Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
Water SA
versão On-line ISSN 1816-7950
versão impressa ISSN 0378-4738
Water SA vol.39 no.3 Pretoria 2013
Treatability of South African surface waters by activated carbon
KP Lobanga; J Haarhoff*; SJ van Staden
Department of Civil Engineering Science, University of Johannesburg, PO Box 524, Auckland Park, 2006, South Africa
ABSTRACT
Natural organic matter (NOM) in water resources for drinking purposes can be removed by different methods, including activated carbon adsorption. Due to the variability of NOM in natural waters, both in terms of its nature and its concentration, a study was undertaken to investigate NOM removal for a wide range of South African surface waters, sampled at different periods, by the use of granular activated carbon (GAC). NOM removal was assessed by measuring the ultraviolet (UV) absorbance at 3 wavelengths, namely, 254 nm (UV254), 272 nm (UV272) and 300 nm (UV300). A comparison of data between the three wavelengths showed that any of the three wavelengths can be used to assess NOM removal by GAC, which is well described by the Freundlich equilibrium equation. A treatment target of 40% removal of initial UV254 absorbance was considered. It was observed that, although the GAC dosage was generally a function of the initial UV254 absorbance, differences existed between waters. This suggests that GAC usage rate is not only a function of the initial UV absorbance but also of the NOM composition, indicating a need for improved NOM characterisation. Comparison between the UV absorbance and dissolved organic carbon (DOC) data suggested that for some waters UV254 absorbance can be used as a rapid substitute for DOC. Finally, the high GAC dosage rates required for the target criterion revealed that the process is inadequate for use at the initial stage of raw water treatment; GAC adsorption should be used at later stages of drinking water treatment.
Keywords: activated carbon, adsorption, Freundlich isotherm, natural organic matter, surface water, ultraviolet absorbance
INTRODUCTION
Natural organic matter is a complex mixture of organic compounds such as humic and fulvic acids, proteins, amino acids and carbohydrates, resulting from the degradation of plants, animals and microorganisms (Cornelissen et al., 2008; Edzwald and Tobiason, 2010; García et al., 2011). Based on its origin, NOM can be placed in two categories, namely autochthonous organic matter (formed within the water body) and allochthonous organic matter (produced elsewhere and transported to the water body) (Edzwald and Tobiason, 2010). It can be further classified as either particulate organic matter (POM) or dissolved organic matter (DOM). NOM in water affects the organoleptic aspects of the drinking water, promotes bacterial regrowth in drinking water distribution systems and reacts with disinfectants and oxidants, producing disinfection by-products and other products (Van der Kooij, 1998; Batterman et al., 2000; Hallam et al., 2001; Melnick et al., 2007; Edzwald and Tobiason, 2010; 0degaard et al., 2010). NOM in raw water can be removed by different methods including the use of activated carbon. Adsorption of DOM by the activated carbon is a function of the NOM composition (nature and concentration), pH of the water, water temperature, molecular size and concentrations of some ions, such as magnesium and calcium (Schreiber et al., 2005).
In drinking water treatment plants, adsorption by activated carbon is a well-established process. The activated carbon is used either as powder (powdered activated carbon - PAC) or as granules (granular activated carbon - GAC). The PAC is generally used at the beginning of the treatment process (at or just after coagulation), while the GAC is used at a later stage of the treatment (generally before disinfection) (Kristiana et al. 2011; Matilainen et al., 2006). PAC is added to the water as slurry, while the GAC is placed in filter beds.
The aim of this study was to investigate NOM removal for a large range of South African surface waters by the use of GAC. The removal was assessed by measuring the UV absorbance at 254 nm (UV254), 272 nm (UV272) and 300 nm (UV300). For some waters, the DOC values were used and compared with the UV absorbance values.
MATERIALS AND METHODS
Source water
Eight surface waters were sampled. As shown in Fig. 1, the sampling sites were chosen from different geographic regions in South Africa in order to include differences in NOM composition. The surface waters were also chosen to account for the main surface water types of South Africa (Oberholster, 2010). The different categories of waters are summarised in Table 1. The raw waters were collected at 5 different times to capture the seasonal variations in NOM composition (Sharp et al., 2006; Uyak et al., 2008). The sampling was done during the following periods: Round 1 from February to April 2010; Rounds 2, 3 and 4 in July 2010, November 2010 and February 2011, respectively. Round 5 waters were sampled in May and June 2011. Raw waters were collected (using two 25 ℓ plastic containers) and stored at approximately 4°C, in the dark. Analysis of the samples was done within 2 months, during which there was no significant change in NOM concentration or composition (Haarhoff et al., 2013).
Granular activated carbon preparation
The activated carbon used was a product commercially available in South Africa and kindly provided by a local supplier.
It is a bituminous, coal-based GAC used in liquid phase applications to remove organic compounds. Figure 2 shows the GAC as supplied (a) and after grinding (b).
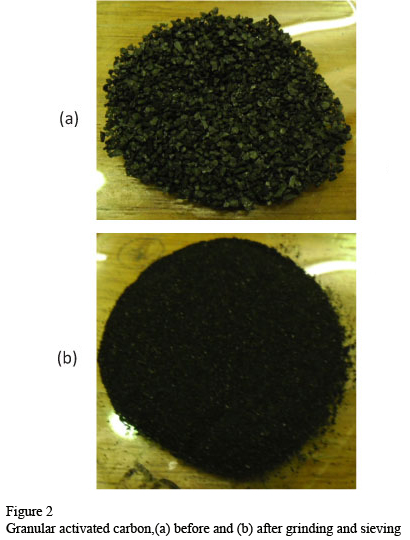
The general practice adopted for activated carbon studies is to grind the virgin GAC and use a fraction of the finely ground GAC. The main advantages of using the fine GAC are that it requires a shorter time to reach equilibrium with a smaller chance of biological interference. The unground GAC requires a longer time to reach equilibrium, which allows biological activity to take place and interfere significantly with the adsorption process (Randtke and Snoeyink, 1983). These authors found that the adsorptive capacity of the activated carbon was not significantly affected by grinding to a smaller size. When GAC is used in a column test, it is washed prior to use in order to remove the fine particles that can contaminate the effluent or plug the underdrain system. However, if the GAC is used in a batch equilibrium test, it is not necessary to apply this rinsing step since the fine particles are a very small fraction of the total mass of the carbon used.
To obtain the powdered activated carbon, 100 g of virgin GAC was crushed with a porcelain mortar and pestle. Using a vibratory sieve shaker, the fraction passing the 300 µm sieve was collected and stored in a glass container. The procedure was repeated until the quantity of fine GAC obtained was sufficient for further testing.
Batch adsorption tests
Batch tests were conducted at room temperature (20-25°C). On the day of testing, at least 3 ℓ of raw water was removed from refrigeration and stirred in the laboratory at 300 r-min-1 for a minimum of 30 min. Different masses of carbon (blank, 6.25, 8.25, 12.50, 16.50, 25.00, 33.25 and 50.00 mg) were each added to 250 mℓ of raw water in 500 mℓ Erlenmeyer flasks. The samples were placed on an orbital shaker table at 140 r-min-1 for 72 h (3 days). The rotation speed of 140 r-min-1 was chosen as it was visually observed to provide good mixing. After 3 days, the samples were filtered through a 0.45 µm hydrophilic Durapore (PVDF) membrane filter (Millipore Millex-HV) before the ultraviolet absorbance was measured at 254 nm (UV254), 272 nm (UV272) and 300 nm (UV300) using a spectro- photometer (Ultrospec II UV/Vis). The DOC values of some samples were also measured using a total organic carbon analyser (Teledyne Tekmar, TOC fusion).
The Freundlich equation was used for modelling the UV254 absorbance and shown in Eq.

where:
qe = equilibrium NOM concentration in the solid phase (ℓ∙mg-1∙m-1)
Ce = equilibrium NOM concentration in water (m-1)
K and n = Freundlich constant and exponent, respectively.
The Freundlich parameters K and n were determined from the results of absorbance at the three wavelengths. The parameters K and n are related to the capacity and affinity of the carbon for NOM molecules, respectively (Cornelissen et al., 2008).
Performance indicator
The required GAC dosage for each raw water was determined for an arbitrary treatment goal of 40% removal of initial UV254 absorbance.
The GAC dosage calculation was derived from Eq. (1) as follows:


where:
Ci = initial NOM concentration in raw water (m-1)
M = GAC dosage (mg.ℓ-1)
RESULTS AND DISCUSSION
Comparison of absorbance results at three wavelengths
The indicators used and reported in this paper are the UV254 absorbance (indicative of the presence of conjugated C=C double bonds or compounds with aromatic structure) (Edzwald and Tobiason, 2010), UV272 absorbance (reported by some as the best indicator of total organic halogen and trihalomethane formation (Korshin et al., 1997)) and UV300 absorbance (used by some South African water treatment plants as an operational parameter) (Haarhoff et al., 2012). Ultraviolet absorbance at 254 nm (UV254) is used by many organisations to characterise NOM (Karanfil et al., 2002). It was found that, when plotting the graphs of equilibrium UV absorbance in water (Ce) versus the GAC dosage, the removal patterns were the same for all wavelengths, as shown in Figs. 3 and 4 (Round 5 Stilfontein and Round 3 Umzoniana waters, respectively).
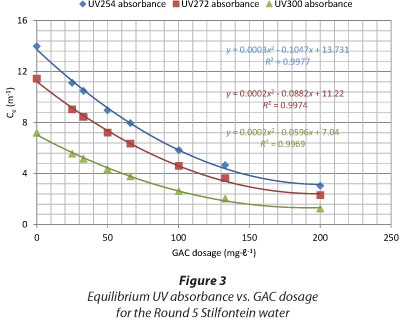
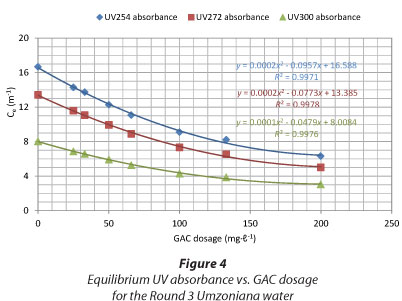
Table 2 gives the ratios between UV272 and UV254 and, UV300 and UV254 for the above-mentioned waters. It demonstrates that the ratios between UV272 and UV254 were practically the same for all the GAC dosages for these two water samples. The same conclusion applied to the ratios between the UV300 and UV254. The average value, for all the water samples, of the ratio between UV272 and UV254 was 0.82 (SD = 0.0006), and for the ratio between UV300 and UV254was 0.53 (SD = 0.0033).

Table 3 presents the UV absorbance removal at different wavelengths for certain waters. UV absorbance removal was the same for a particular water at the same GAC dosage for the three wavelengths.
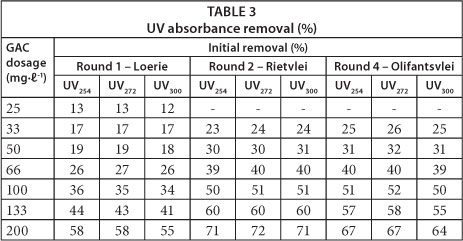
Table 4 compares the GAC dosages required to remove 40% of the initial UV absorbance at 3 wavelengths for 3 different waters. The dosages were calculated by applying the Freundlich equation.
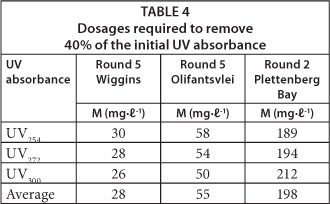
Table 4 indicates that the dosage required to remove the initial UV absorbance by a fixed percentage (in this case 40%) was approximately the same for all three wavelengths for each water sample. It also shows that the required dosage varied with the water type. To remove 40% of initial UV absorbance, Round 5 Olifantsvlei water required almost twice the dosage of the Wiggins Round 5 water, whilst the Plettenberg Bay Round 2 needed approximately 7 times more GAC than the Round 5 Wiggins water. These differences of dosages show that NOM composition varies with water source. Thus, improved NOM characterisation is required.
UV254 absorbance values
Based on all the findings presented above, it appeared that any of the three wavelengths investigated could be used to assess NOM removal. In this paper, the rest of the data analysis was done with the UV254 absorbance value, as this is the most popular wavelength used in the published literature (Karanfil et al., 2002). The Freundlich equilibrium equation was then applied to UV254 absorbance data. Table 5 presents the Freundlich parameters of the water samples. Round 1 Loerie and Vereeniging had an R2 of 0.80 and 0.73, respectively, while Round 3 of the Vereeniging water had an R2 of 0.78. The lowest R2, 0.61, for Round 4 was found for the Stilfontein water. For Round 5, the Umzoniana, Vereeniging and Plettenberg Bay waters displayed R2 values of 0.78, 0.83 and 0.66, respectively.
Performance criterion
The required GAC dosage was calculated, i.e. the carbon dosage required to remove the initial UV254 absorbance (Ci) by 40%. Table 6 represents the calculated GAC dosage M (mg.ℓ-1) to meet the required criterion.
In general, the smaller the initial UV254 absorbance, the smaller the required GAC dosage, e.g. all of the Wiggins water samples. When the UV absorbance increased the dosage also increased. There were, however, some notable exceptions: Round 1 Olifantsvlei water with initial UV254 absorbance of 13.1 m-1 required a lower dosage of 48 mg.ℓ-1 and Umzoniana Round 2 with initial UV254 absorbance of 14.8 m-1 required a higher dose of 75 mg.ℓ-1. The GAC usage of all of the samples with R2 < 0.90 is not shown in Table 6.
Figure 5 presents initial UV254 absorbance versus GAC dosage. Some waters displayed a clear relationship between the activated carbon dosage and the initial UV absorbance. It was, therefore, possible to predict the GAC usage rate of some waters, but for others it was difficult. The data of the Plettenberg Bay water Round 2 (initial UV254 absorbance and GAC usage of 30.4 m-1 and 189.4 mg.ℓ-1, respectively) was not included in Fig. 6 because it was out of the range of the figure.
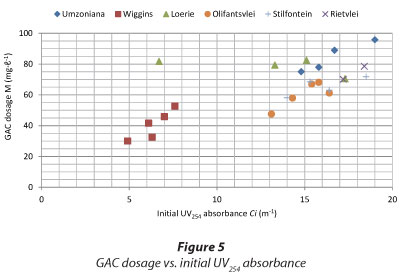
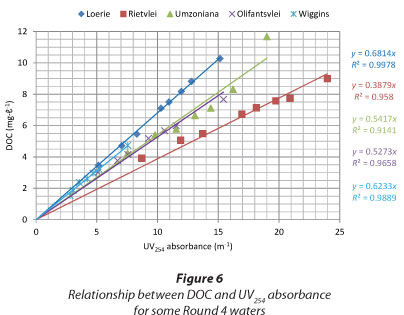
However, it appears that the dosages required for achieving the target criterion are high compared to what can be tolerated by water treatment plants.
Comparison of DOC and UV254 absorbance
Parallel DOC analysis was performed on the Round 4 waters (i.e. Loerie, Rietvlei, Umzoniana, Olifantsvlei and Wiggins). Figure 6, representing the comparison between the DOC and UV254 absorbance data of the selected waters, showed that there was a strong linear relationship between the two surrogate parameters, for NOM characterisation.
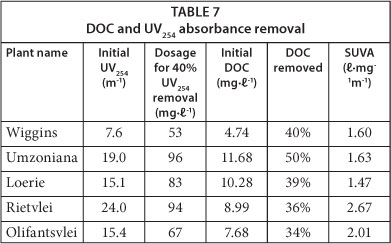
It was also found that the GAC dosage required to remove 40% of UV254 absorbance also removed about 40% of the DOC (see Table 7). This suggests that UV254 absorbance might be used as a quick and reliable characterisation parameter for some South African waters. The specific UV absorbance (SUVA) values of the waters presented in the last column of Table 7 are good indicators of the humic acid content in water (USEPA, 1999). The SUVA values suggested that there were two categories of waters: those with a high fraction of non-humic matter (SUVA < 2) and those with a mixture of aquatic humic and non-humic matter (SUVA between 2 and 4).
CONCLUSION
The aim of the study was to investigate the removal of NOM by the use of GAC by measuring the UV absorbance at 254 nm (UV254), 272 nm (UV272) and 300 nm (UV300), for a representative selection of South African surface waters, in batch experiments. The performance of activated carbon was considered over a range of dosages in order to detect some general patterns - not to suggest practical or economical values. The data for the three wavelengths were compared first:
- It was found that, at all three wavelengths, the percentage of NOM removal was practically the same. The differences amongst these wavelengths can, therefore, not be used to characterise the differences in NOM composition of the different sources. Any of the three wavelengths can be used to assess the NOM removal by GAC.
- The UV272 and UV300 absorbance values were, on average, 0.82 and 0.53 times the UV254 absorbance data, respectively. The UV254 absorbance data were chosen for the remainder of the analysis, as it is the most popular wavelength used in the published literature.
The Freundlich equilibrium equation provided a good mathematical description of the adsorption. The granular activated carbon dosage could be calculated for an arbitrary goal of 40% of UV absorbance removal by fitting this isotherm to the UV254 absorbance data. It was found that:
- The value of the initial UV absorbance value impacts on the dosage rate. Results suggested that when the initial UV absorbance is low, the GAC dosage required is also likely to be small.
- The GAC usage rate is not only dependent on the initial UV254 absorbance of the water but also on the composition (nature and concentration) of the NOM indicating a need for improved NOM characterisation.
Dissolved organic carbon analysis was conducted on some waters:
- Strong linear relations were found between the DOC and the UV254 absorbance values, suggesting that, for those waters, the latter could be used as a quick and reliable characterisation surrogate parameter for NOM removal.
- The dosages required to remove 40% of UV absorbance also removed approximately the same percentage of DOC.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the financial support from the University of Johannesburg and the South African Water Research Commission.
REFERENCES
BATTERMAN S, ZHANG L and WANG S (2000) Quenching of chlorination disinfection by-product formation in drinking water by hydrogen peroxide. Water Res. 34 (5) 1652-1658. [ Links ]
CORNELISSEN ER, MOREAU N, SIEGERS WG, ABRAHAMSE AJ, RIETVELD LC, GREFTE A, DIGNUM M, AMY G and WESSELS LP (2008) Selection of anionic exchange resins for removal of natural organic matter (NOM) fractions. Water Res. 42 (1-2) 413-423. [ Links ]
EDZWALD JK and TOBIASON JE (2010) Chemical principles, source water composition, and watershed protection. In: Edzwald JK (ed.) Water Quality & Treatment - A Handbook on Drinking Water (6th edn.). AWWA and McGraw-Hill, New York. [ Links ]
GARCÍA I (2011) Removal of natural organic matter to reduce the presence of trihalomethanes in drinking water. Doctoral thesis, School of Chemical Science and Engineering, Royal Institute of Technology, Stockholm. [ Links ]
HAARHOFF J, MAMBA B, KRAUSE R, VAN STADEN S, NKAM-BULE T, DLAMINI S and LOBANGA KP (2013) Natural organic matter in drinking water sources: Its characterisation and treat-ability. WRC Report No. 1883/1/12. Water Research Commission, Pretoria. [ Links ]
HALLAM NB, WEST JR, FORSTER CF and SIMMS J (2001) The potential for biofilm growth in water distribution systems. Water Res. 35 (17) 4063-4071. [ Links ]
KARANFIL T, SCHLAUTMAN MA and ERDOGAN I (2002) Survey of DOC and UV measurement practices with implications for SUVA determination. J. AWWA 94 (12) 68-80. [ Links ] KORSHIN GV, LI C-W and BENJAMIN MM (1997) The decrease of UV absorbance as an indicator of TOX formation. Water Res. 31 (4) 946-949. [ Links ]
KRISTIANA I, JOLL C and HEITZ A (2011) Powdered activated carbon coupled with enhanced coagulation for natural organic matter removal and disinfection by-product control: Application in a Western Australian water treatment plant. Chemosphere 82 (5) 661-667. [ Links ]
MATILAINEN A, VIENO N and TUHKANEN T (2006) Efficiency of the activated carbon filtration in the natural organic matter removal. Environ. Int. 32 (3) 324-331. [ Links ]
MELNICK RL, NYSKA A, FOSTER PM, ROYCROFT JH and KISSLING GE (2007) Toxicity and carcinogenicity of the water disinfection byproduct, dibromoacetic acid, in rats and mice. Toxicology 230 (2-3) 126-136. [ Links ]
OBERHOLSTER P (2010) The current status of water quality in South Africa. In: A CSIR perspective on water in South Africa - 2010. CSIR Report No. CSIR/NRE/PW/IR/2011/0012/A. [ Links ]
ØDEGAARD H, ØSTERHUS S, MELIN E and EIKEBROKK B (2010)) NOM removal technologies - Norwegian experiences. Drink. Water Eng. Sci. 3 (1) 1-9. [ Links ]
RANDTKE SJ and SNOEYINK VL (1983) Evaluating GAC adsorptive capacity. Jour. AWWA 75 (8) 406-413. [ Links ]
SCHREIBER B, BRINKMANN T, SCHMALTZ V and WORCH E (2005) Adsorption of dissolved organic matter onto activated carbon - the influence of temperature, absorption wavelength, and molecular size. Water Res. 39 (15) 3449-3456. [ Links ]
SHARP EL, PARSONS SA and JEFFERSON B (2006) Seasonal variations in natural organic matter and its impact on coagulation in water treatment. Sci. Total Environ. 363 (1-3) 183-194. [ Links ]
USEPA (UNITED STATES ENVIRONMENTAL PROTECTION AGENCY) (1999) Enhanced Coagulation and Enhanced Precipitative Softening Guidance Manual. EPA 815-R-99-012. US EPA, Washington. [ Links ]
UYAK V, OZDEMIR K and TOROZ I (2008) Seasonal variations of disinfection by-product precursors profile and their removal through surface water treatment plants. Sci. Total Environ. 390 (2-3) 417-424. [ Links ]
VAN DER KOOIJ D (1998) Potential for biofilm development in drinking water distribution systems. J. Appl. Microbiol. 85 (S1) 39S-44S. [ Links ]
This paper was originally presented at the 2012 Water Institute of Southern Africa (WISA) Biennial Conference, Cape Town, 6-10 May 2012.
* To whom all correspondence should be addressed. +27 11 559 2148; e-mail: jhaarhoff@uj.ac.za














