Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Water SA
On-line version ISSN 1816-7950
Print version ISSN 0378-4738
Water SA vol.40 n.1 Pretoria Jan. 2014
ARTICLES
Anaerobic ammonium oxidation in the old trickling filters at Daspoort Wastewater Treatment Works
J WilsenachI; L BurkeII; V RadebeII; M MashegoII; W StoneIII; M MoutonIII; A BothaIII
IVirtual Consulting Engineers, PO Box323, Groenkloof, Pretoria, 0027, South Africa
IICouncil for Scientific and Industrial Research, Meiring Naudé Road, Brummeria, South Africa
IIIDepartment of Microbiology, University of Stellenbosch, Private Bag X1, Matieland 7602, South Africa
ABSTRACT
The century-old trickling filters at the Daspoort Wastewater Treatment Works in Pretoria (Gauteng, South Africa) are known for their remarkable removal of nitrogen from municipal wastewater. Our study was conducted to identify the microbiological processes responsible for this phenomenon and to establish whether anammox bacteria may be involved. An aerobic and anaerobic bench top reactor, both inoculated with biofilm-covered stones from one of the filters, were spiked with ammonia-nitrogen (NH4+-N) and nitrite-nitrogen (NO2--N). These reactors were subsequently monitored by conducting stoichiometric analyses of chemical oxygen demand (COD), NH4+-N, NO2--N, and nitrate-nitrogen (NO3--N). In the aerobic reactor, the COD concentration decreased over the 56 h batch reaction period and nitrification was revealed by a decrease in NH4+-N and NO2--N concentrations. However, the NO3--N concentration showed no notable decrease. In contrast, in the anaerobic reactor the concentrations of COD, NH.+-N, NO2 -N, NO3 -N, as well as total nitrogen decreased during the batch reaction period. The decrease of both the NH4+-N and NO2--N concentrations, the latter to zero under anaerobic conditions, suggested that, in addition to heterotrophic denitrification, anaerobic ammonium oxidation (anammox) may also occur in the trickling filter biofilm. This was highlighted by the observation that ammonium removal in the anaerobic reactor stopped as soon as the nitrite concentration became zero. The ratio of nitrite:ammonium removal was 1.33 on average, which conforms to anammox behaviour. Gene sequence analysis was used to test for the possible presence of anammox bacteria in the trickling filter biofilm. Genomic DNA was extracted from trickling filter humus sludge and the polymerase chain reaction (PCR) was used to amplify taxonomically informative 16S rRNA gene sequences, using primers specific for selected anammox species. Subsequent sequence analysis, including using the online Basic Local Alignment Search Tool (BLAST), and constructing a phylogenic tree using a heuristic search strategy for Maximum Parsimony analysis, confirmed the presence of an anammox bacterium closely related to Candidatus 'Brocadia anammoxidans' and Candidatus 'Brocadia fulgida' on the biofilm-covered stones of the Daspoort trickling filters.
Keywords: Daspoort, trickling filter, anammox, nitrification, denitrification, stoichiometric analyses, polymerase chain reaction
INTRODUCTION
The relationships between hydraulic loading rate, organic loading rate, and efficiency of removal of biochemical oxygen demand (BOD) in trickling filters was first quantified by Schulze (1957) more than 50 years ago. From Schulze's references, it is evident that the formal investigation of these systems had been quite productive since the 1940's. During this period, much of the basic understanding of trickling filters was established; specifically that treatment capacity depends on the mass of active biofilm and the contact time between the liquid and this biofilm. The effect of biofilm thickness on various biochemical processes also seems to be important in understanding trickling filters (Nielsen et al., 1990).
The inner layer of the biofilm in trickling filters is almost always anaerobic, although this zone could also become anoxic, depending on nitrate concentration in bulk liquid, and diffusion into the biofilm. Nielsen et al. (1990), Kuhl and Jorgensen (1992) and Persson et al. (2002) used micro-sensors with high spatial resolution to quantify the concentration of substances at various depths in biofilms. The same overall picture emerged from their work: oxygen was found only in the upper 0.5 mm of a biofilm, below which anoxic (nitrate reduction) and anaerobic (sulphate reduction) processes occurred, depending on the concentration of diffused nitrate or sulphate. Dalsgaard and Revsbech (1992) studied the effect of micro-zonation on the performance of different processes, especially denitrification, in biofilms. Denitrification was measured as a function of oxygen and nitrate concentrations, organic matter and ammonium. It was found that increased bulk liquid concentrations of nitrate increased the zone within the biofilm in which denitrification took place. In contrast, higher bulk liquid oxygen concentrations increased the aerobic zone thickness within the biofilm, and decreased the overall denitrification rate. This aerobic zone normally extended 0.2-0.3 mm into the biofilm, below which a mostly anoxic/anaerobic zone could be detected. If the oxygen penetration depth was increased artificially, denitrification stopped, but resumed immediately when anoxic conditions reestablished. This suggested that the same organisms responsible for aerobic removal of COD were responsible for COD removal with nitrate reduction (denitrification).
Intact biofilm samples from a carbonaceous trickling filter in a bench-scale reactor were investigated by Biesterfeld et al. (2003). The reactors were fed with sterilised wastewater effluent and spiked with nitrate to a final concentration of 16-18 mg.ℓ-1 N03-N. Dissolved oxygen concentrations in the bulk liquid were kept at 2-4 mg.ℓ_1. Denitrification took place immediately, at rates of 3 to 5 g.m-2day-1, indicating that denitrification is possible inside the biofilm of systems with dissolved oxygen in the bulk liquid. Similarly, Szewczyk and Kuroή-Kulig (2007) found that oxygen diffusion limitation inside the flocs of activated sludge 'created significant anoxic micro-zones' to allow for denitrification of nitrite and nitrate produced in aerobic zones of the flocs, even with bulk liquid oxygen concentrations up to 5 mg.ℓ-1.
From the above it is evident that both nitrification and denitrification may occur simultaneously in the biofilms of trickling filters, which have been used for decades to remove nitrogen from wastewater. Such an example is the old trickling filters at the Daspoort Wastewater Treatment Works (WTWW), built in 1910, which have always been operated as single-pass reactors (i.e. no recirculation, or filters in series). Recently, the remarkable nitrogen removal of these trickling filters became of interest (Radebe et al., 2012). It was found that up to 25 mg.ℓ-1 N was removed across the filters: with total Kjeldahl nitrogen (TKN) concentration of 45 mg.ℓ-1 N in the influent, an effluent was produced with NO3- concentration of around 15 mg-£-1 N (with lows of 8-10 mg.ℓ-1 N), an NH4+ concentration of less than 1 mg.ℓ-1 N, and TKN associated with solids in effluent of between 2-3 mg.ℓ-1 N. A total nitrogen effluent concentration of around 20 mg.ℓ-1 is common for these trickling filters. Nitrogen removal efficiency is therefore between 50 and 60%.
The stoichiometric ratio of COD to nitrogen, typically calculated using the metabolic reactions of Proteobacteria and Firmicutes and based on the thermodynamics of growth (Eqs.(1) and (2); Heijnen et al., 1999), dictates that 25 mg.ℓ-1 N removal, via denitrification over nitrite or nitrate, requires at least 90 or 150 mg.ℓ-1 COD, respectively, under anoxic conditions.
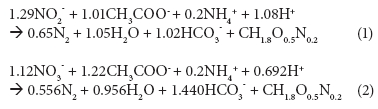
However, the Daspoort filters are mostly aerobic, because nitrification is nearly complete. If the environment is mostly aerobic, one would expect that most of the COD would be removed using oxygen, and not nitrate or nitrite, as electron acceptor. Settled wastewater COD concentrations (influent to the trickling filters) were mostly between 250 and 300 mg.ℓ_1.
If 150 mg.ℓ_1 COD was removed via nitrate reduction, and assuming 50 mg.ℓ_1 COD in the final effluent, then only 50 to 100 mg.ℓ_1 COD was removed using oxygen. This seems unlikely in a single-pass aerobic system. It was therefore suspected that another mechanism, unlike the heterotrophic denitrification of Proteobacteria and Firmicutes, was responsible for some of the nitrogen removal. Such a process, called anaerobic ammonium oxidation (anammox), is known in bacteria belonging to the order Planctomycetales (Strous et al., 1999) and was previously found in the inner layers of biofilms where an anaerobic niche environment may exist in an otherwise predominantly aerobic environment (Egli et al., 2001).
Anammox bacteria facilitate complete ammonia removal and denitrification of nitrite occurs with ammonia as the electron donor (Van Loosdrecht et al., 1997), via autotrophic pathways without the requirement for organic carbon (Van Rijn et al., 2006). Despite a considerable body of work on using anammox as an additional process unit to improve the nitrogen removal efficiency of conventional activated sludge wastewater treatment processes, relatively few documented examples exist of anammox within trickling filters. Lydmark et al. (2006) studied the distribution of nitrifying organisms at different depths in a full-scale nitrifying trickling filter. They found that ammonium-oxidising bacteria (Nitrosomonas), as well as nitrite-oxidising bacteria (Nitrospira) occurred at all depths in the filter, with Nitrospira species generally found closer to the biofilm base and Nitrosomonas on the biofilm surface. Small populations of anammox bacteria were only found at a depth of 6 m in the trickling filter.
The aim of this study was to identify the microbiological processes responsible for nitrogen removal in the Daspoort trickling filters and to establish whether anammox bacteria, specifically, play an important role.
EXPERIMENTAL
Sampling from the trickling filters
The old trickling filter system at Daspoort WTWW consists of 4 units with 4 trickling filters each, 1.8 m in height (Fig. 1). The surface area of each trickling filter is 452 m2, consisting of gravel and stone filter media, with a bulk volume of 814 m3. The top layer of the trickling filters (above 1.2 m high) is filled with stone media, 38 to 63 mm in diameter, to create an attached growth treatment process. To promote ventilation through the trickling filter the bottom part is filled with stone, 101 to 127 mm in diameter.
To confirm the presence of anammox bacteria using specific molecular probes, a representative sample of the microbial consortia in the biofilm on filter stones was obtained by collecting sludge from the sludge withdrawal valve of the humus tanks (Fig. 1). However, prior to sampling, filter stones containing biofilm were removed from the trickling filter, obtained at a depth of 0.9 m into the filters, to act as inoculum for in-vitro experiments aimed to indicate the presence of anammox bacteria via stoichiometric analyses.
Stoichiometric analyses of nitrogen removal with in-vitro experiments
Reactor configuration and experimental design
Two glass vessels, each with a volume of 7ℓ (diameter = 18 cm; height = 30 cm), were used as aerobic and anaerobic reactors. Equal masses of trickling filter stones (7.8 kg) with existing biomass, were packed into the two reactors. Packed reactors were filled with Daspoort trickling filter effluent to an active volume of 3.5ℓ. Reactor liquid was circulated from bottom to top at a rate of 450 mℓ-min-1. The anaerobic reactor was connected to a recycle pump (Watson-Marlow) with marprene tubing to ensure a low permeability to oxygen, while the aerobic reactor was fitted with a pH-correction mechanism, dosing NaHCO3 when the pH dropped below 7.5.
The aerobic reactor was sparged with compressed air (resulting in an average DO concentration of 4.5 mg.ℓ-1), and the anaerobic reactor with 99% nitrogen gas (resulting in an average DO concentration of 0.17 mg.ℓ-1). The gas pipes that supplied compressed air/nitrogen gas extended through the bottom of the reactor, and were attached to diffusers. A mesh sieve was placed above the diffusers for optimal gas distribution.
The reactors were incubated at 24°C to 28°C for a period of 51 days. This period included an initial adaptation period of 35 days, during which the reactor liquid of each reactor was periodically spiked with approx. 30 mg.ℓ-1 NH4+-N and approx. 25 mg.ℓ-1 NO2--N. Immediately after the addition of these compounds on Day 35, the reactors were operated as sequential batch reactors and again spiked with the substrates of the nitrification process, i.e. ammonia-nitrogen (NH4+-N) and nitrite-nitrogen (NO2--N). Both reactors were monitored by analysing pH, COD, NH4+-N, nitrate-nitrogen (NO3--N), nitrite-nitrogen (NO2--N) and total nitrogen content for a period of 56 h. This first batch experiment, including the spiking event, was performed in duplicate. To prevent loss of biomass and oxygen contamination, the anaerobic reactor contents were not drained between these batch experiments.
To determine the relative utilisation of nitrogenous compounds by the microbial consortia in each of the two reactors, data were analysed using scatterplots with regression lines. In addition, the data were analysed by constructing correlation matrixes comparing the levels of different nitrogenous compounds within each reactor. The software program STATISTICA version 10 (http://www.statsoft.com; Statsoft, Inc., Tulsa, Oklahoma, USA) was used for this purpose.
Sample analyses
All samples, obtained from the recycle pipes, were first filtered (Whatman No. 1) before analysis. COD analysis was carried out using low-range COD vials (15-160 mg.ℓ-1) (Macherey-Nagel) and measured with a 500D Nanocolour Universal Photometer. Total nitrogen analysis was also performed using Nanocolour vials (Macherey-Nagel) and measured using the 500D Nanocolour Universal Photometer. Total nitrogen includes the species total Kjeldahl nitrogen, nitrate and nitrite. Ammonia, nitrite and nitrate analyses were performed using a Hach spectrophotometer DR/2010.
Testing for anammox bacteria using molecular probes
DNA extraction and amplification of relevant gene sequences
The GeneJET Genomic DNA Purification Kit (Fermentas Life Sciences, Inqaba Biotec, South Africa) was used for extraction of genomic DNA from the humus sludge sampled from the trickling filters.
Amplification of relevant gene sequences
The polymerase chain reaction (PCR) was used to amplify taxonomically informative 16S rRNA gene sequences for the identification and classification of bacterial species. Twelve primer combinations (Table 1; Inqaba Biotec, South Africa) obtained from literature were selected to test for the presence of different anammox species. The 50 µℓ PCR reaction contained 200 µM of each deoxynucleotide triphosphate, 0.25 µM of each primer, 2 mM MgCl2, 2.5 U Taq DNA poly-merase (Fermentas Life Sciences, EU) and approximately 100 ng of template DNA (adapted from Amano et al., 2007). The PCR amplification was initiated with a 4 min denatura-tion step at 94°C and concluded with a final 7 min elongation at 72°C. The reaction comprised 30 cycles, each with a 30 s denaturation at 94°C, a 30 s annealing at 56°C and a 60 s elongation at 70°C. The reactions were performed in a 2527 Thermal Cycler (Applied Biosystems, Singapore). PCR products were visualised under UV light in a 0.8% agarose gel. Bovine serum albumin was added (2.5 µℓ at 2 mg.mℓ-1) in order to overcome inhibition by wastewater contaminants. Negative controls were included in all instances.
Gene sequencing and phylogenetic analyses
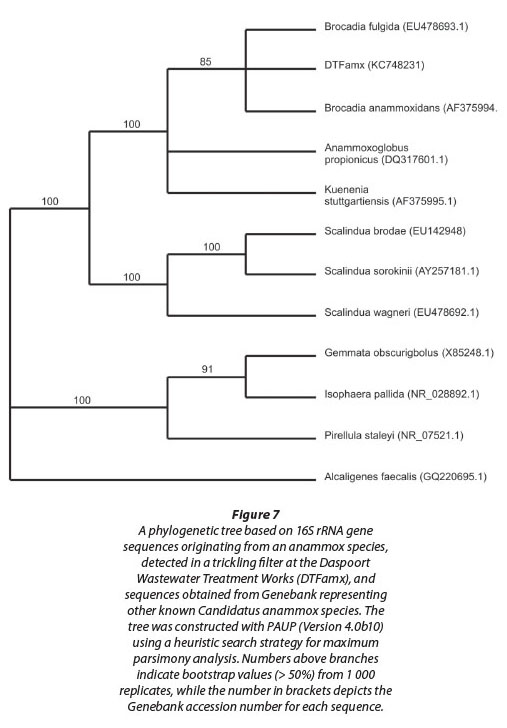
An ABI PRISM (model 3100) genetic sequencer was used for sequencing and the online Basic Local Alignment Search Tool (BLAST) was used to detect homology with known anammox species on the NCBI (National Centre for Biotechnology Information) database website (http://www.ncbi.nlm.nih.gov/BLAST/). Sequences with homology above 98% were regarded as meaningful for identification and were imported into PAUP (Version 4.0b10), and analysed using a heuristic search strategy for maximum parsimony analysis (Swofford, 2002). The quality of the branching patterns was assessed by bootstrap resampling of the data sets with 1 000 replications.
RESULTS AND DISCUSSION
Stoichiometric monitoring during batch experiments Aerobic conditions
Results obtained during the three aerobic batch experiments are depicted in Figs. 2 to 4 and Table 2 which gives the rates (slopes of the lines). The COD concentration in the aerobic reactor (Fig. 2; aCOD(ï)] notably decreased during all three experiments. The final COD appears to be the residual inert soluble COD, which is typically between 30 and 40 mg∙ℓ-1 for domestic wastewater. It therefore seemed that the COD removed was very slowly degradable. No COD was added to the system, but concentrations increased between successive batch reactions. This slowly biodegradable COD must have originated from the decaying biomass.
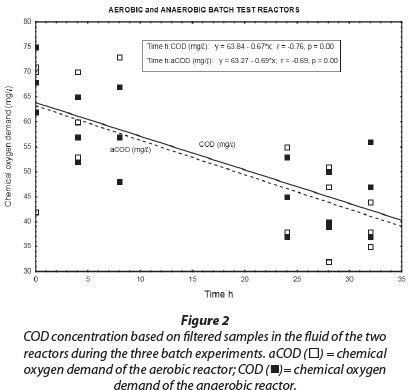
Bacteria that oxidise ammonia to nitrate are autotrophic and do not require an organic carbon source (Wezernak and Gannon, 1967), The decrease in COD concentration in the aerobic reactor was therefore most likely due to utilisation by aerobic heterotrophs present in the biofilm consortia. Heterotrophic nitrifiers occur in some ecological systems, but their nitrification rates are much lower than that of autotrophic nitrifiers (Robertson and Kuenen, 1990).
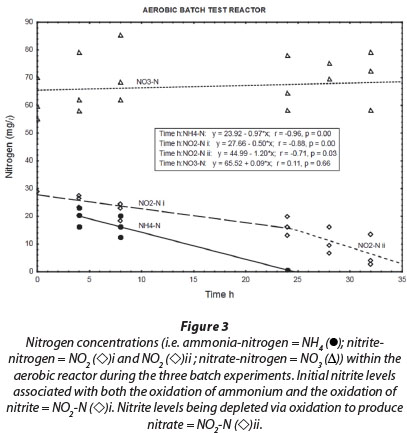
Most of the NH4+-N in the aerobic reactor was removed within 24 h (Fig. 3; NH4+-N (•)). A positive correlation (r = 0.82, p = 0.00) was found between NH4+-N and NO2--N in the reactor liquid. Thus, it seems that the aerobic conditions in the reactor promoted biofilm-associated nitrification, which involved oxidation of NH4+-N to NO2--N by ammonia-oxidising bacteria, followed by oxidation of NO2--N to NO3--N by nitrite-oxidising bacteria (Vayenas et al., 1997). It must be noted, however, that both nitrite and ammonium were initially added to the system. Therefore; changes in the nitrite concentrations (Fig. 3; NO2--N (◊)i) are always the result of two biochemical processes, i.e., ammonium oxidation that produces nitrite and nitrite oxidation that consumes nitrite, at different rates. As soon as ammonium concentrations reached zero, the observable nitrite removal rate increased markedly (Fig. 3; NO2-N(◊)ii) The true nitrite oxidation rate is therefore seen between 24 h and 32 h, which was 1.2 mg∙ℓ-1-h-1 N. Although Fig. 3 shows an overall increase of nitrate concentration during the aerobic batch experiment (NO3-N (Δ)), this could not be balanced with the presumed oxidation of both ammonium and nitrite. At this point, nitrate was already at a higher concentration, being a residue of the incubation period, which can be misleading during comparison. However, nitrate concentrations did increase notably from one batch reaction to the next, indicating the effect of nitrification.
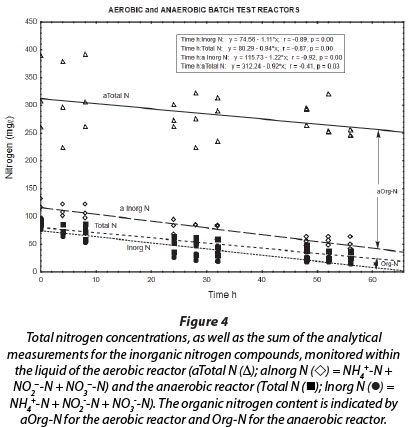
The total nitrogen concentrations (NH4++ NO2- + NO3- + OrgN) of the reactor liquid changed little during the 56-h duration of batch reactions (Fig. 4; aTotal N (Δ)). This means that no net nitrogen removal occurred, and nitrogen was only converted from the reduced species to the oxidised species, i.e., ammonium and nitrite were ultimately oxidised to nitrate. The analytical measurement of total nitrogen differed from the sum of analytical measurements for ammonium, nitrite and nitrate, because of the difference between Total Kjeldahl Nitrogen (TKN) and NH4+-N. Consequently, it seems that at least 220 mg∙ℓ-1 nitrogen remained in the reactor liquid as reduced organic nitrogen. This organic nitrogen may have been associated with planktonic bacterial cell biomass that formed during aerobic incubation of the reactor; however, these findings should be further investigated in future.
Anaerobic conditions
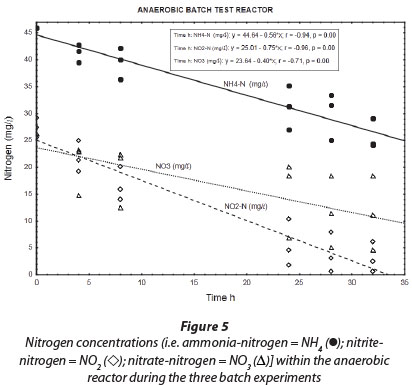
Results obtained during the three anaerobic batch experiments are depicted in Figs 2, 4 and 5, while Fig. 6 illustrates the results obtained during the monitoring of the anaerobic reactor during the first batch reaction. The pH of the reactor mostly remained between pH 8 and 8.5 (Fig. 6; pH (◊)) and no adjustment was necessary to accommodate denitrifying bacteria. The optimum pH for denitrification is between 7 and 8, with different optimums for different bacterial populations (Yoo et al., 1999).
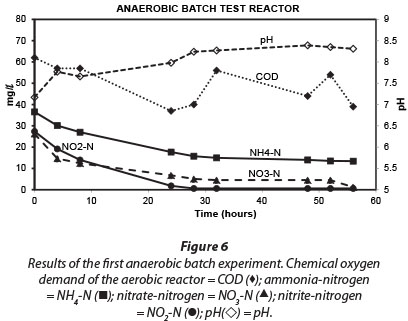
Similar to the results obtained for the aerobic reactor, the COD concentration in the reactor content of the anaerobic reactor notability decreased during all three experiments (Fig. 2; COD (■)). At the onset of each consecutive experiment the COD was approx. 20 mg∙ℓ-1 higher than the final COD concentration in the reactor which amounted to approx. 40 mg∙ℓ-1. This decrease in COD indicated that heterotrophic denitrification may have contributed to nitrogen removal from the reactor. As speculated in the case of the aerobic reactor, the COD would be very slowly degradable, and would be present only through the slow decay of trickling filter humus sludge. Normally nitrite and nitrate in an anaerobic reactor would have been reduced via heterotrophic denitrification. Thus, the theoretical amount of COD that was required for the catabolic reaction only (assuming decaying bacteria in maintenance mode, without growth) was calculated for heterotrophic denitrification via nitrite and nitrate, based on 2.86 g COD∙g-1 NO3--N, and 1.71 g COD∙g-1 NO2--N, respectively. The theoretical COD requirement of 2.42 mg∙ℓ-1∙h-1 was calculated based on 0.40 mg∙ℓ-1∙h-1 NO3--N and 0.75 mg∙ℓ-1∙h-1 NO2--N, and was subsequently compared to the measured COD removal of only 0.67 mg∙ℓ-1∙h-1 during the first 32 h of batch reactions. Interestingly, Ekama and Wentzel (1999) found that in denitrifying activated sludge systems, nitrite concentrations remained steady while nitrate was being reduced, and that nitrite was only reduced once the nitrate had all been removed. One should then compare the COD removal (measured) with the COD removal required for nitrate reduction only, an amount of 1.1 mg∙ℓ-1∙h-1 COD. This suggests that nitrate removal only could be explained by normal denitrification and COD removal.
Regardless of nitrate removal, removal of ammonium was clearly observed in the anaerobic reactor (Fig. 5; NH4 (•)). Important to note is that the final ammonium concentration after each consecutive experiment (approx. 10 mg∙ℓ-1 N) was replenished to approx. 35 mg∙ℓ-1 N with the addition of 25-26 mg∙ℓ-1 N ammonium at the onset of each batch experiment. In the anaerobic reactor, where no nitrification was expected to take place due to lack of oxygen, and where COD was insufficient for denitrification, NO2--N was nevertheless removed, with most of the NO2--N removed within 32 h and reduced to nearly zero after 48 h (Fig. 5; NO2 (◊)). Interestingly, a positive correlation existed between NH4+-N and NO2--N (r = 0.91, p = 0.00), indicating a close metabolic link between these two inorganic nitrogen compounds. Figure 6 shows data for one batch experiment only (for graphical clarity). It can be seen that ammonium removal stopped as soon as the nitrite concentration reached zero. Figure 6 also shows an increase in pH over the batch reaction, which is in line with the removal of a strong acid (HNO2).
The above-mentioned results provided strong indications that heterotrophic denitrification was not the primary nitrogen removal process in the anaerobic reactor. Firstly, during heterotrophic denitrification, nitrite denitrification commences only once the nitrate concentration nears zero (Ekama and
Wentzel, 1999). However, during our anaerobic batch experiments NO2~-N levels (Fig. 5; NO2 (◊)) were reduced to near zero while NO3-N levels (Fig. 5; NO3 (Δ)) were still relatively high. Secondly, anaerobic removal of nitrite was clearly linked to the removal of ammonium. In addition, the ratio of NO2--N to NH4+-N removal (as calculated from the slope of the graphs) was found to be 1:1.33 (Fig. 5). The latter is nearly identical to the stoichiometric ratio for NO2--N and NH4+-N removal to N2 during the anammox process (Strous et al. 1998).
Testing for anammox bacteria using molecular probes
Evidence for the presence of anammox bacteria within sludge sampled from the sludge withdrawal valve, and hence within the biofilm on the filter stones, was obtained when PCR products of taxonomically informative 16S rRNA gene sequences in an agarose gel were visualised under UV light. Subsequent sequence analysis of the amplification product, obtained using the Amx60f /Amx820r primer combination (Table 1), confirmed the presence of an anammox bacterium in the sludge (Fig. 7). This amplified sequence (DTFamx KC748231) showed significant homology to that of Candidatus 'Brocadia anammoxidans' and Candidatus 'Brocadia fulgida'. The latter species, previously detected within microbial consortia containing heterotrophic bacteria, was found by others to exhibit the typical features of anammox bacteria such as the presence of an anammoxosome, ladderane lipids, as well as the ability to produce hydrazine in the presence of hydroxylamine (Kartal et al., 2008).
Process evaluation
The liquid of the anaerobic reactor contained less organic nitrogen (4 to 16 mg∙ℓ-1) than the liquid of the aerobic reactor (approx. 220 mg∙ℓ-1) (Fig. 4). Also, during all the experimental periods, the total nitrogen concentration was notably higher in the aerobic reactor than in the anaerobic reactor. This may be ascribed to denitrification conducted by autotrophic and/or heterotrophic bacteria in the anaerobic reactor, which resulted in a decrease in nitrate, nitrite and ammonium, and therefore total nitrogen, compared to the inhibition of this activity in the aerobic reactor, where ammonium and nitrite were ultimately oxidised to nitrate. The lack of nitrogen removal in the aerobic reactor may be ascribed to high airflow rates in this reactor, resulting in declining anaerobic or anoxic zones within the biofilm and the concomitant inhibition of anaerobic denitrification in these zones situated below the aerobic nitrifying zones (Holman and Wareham, 2005).
As demonstrated in Table 2, the rates of ammonium oxidation under aerobic and anaerobic conditions were found to be comparable during the batch reactions. Similarly, the rate of nitrite removal, under optimum anaerobic conditions, was comparable with the rate of nitrite oxidation, under optimum aerobic conditions. However, nitrate seemed to accumulate during aerobic batch reactions, while it was reduced under anaerobic conditions. All of these reactions caused the overall nitrogen conversion during the batch reactions to be more under anaerobic (1.70 mg∙ℓ-1∙h-1) than aerobic conditions (1.00 mg∙ℓ-1∙h-1).
If one considers that conditions inside full-scale trickling filters would vary according to the diurnal load distribution, then it seems plausible that predominant aerobic or anaerobic conditions would prevail at different times. At the same time, the physical distribution of bacteria within the biofilms creates simultaneous aerobic and anaerobic microclimates. Based on the rates observed, we believe that the anaerobic oxidation of ammonium and removal of nitrite could play an equally important role in the overall nitrogen removal processes as aerobic nitrification.
CONCLUSIONS
The results obtained with the aerobic reactor showed that microbial consortia in the Daspoort trickling filter biofilm were capable of nitrification under aerobic conditions. Findings obtained with the anaerobic reactor also revealed the presence of microbes capable of denitrification in this trickling filter biofilm. The latter microbes may be heterotrophic or autotrophic. Heterotrophic activity was indicated by the reduction of COD and nitrate levels, while a number of observations pointed to the existence of anammox bacteria in the full-scale trickling filters at Daspoort, and the important role these bacteria may play in the overall removal of nitrogen from settled wastewater. These observations are summarised below:
•The measured availability, as well as the removal, of COD from the bulk liquid was insufficient for the removal of nitrite and nitrate via ordinary heterotrophic denitrification, even considering only the catabolic reactions for nitrite/nitrate reduction.
•Nitrite was reduced to near-zero (below detection limit) in anaerobic batch experiments, whereas nitrate was not reduced to zero in any of the anaerobic experiments. Under normal heterotrophic denitrification, one expects nitrate to be removed completely before nitrite is removed.
• Anammox bacteria are known to have a high affinity for nitrite, resulting in complete nitrite removal with sufficient ammonium. When nitrite concentrations in the anaerobic experiments reached zero, the removal of ammonium stopped altogether, and the concentration remained near constant. Anaerobic removal of ammonium was clearly correlated with the removal of nitrite.
• The average ratio between nitrite removal and ammonium removal was around 1.3, which has been observed the world over for anammox bacteria.
• The reaction rate for anaerobic ammonium removal was relatively high (around 50% of the rate of aerobic ammonium oxidation), and the reaction rate of anaerobic nitrite removal was comparable with the rate of aerobic nitrite oxidation.
• The use of a PCR detection technique, based on primers for taxonomic informative gene sequences, confirmed the presence of an anammox bacterium, closely related to Candidatus 'Brocadia anammoxidans' and Candidatus 'Brocadia fulgida', within the Daspoort humus sludge.
Thus, nitrogen removal over the Daspoort trickling filters is not only a function of conventional heterotrophic denitrification; an anaerobic ammonium oxidation process also seems to play an important role in nitrogen removal. As far as we know this is the first published report of an anammox bacterial species in South Africa.
Although the slow growth rate of anammox bacteria is often seen as a limitation in process technology (Van Rijn et al., 2006), these ubiquitous bacteria are known to play a pivotal role in the biogeochemical cycling of nitrogen (Kuenen, 2008). This natural occurrence is underlined by our finding of anammox in Daspoort's old trickling filters, which have been functional since long before the discovery of anammox bacteria (Kuenen, 2008). We envisage that with more knowledge it might be possible to optimise growth conditions for annamox bacteria within trickling filters thereby creating superior biofilm consortia via selection and evolution. The latter will render trickling filters an appropriate technology when considering upgrade or construction of new wastewater treatment works.
ACKNOWLEDGEMENTS
This work was initiated through the CSIR's young researcher establishment fund. We gratefully acknowledge the Water Research Commission of South Africa for continued financial support. We also thank the City of Tshwane and personnel at Daspoort Wastewater Treatment Works for permission and support.
REFERENCES
AMANO T, YOSHINAGA I, OKADA K, YAMAGISHI T, UEDA S, OBUCHI A, SAKO Y and SUWA Y (2007) Detection of anammox activity and diversity of anammox bacteria-related 16S rRNA genes in coastal marine sediment in Japan. Microbes Environ. 22 232-242. [ Links ]
BIESTERFELD S, FARMER G, FIGUEROA L, PARKER D and RUSSELL P (2003) Quantification of denitrification potential in carbonaceous trickling filters. Water Res. 37 4011-4017. [ Links ]
DALSGAARD T and REVSBECH NP (1992) Regulating factors of denitrification in trickling filter biofilms as measured with the oxygen/nitrous oxide microsensor. FEMS Microbiol. Ecol. 101 151-164. [ Links ]
EGLI K, FANGER U, ALVAREZ PJJ, SIEGRIST H, VAN DER MEER JR and ZEHNDER AJB (2001) Enrichment and characterization of an anammox bacterium from a rotating biological contactor treating ammonium-rich leachate. Arch. Microbiol. 175 198-207. [ Links ]
EKAMA GA and WENTZEL MC (1999) Denitrification kinetics in biological N and P removal activated sludge systems treating municipal waste waters. Water Sci. Technol. 39 69-77. [ Links ]
HEIJNEN JJ (1999) Bioenergetics of microbial growth In: Encyclopaedia of Bioprocess Technology: Fermentation, Biocatalysis and Bioseparation. John Wiley and Sons, Inc., Rahway. [ Links ]
HOLMAN JB and WAREHAM DG (2005) COD, ammonia and dissolved oxygen time profiles in the simultaneous nitrification/denitrification process. Biochem. Eng. J. 22 125-133. [ Links ]
KARTAL B, VAN NIFTRIK L, RATTRAY J, VAN DE VOSSENBERG JLCM, SCHMID MC, DAMSTÉ JS, JETTEN MSM and STROUS M (2008) Candidatus 'Brocadia fulgida': an autofluorescent anaerobic ammonium oxidizing bacterium. FEMS Microbiol. Ecol. 63 46-55. [ Links ]
KUENEN JG (2008) Anammox bacteria: from discovery to application. Nat. Rev. Microbiol. 6 320-326. [ Links ]
KUHL M and JORGENSON BB (1992) Microsensor measurements of sulphate reduction and sulphide oxidation in compact microbial communities of aerobic biofilms. Appl. Environ. Microbiol. 58 1164-1174. [ Links ]
LYDMARK P, LIND M, SÖRENSSON F and HERMANSSON M (2006) Vertical distribution of nitrifying populations in bacterial biofilms from a full-scale nitrifying trickling filter. Environ. Microbiol. 8 2036-2049. [ Links ]
NIELSEN LP, CHRISTENSEN PB, REVSBECH NP and SORENSEN J (1990) Denitrification and oxygen respiration in biofilms studied with a microsensor for nitrous oxide and oxygen. Microb. Ecol. 19 63-72. [ Links ]
PERSSON F, WIK T, SÖRENSSON F and HERMANSSON M (2002) Distribution and activity of ammonia oxidizing bacteria in a large full-scale trickling filter. Water Res. 361439-1448. [ Links ]
RADEBE VB, MASHEGO M, MAREE JP and WILSENACH J (2012) Simultaneous nitrification and denitrification using trickling filters. Paper presented at WISA 2012, 6-10 May 2012, Cape Town. URL: http://www.ewisa.co.za/literature/literaturedetails.aspx?id=d601ed14-bf6f-47ea-8697-453a170057cf (Acessed 17 December 2013). [ Links ]
REVSBECH NP, CHRISTENSEN PB, NIELSEN LP and SORENSEN J (1989) Denitrification in a trickling filter biofilm studied by a microsensor for oxygen and nitrous oxide. Water Res. 23 867-871. [ Links ]
ROBERTSON LA and KUENEN JS (1990) Combined heterotrophic nitrification and aerobic denitrification in Thiosphaera pantotropha and other bacteria. Antonie van Leeuwenhoek 57 139-152. [ Links ]
SCHULZE KL (1957) Experimental vertical screen trickling filter. Sewage Ind. Wastes 29 458-467. [ Links ]
STROUS M, FUERST JA, KRAMER EHM, LOGEMANN S, MUYZER G, VAN DE PAS-SCHOONEN KT, WEBB R, KUENEN JG and JETTEN MSM (1999) Missing lithotroph identified as new Planctomycete. Lett. Nature 400 446-449. [ Links ]
STROUS M, HEIJNEN JJ, KUENEN JG, JETTEN MSM (1998) The sequencing batch reactor as a powerful tool for the study of slowly growing anaerobic ammonium-oxidizing microorganisms. Appl. Microbiol. Biotechnol. 50 589-596. [ Links ]
SWOFFORD DL (2002) PAUP*: Phylogenetic Analysis Using Parsimony (*and Other Methods). Sinauer Associates, Sunderland, Massachusetts. [ Links ]
VAN LOOSDRECHT MCM and JETTEN MSM (1997) Method for treating ammonia-comprising wastewater. Patent PCT/ NL97/00482. [ Links ]
VAN RIJN J, TAL Y and SCHREIER J (2006) Denitrification in recirculating systems: theory and applications. Aquacult. Eng. 34 346-376. [ Links ]
VAYENAS DV, PAVLOU S and LYBERATOS G (1997) Development of a dynamic model describing nitrification and nitrification in trickling filters. Water Res. 31 1135-1147. [ Links ]
WEZERNAK CT and GANNON JJ (1967) Oxygen-nitrogen relationships in autotrophic nitrification. Appl. Microbiol. 15 1211-1215 [ Links ]
YOO H, AHN K-H, LEE H-J, LEE K-H, KWAK Y-J and SONG K-G (1999) Nitrogen removal from synthetic wastewater by simultaneous nitrification and denitrification (SND) via nitrite in an intermittently-aerated reactor. Water Res. 33 145-154. [ Links ]
 Correspondence:
Correspondence:
A Botha
+27 21 808 5856
e-mail: abo@sun.ac.za
Received 19 March 2013
accepted in revised form 17 December 2013














