson et al., 1983). The heterotrophic capabilities of C. glomerata refer to its ability to meet energy requirements through the assimilation of dissolved organic compounds in the absence of light (Quesada et al., 2002). Furthermore, the thallus of C. glomerata is generally only a few cell layers thick with all of them being photosyn-thetic and in direct contact with the surrounding medium, which enables prolonged periods of photosynthetic activity (Hanisak, 1993; Paalme et al., 2002). In contrast, the initially quicker decomposition (ca. 3 d) of the submerged macrophytes (Z. capensis and R. cirrhosa) may be a result of the increased respiratory demand of their internal, non-photosynthetic structural cells (Hanisak, 1993).
When addressing the aspects of this study that could be improved, it is important to realise that there are numerous methodologies with which to assess macrophyte decomposition (Lomstein et al., 2006): (i) biomass and nutrient loss during decomposition, (ii) decay rates and inorganic nutrient cycling, (iii) nutrient fluxes between the macrophyte and the surrounding water column, and (iv) benthic fluxes of oxygen, nutrients and sulphides. In addition to this, the selection of an in-situ or laboratory-based experimental design poses another challenge (Twilley et al., 1986), as it can be difficult to determine the mechanisms of plant decomposition on the basis of a single study type (Twilley et al., 1986). However, it is important to continue with these types of studies to further our knowledge of estuarine structure and functioning under different environmental scenarios.
CONCLUSION
In temporarily open/closed estuaries, such as the Great Brak Estuary, with low freshwater inflow due to low annual rainfall and dam construction, and substantial nutrient inputs from sewage discharges, the probability of macroalgal blooms is high. The most prominent bloom-forming macroalgal species found in the Great Brak Estuary, during periods of eutrophication, is Cladophora glomerata. By influencing benthic nutrient processes through interception of light and nutrients in the water column, the filamentous macroalgae C. glomerata is able to out-compete long-lived species such as Zostera capensis and Ruppia cirrhosa (Leston et al., 2008). These submerged macrophyte communities are responsible for providing unique ecosystem services, such as nursery areas for various marine species, enhancement of biodiversity and the stabilisation of sediments (Carstensen et al., 2012). However, under bloom conditions estuarine health deteriorates due to a loss of these important services.
Consequently, it is important to implement mitigation measures to minimise and reverse the effects of eutrophication, and return the system to a previous state of 'stability' (Leston et al., 2008). Possible mitigation measures include: reduction of external nutrient inputs, deliberate alteration of an ecosystem by adding or removing species, plant harvesting to remove nutrients, artificial mouth breaching, and an increase in freshwater inflow to reduce water residence time (Marion and Paillisson, 2003; Leston et al., 2008). However, in order for such measures to be successful it is important to understand the mechanisms that have led to the ecological changes (Leston et al., 2008). It is for this reason that studies such as these are important as they provide information that can be incorporated into nutrient budgets and water quality models, for example, which ultimately enable more effective management of estuaries.
REFERENCES
ANESIO AM, ABREU PC and BIDDANDA BA (2003) The role of free and attached microorganisms in the decomposition of estuarine macrophyte detritus. Estuar. Coast. Shelf Sci. 56 197-201. [ Links ]
BATE GC and HEELAS BV (1975) Studies on the nitrate nutrition of two indigenous Rhodesian grasses. J. Appl. Ecol. 12941-952. [ Links ]
BEREZINA NA and GOLUBKOV SM (2008) Effect of drifting macro-algae Cladophora glomerata on benthic community dynamics in the easternmost Baltic Sea. J. Mar. Syst. 74 80-85. [ Links ]
BOURGUÈS S, AUBY I, DE WIT R and LABOURG P-J (1996) Differential anaerobic decomposition of seagrass (Zostera noltii) and macroalgal (Monostroma obscurum) biomass from Arcachon Bay (France). Hydrobiologia 329 121-131. [ Links ]
BUCHSBAUM R, VALIELA I, SWAIN T, DZIERZESKI M and ALLEN S (1991) Available and refractory nitrogen in detritus of coastal vascular plants and macroalgae. Mar. Ecol. Prog. Ser. 72 131-143. [ Links ]
CARSTENSEN J, DAHL K, HENRIKSEN P, HJORTH M, JOSEFSON A and KRAUSE-JENSEN D (2012) Chapter 7.08. Coastal monitoring programs. In: McLusky D and Wolanski E (eds.) Treatise on Estuarine and Coastal Science, Volume 7: Functioning of Ecosystems at the Land-Ocean Interface. Elsevier, Oxford. [ Links ]
DWAF (DEPARTMENT OF WATER AFFAIRS AND FORESTRY, SOUTH AFRICA) (2009) Reserve determination studies for selected surface water, groundwater, estuaries and wetlands in the Outeniqua (Groot Brak and other water resources, excluding wetlands) catchment: Ecological Water Requirements Study - Estuarine RDM Report: Groot Brak Assessment. Report RDM/ K10 - K30, K40E/00/CON/0307. Department of Water Affairs and Forestry, Pretoria. [ Links ]
DUARTE CM (1992) Nutrient concentration of aquatic plants: patterns across species. Limnol. Oceanogr. 37 882-889. [ Links ]
GABRIELSON JO, BIRCH PB and HAMEL KS (1983) Decomposition of Cladophora II. In vitro studies of nitrogen and phosphorus regeneration. Bot. Mar. 26 173-179. [ Links ]
GUBELIT YI and BEREZINA NA (2010) The causes and consequences of algal blooms: The Cladophora glomerata bloom and the Neva estuary (eastern Baltic Sea). Mar. Pollut. Bull. 61 183-188. [ Links ]
HANISAK MD (1993) Nitrogen release from decomposing seaweeds: species and temperature effects. J. Appl. Phycol. 5 175-181. [ Links ]
KOROLEFF F (1983) Simultaneous oxidation of nitrogen and phosphorus compounds by persulfate. In Grasshoff K, Eberhardt M and Kremling K (eds.) Methods of Seawater Analysis (2nd edn.). Verlag Chemie, Weinheimer, FRG. 168-169. [ Links ]
LESTON S, LILLEB0 AI and PARDAL MA (2008) The response of primary producer assemblages to mitigation measures to reduce eutrophication in a temperate estuary. Estuar. Coast. Shelf Sci. 77 688-696. [ Links ]
LOMSTEIN BA, GULDBERG LB, NEUBAUER AA, HANSEN J, DONNELLY A, HERBERT RA, VIAROLI P, GIORDANI G, AZZONI R, DE WIT R and FINSTER K (2006) Benthic decomposition of Ulva lactuca: A controlled laboratory experiment. Aquat. Bot. 85 271-281. [ Links ]
MARION L and PAILLISSON J-M (2003) A mass balance assessment of the contribution of floating leaved-macrophytes in nutrient stocks in an eutrophic macrophyte-dominated lake. Aquat. Bot. 74 249-260. [ Links ]
PAALME T, KUKK H, KOTTA J and ORAV H (2002) 'In vitro and 'in situ decomposition of nuisance macroalgae Cladophora glomerata and Pilayella littoralis. Hydrobiologia 475/476 469-476. [ Links ]
PARSONS TR, MAITA Y and LALLI CM (1984) A Manual of Chemical and Biological Methods for Seawater Analysis. Pergamon Press, New York. 173 pp. [ Links ]
PEDERSEN MF and BORUM J (1996) Nutrient control of algal growth in estuarine waters. Nutrient limitation and the importance of nitrogen requirements and nitrogen storage among phytoplankton and species of macroalgae. Mar. Ecol. Prog. Ser. 142 261-272. [ Links ]
QUESADA A, JÜTTNER F, ZOTINA T, TOLOMEYEV AP and DEGERMENDZHY AG (2002) Heterotrophic capability of a metalimnetic plankton population in saline Lake Shira (Siberia, Khakasia). Aquat. Ecol. 36 219-227. [ Links ]
ROMANI AM, FISCHER H, MILLE-LINDBLOM C and TRANVIK LJ (2006) Interactions of bacteria and fungi on decomposing litter: Differential extracellular enzyme activities. Ecology 87 2559-2569. [ Links ]
SNOW GC and ADAMS JB (2007) Relating microalgal spatial patterns to flow, mouth and nutrient status in the temporarily open/closed Mngazi estuary, South Africa. Mar. Freshwater Res. 58 1032-1043. [ Links ]
TWILLEY RR, EJDUNG G, ROMARE P and KEMP WM (1986) A comparative study of decomposition, oxygen consumption and nutrient release for selected aquatic plants occurring in an estuarine environment. Oikos 47 190-198. [ Links ]
VAN NIEKERK L and TURPIE JK (eds) (2012) South African National Biodiversity Assessment 2011: Technical Report. Volume 3: Estuary Component. CSIR Report Number CSIR/NRE/ECOS/ ER/2011/0045/B. Council for Scientific and Industrial Research, Stellenbosch. [ Links ]
WHITFIELD AK (1992) A characterization of South African estuaries. S. Af. J. Aquat. Sci. 18 89-103. [ Links ]
 Correspondence:
Correspondence:
GC Snow
+27 84 603 5548
e-mail: gavin.snow@nmmu.ac.za
Received 29 April 2013
Accepted in revised form 9 January 2013