Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Water SA
On-line version ISSN 1816-7950
Print version ISSN 0378-4738
Water SA vol.39 n.2 Pretoria Jan. 2013
Sources of manganese in the residue from a water treatment plant
DL Trollip; JC Hughes; LW Titshall*
Soil Science, School of Agricultural, Earth and Environmental Sciences, University of KwaZulu-Natal, Private Bag X01, Scottsville 3209, South Africa
ABSTRACT
Disposal of water treatment residue (WTR), the by-product from the production of potable water, has traditionally been to landfill. The shortage of suitable landfill sites has led to the proposal that WTR be applied to land. Such disposal is only possible if the WTR contains no toxic elements that may contaminate soil, water or vegetation. Previous studies have shown that most WTRs in South Africa contain a high concentration of Mn, which was assumed to be from the drinking water treatment chemicals. This study investigated this assumption at one water treatment plant (WTP) in KwaZulu-Natal. Chemical analysis of drinking water treatment chemicals and a mass balance for Mn at the WTP showed that the main source of Mn was brown lime (added during the treatment process), although the raw water also added appreciable amounts of Mn to the WTR due to the volume of water treated. The concentration of Mn in the organic polymers, bentonite, ferric chloride, ferric sulphate and alum was negligible or very low. It is unlikely that the cost increase associated with changing from brown lime to white lime could be justified, given that the environmental impact of Mn is unclear and is generally not considered to be a problem internationally. Different ecosystems will respond differently to Mn loading and deriving a single, national, maximum permissible level for Mn within a WTR to permit land application is thus difficult and inappropriate.
Keywords: drinking water treatment chemicals, land application, manganese, water treatment residue
Introduction
One of the principal objectives of potable water treatment is purification by removal of suspended solids and dissolved matter (e.g. clay, fine particulate matter, organic and inorganic components, algae, bacteria and viruses) from the raw water. At a conventional water treatment plant (WTP), the suspended solids are removed in clarifiers and sand filters, after prior coagulation with long-chain polymers, addition of flocculation agents such as bentonite and polyacrylamide, and pH correction utilising lime, sodium hydroxide or soda ash. Filter wash-water contains suspended matter removed by the filters. In South Africa, this waste by-product from the clarifiers and wash-water (here termed water treatment residue (WTR) to distinguish it from wastewater sludge) has historically either been disposed of by release into the nearest watercourse, into evaporation ponds or, after partial dewatering, to landfill. Due to high evaporation rates and the perceived availability of land in South Africa (though this availability is decreasing close to urban areas), the use of evaporating ponds is often favoured, while the direct disposal of WTR to watercourses is now illegal (National Water Act (NWA); Act No. 36 of 1998).
Internationally, techniques for disposal of WTR have included direct discharge to a watercourse, disposal to sanitary landfills, and co-disposal with municipal sewage (Elliot and Dempsey, 1991). In South Africa, the reason for the previous popularity of landfills was that, with environmental concerns being of low priority, landfill constituted a convenient method of waste disposal. Since 1994, however, South Africa has seen the closure of numerous landfill sites for both social and environmental reasons. With increasing transport costs to the nearest acceptable landfill, land disposal systems are becoming more popular for the ultimate disposal of WTRs, with a view to make use of their possible beneficial aspects (Basta, 2000). Elliot and Dempsey (1991 p. 126) define land treatment as 'the controlled spreading of sludge onto or incorporation into the surface layer of soil to stabilise, degrade and immobilise sludge constituents'. Furthermore, land application systems may be engineered to favourably modify soil properties and recycle valuable components of the applied waste (Overcash and Pal, 1979).
Hughes et al. (2005), in an extensive study, detailed the effect of land application of WTR for South African conditions. This study concluded that land application of WTRs is safe and is likely to have no negative impacts on soils, vegetation or groundwater even at very high disposal rates that are unlikely to occur in the field (Hughes et al., 2005). In a follow-up risk assessment, Hughes et al. (2007) recommended that WTR be reclassified to allow its disposal under a general authorisation (NWA, Act 36 of 1998) with consideration being given to the nature of the WTR to determine any potential negative impacts the material may have if applied to land. These studies suggested that a possible area of concern was the elevated manganese (Mn) concentration found in WTRs collected from a large number of WTPs in South Africa. This concern was also highlighted by Novak et al. (2007) who found that soil incubated with a WTR released Mn after 60 days. They concluded that slight soil acidification and reducing conditions were the cause. Furthermore, the raw water was treated with potassium permanganate which would have contributed Mn to the WTR.
Manganese is often perceived as a toxic heavy metal and its presence in the environment at elevated levels may be potentially lethal (Kabata-Pendias and Pendias, 2001). However, Mn is an essential nutrient for microorganisms, plants, birds and animals. Terrestrial plants have a nutritional requirement of approximately 10 to 50 mg Mn kg-1 of tissue (Howe et al., 2004), with average Mn concentrations in plants ranging from 15 to 1 000 mg-kg-1 (Ross, 1994).
In soils, natural background levels of total Mn range from <1 to 4 000 mg-kg-1, with mean values of 300 to 600 mg-kg-1 (Howe et al., 2004). The major pool of Mn in soil is inherited directly from the soil's parent material; approximately 0.1% of the Earth's crust is Mn (Howe et al., 2004). Addition of Mn to soil can also result from direct atmospheric deposition, wash-off from plants and other surfaces, leaching from plant tissues and the decomposition of organic material (Stokes et al., 1998). Manganese solubility in soil is governed by two major variables, namely, pH and redox potential (McBride, 1994). The three most common valence states in soil are Mn2+, Mn3+ and Mn4*. Manganese solubility, particularly Mn2+, which is the primary plant micronutrient, is increased as the soil pH falls below 5.5, often leading to Mn toxicity in plants and potential leaching to groundwater.
Titshall and Hughes (2005) and Hughes et al. (2007) proposed that the probable source of the high concentrations of Mn in WTR was the drinking water treatment chemicals (DWTCs) used at the WTPs from which their samples were taken. Hughes et al. (2007) suggested that a change to cleaner chemicals could alleviate or remove this problem entirely, but recognised that there may be economic implications.
The objective of this study was to evaluate the role played by DWTCs utilised at a WTP in determining the concentration of total Mn that accumulates in the WTR produced from the purification of the raw water source.
Materials and methods
Drinking water treatment chemicals
The various DWTCs used by Umgeni Water's Midmar Water Treatment Plant, situated outside Howick (approx. 30 km from Pietermaritzburg) in KwaZulu-Natal, were used in this study. The DWTCs received by the Midmar WTP for the period January to June 2007 consisted of lime and bentonite samples and two types of long-chain polymeric coagulants (a raw water treatment polymer and a sludge-thickening polymer). The lime samples consisted of 7 brown and 1 white lime submitted for tender evaluation. These samples were included as they represent the main lime suppliers to all WTPs in South Africa. Samples of other treatment chemicals (taken from existing stock at the WTP), namely, ferric chloride, ferric sulphate and alum, were also analysed, to investigate the possibility of their contributing to the concentration of manganese in the WTR.
Raw and final water and water treatment residue
Samples of the raw and final water and WTR were taken at weekly intervals between January and June 2007 for measurement of Mn.
Analytical procedures
A modification of the ISO 11466: 1995 (E) method for the extraction of trace elements soluble in aqua regia (Snyman and Herselman, 2006) was performed. The modification was necessary as following the ISO 11466 method resulted in all samples burning within the 2 h digestion period. The lime samples and WTR were dried at room temperature prior to milling and sieving through a 425 µm stainless steel sieve. A representative sample of 1 g of each DWTC was digested in 30 mℓ of distilled water and 10 mℓ of aqua regia on a hot plate for 3 h at 110°C. After evaporation to near dryness, the sample was re-suspended with distilled water and filtered through a Whatman No 42 filter paper and diluted to 500 mℓ. The sample was then transferred to a high-density polyethylene (HDPE) bottle containing 2% HNO3. Similar HDPE sample bottles containing 2% HNO3 acid were used to preserve the raw and final water samples. All analyses were conducted in triplicate.
The HNO3 and HCl used to prepare fresh aqua regia for each batch of digestions was of trace metal grade. The standard solutions for Mn were of spectroscopic grade. Working standards were prepared weekly by dilution from the stock standard solutions along with digestion blanks. All Mn concentrations were determined by inductively coupled plasma spectrometry (ICP) on a 30-Perkin Elmer ICP-Optima 5300, with a detection limit of <0.01 mg-ℓ-1.
A mass balance for Mn was calculated utilising operational data from Midmar WTP.
RESULTS AND DISCUSSION
Ferric chloride, ferric sulphate and alum
Aqua-regia digested samples of alum, ferric sulphate, and ferric chloride contained low concentrations of Mn i.e., <0.01, 0.5 and 0.7 g-kg-1, respectively. It is unlikely that these chemicals contribute significantly to the Mn in the WTR.
Lime, bentonite and polymers
The brown lime utilised for pH correction of the raw water at the WTP had the highest average Mn concentration (about 6 mg-kg-1) of the DWTCs tested (Fig. 1). The concentrations of Mn in the long-chain organic polymer, the polyacrylamide polymer (sludge polymer; used in the sludge thickening process) and bentonite were low in comparison to the brown lime, typically below 1 mg-kg-1 (Fig. 1).

Figure 1
Concentrations of total manganese in drinking water treatment chemicals supplied to Midmar Water Treatment Plant (several suppliers) for the months January to June 2007 (Lime refers brown lime samples, sludge polymer to the sludge thickening polymer and polymer to the raw water treatment polymer)
The addition of lime to Midmar raw water is important as it raises the pH, subsequently increasing the total hardness and alkalinity of the system, thus promoting the formation of CaCO3 which provides a protective coating to the inside of the water pipes. The increased pH also promotes the precipitation of hydrous manganese oxides that are thus removed from the drinking water and accumulate in the WTR. Approximately 50 Mg of lime are purchased monthly, with daily dosages ranging between 5.52 and 8.35 mg-ℓ-1, depending on the season, to maintain the pH between 8.8 and 9.0.
The white lime (Supplier A in Fig. 2) had the lowest Mn concentration, of 0.02 g-kg-1. The concentration of Mn in the brown lime samples (Samples B to H in Fig. 2) was considerably higher than that of the white lime sample and ranged between about 6 and 7 g-kg-1. Although the white lime contained a very low concentration of Mn in comparison to the brown lime samples (Samples B to H in Fig. 2), the high cost and low availability of this product limits its widespread use in water purification plants.
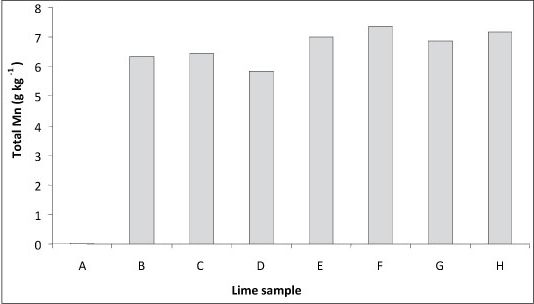
Figure 2
Concentration of total manganese in the white lime (Sample A) and brown lime samples received from 8 suppliers by the Midmar Water Treatment Plant in 2007
Mass balance
A flowchart of the Midmar WTP (Fig. 3) indicates the inputs are raw water, polymers, lime and bentonite, with the outputs being final water (delivered to the consumer), combined backwash water and supernatant from the centrifuge (chlorinated and discharged back to river) and the WTR (disposed of by land application).

Figure 3
A generalised schematic of Midmar Water Treatment Plant processes that result in the production of the thickened water treatment residue (WTR)
A simple mass balance of the Mn outputs relative to the Mn inputs for Midmar WTP is presented in Table 1.
These results indicate that, although the brown lime is generally the single major source of Mn, another significant source is the raw water, which had Mn values ranging from 211 to 357 kg-month-1 (Table 1), and for two of the months studied contributed more Mn than the brown lime. For the period under study, the WTP obtained its raw water from Midmar Dam's Level 2-abstraction point and for this period the depth from the surface varied between 9 and 11 m, depending on the gauge height at the time of sampling. The pH and Mn concentration of the raw water for the study period ranged from 7.3 to 7.9 and 0.035 to 0.055 mg-ℓ-1, respectively. The very low Mn concentrations in the raw water indicate that the contribution of Mn from the raw water to the WTR is due to a concentrating effect of the high volume of water treated rather than from high soluble Mn concentrations in the water source. The concentration of Mn in the raw water reflects Mn concentrations in the rivers feeding Midmar Dam, which range between 0.01 and 0.07 mg-ℓ-1. The Midmar Dam catchment area can be considered to be pristine as a source of natural surface water, since catchments recording Mn concentrations of between 0.2 and 1 mg-ℓ-1 are considered to essentially be free of anthropogenic sources of Mn contamination (Howe et al., 2004).
The mass balance recovery was between 61 and 84%. Possible sources of error include:
• Detailed and accurate records are kept of volumes of raw water treated and amounts of chemicals consumed in a month (inputs). However, the only detailed records of outputs are the final water volume and the Mn concentrations in the output water, which, like the raw water, are routinely monitored.
• Volumes used to calculate the mass of Mn in the backwash water and supernatant are estimated. No routine samples are taken of the supernatant discharged to river or backwash and samples were taken on an ad hoc basis over the 6-month period.
• The mass of WTR removed from the plant monthly was estimated from the volume of the skip used to remove it from the site and the number of loads delivered to the land treatment site. Overfilling/underfilling of the skip could easily occur and accurate records of the number of skips removed were not always available.
International and local legislation
In South Australia, WTR is permitted for agricultural use provided that it does not exceed maximum contaminant levels of 20 mg-kg-1 arsenic, 11 mg-kg-1 cadmium, 750 mg kg-1 copper, 300 mg kg-1 lead, 9 mg-kg-1 mercury, 145 mg-kg-1 nickel and 1 400 mg-kg-1 zinc. Its utilisation should not cause harm to groundwater or surface waters, and should not create dust, noise or an odour problem (Australian EPA, 2007). The New Zealand regulations are modelled on the Australian, while the Japanese, due to space constraints, favour incineration. For the European Union (EU) and the United States of America (USA) no specific guidelines for the use or disposal of WTR could be found, although the United States Environmental Protection Agency is collecting and analysing data on WTR in order to decide whether the promulgation of effluent guidelines is necessary (USEPA, 2007).
The quality criteria for DWTCs are only legislated in England and The Netherlands, and in New Zealand 5 DWTCs have quality standards (Drew and Frangor, 2003). However, in South Africa and in some states in Australia, the EU and USA, the quality of the drinking water is legislated, thereby indirectly regulating the quality of the DWTCs. The World Health Organisation (2004, 2006) recommends that DWTC suppliers have their products certified by the National Standards Foundation. However, none of these international standards specify a minimum contaminant level for Mn. Current South African legislation relating to DWTCs does not give minimum contaminant levels of Mn.
Conclusions
This study has confirmed that the main source of Mn in the WTR produced from the Midmar WTP is the drinking water treatment chemicals, especially the brown lime, but that the volume of raw water treated also contributes markedly. The major issue that requires further investigation is consideration of the economics of utilising white lime with a low Mn concentration compared to the potential environmental risk (with associated economics) of continuing to use brown lime with elevated Mn concentrations. The increase in cost of white lime relative to brown lime is not likely to increase the cost of water to the consumer significantly, but there are other important reasons why white lime is not likely to be considered for purchase and use at WTPs. These relate primarily to the reliability and long-term availability of supply. South Africa's resources of white lime are rapidly diminishing (Mokaila, 2003), and its high purity makes it an extremely sought after product, especially for the food and beverage industry. In addition, there is no requirement to monitor Mn in either the DWTCs or WTR. Environmentally, Mn is usually not considered a major risk, which further reduces the justification for the increased cost (and associated supply risk) of changing to white lime in order to reduce concentrations of Mn in the WTR. Internationally, Mn is not considered an environmental problem since the risk it poses is perceived to be minimal. Thus, although the Mn level may be high in South African WTRs, whether it is of environmental concern should these residues be land treated, remains unclear.
Due to the diverse soil types and ecosystems within South Africa each application for land disposal of WTR should be considered independently, as natural concentrations of Mn vary throughout the country, and are controlled by a variety of physical and chemical parameters. Different ecosystems will respond differently to Mn loading. For these reasons, deriving a single national maximum permissible level for Mn within a WTR is possibly inappropriate, and, given the international perspective, probably unnecessary.
Acknowledgements
The authors would like to acknowledge funding from Umgeni Water for this project. Special thanks go to Zanele Hadebe, Nathi Sithole of Umgeni Water Laboratory Services for the ICP analysis, to TC Sithole for sample collection and Themba Mdlalose and Ashwin Singh of the Midmar WTP for data.
References
AUSTRALIAN EPA (2007) Interim guideline for the use of water treatment solids.URL: www.epa.sa.gov.au (Accessed 15 November 2007). [ Links ]
BASTA NT (2000) Examples and case studies of beneficial re-use of municipal by-products. In: Power JF and Dick WA (eds.) Land Application of Agricultural, Industrial and Municipal By-Product. ASA, CSSA and SSSA, Madison, WI. 492-504. [ Links ]
DREW R and FRANGOR R (2003) Overview of national and international guidelines and recommendations on the assessment and approval of chemicals used in the treatment of drinking water. URL: http://www.nhmrc.gov.au/ files nhmrc/publications/attachments/watergde.pdf (Accessed 17 February 2012). [ Links ]
ELLIOT HA and DEMPSEY BA (1991) Agronomic effects of land application of water treatment sludges. J. Am. Water Works Assoc. 84 126-131. [ Links ]
HOWE PD, MALCOLM HM and DOBSON S (2004) Manganese and its Compounds: Environmental Aspects. Concise International Chemical Assessment Document 63. World Health Organisation, Geneva. [ Links ]
HUGHES JC, SYNMAN HG, HERSLEMAN JE and NDORO ET (2007) A Scoping Report towards the Development of Guidelines for the Disposal of Water Treatment Residue to Land. WRC Report No. 1601/1/07. Water Research Commission, Pretoria, South Africa. [ Links ]
HUGHES JC, TITSHALL LW, BUYEYE M, JOHNSTON MA, MOODLEY M and PECKU S (2005) Effects of Land Application of Water Treatment Residue. WRC Report No. 1148/1/05. Water Research Commission, Pretoria, South Africa. [ Links ]
KABATA-PENDIAS A and PENDIAS H (2001) Trace Elements in Soils and Plants (3rd edn.). CRC Press, Boca Raton, Florida. [ Links ]
McBRIDE MB (1994) Environmental Chemistry of Soils. Oxford University Press, New York. [ Links ]
MOKAILA GE (2003) An overview of South Africa's primary industrial mineral imports and exports. Directorate: Mineral Economics (Minerals Bureau) Report R42/2003. Department of Minerals and Energy, Pretoria, South Africa. [ Links ]
NOVAK JM, SZOGI AA, WATTS DW and BUSSCHER WJ (2007) Water treatment residuals amended soils release Mn, Na, S, and C. Soil Sci. 172 (12) 992-1000. [ Links ]
OVERCASH MR and PAL D (1979) Design of Land Treatment Systems for Industrial Wastes - Theory and Practice. Ann Arbor Science Publishers Inc., Michigan. [ Links ]
ROSS SM (1994) Sources and forms of potentially toxic metals in soil- plant systems. In: Ross SM (ed.) Toxic Metals in Soil-Plant Systems. John Wiley and Sons Ltd, West Sussex, England. [ Links ]
SNYMAN HG and HERSELMAN JE (2006) Guidelines for the Utilisation and Disposal of Wastewater Sludge Volume 1: Selection of Management Options. WRC Report No. TT 261/06. Water Research Commission, Pretoria, South Africa. [ Links ]
STOKES PM, CAMPBELL PGC, SCHROEDER WH, TRICK C, FRANCE RL, PUCKETT KJ, LAZERTE B, SPEYER M, HANNA JE and DONALDSON J (1998) Manganese in the Canadian environment. NRCC Report No. 26193. National Research Council of Canada, Associate Committee on Scientific Criteria for Environmental Quality, Ottawa, Ontario. [ Links ]
TITSHALL LW and HUGHES JC (2005) Characterisation of some South African water treatment residues and implications for land application. Water SA 31 299-307. [ Links ]
USEPA (UNITED STATES ENVIRONMENTAL PROTECTION AGENCY) (2007) Effluent guidelines. Data needs for determining whether effluent guidelines for drinking water treatment facilities are warranted. URL: http://water.epa.gov/scitech/wastetech/guide/treatment/index.cfm (Accessed 17 February 2012). [ Links ]
WORLD HEALTH ORGANISATION (2004) Guidelines for Drinking-Water Quality (3rd edn.) Volume 1: Recommendations. World Health Organisation, Geneva. [ Links ]
WORLD HEALTH ORGANISATION (2006) Guidelines for Drinking-Water Quality (3rd edn.). First Addendum to Volume 1. World Health Organisation, Geneva. [ Links ]
Received 13 April 2012
Accepted in revised form 25 February 2013.
* To whom all correspondence should be addressed. Current affiliation: Institute for Commercial Forestry Research, PO Box 100281, Scottsville 3209, South Africa +27 33 386 2314; fax: +27 33 386 8905; e-mail: louis.titshall@icfr.ukzn.ac.za














