Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
Water SA
versão On-line ISSN 1816-7950
versão impressa ISSN 0378-4738
Water SA vol.39 no.2 Pretoria Jan. 2013
Assessment of locally available reactive materials for use in permeable reactive barriers (PRBs) in remediating acid mine drainage
Ayanda N Shabalala*
Council for Geoscience, Private Bag X112, Pretoria, 0001, South Africa
ABSTRACT
The management and treatment of contaminated mine water is one of the most urgent problems facing the South African mining industry. The cost advantage of permeable reactive barriers (PRBs) has seen their increased application as means of passively treating mine drainage. A PRB is built by placing a reactive material in the path of polluted groundwater. As the contaminant moves through the material, reactions occur that transform it into an environmentally acceptable form. Batch tests were carried out on limestone, dolomite, fly ash, concrete and wood chips to find a reactive material and/or reactive mixture, for use in a PRB, which can neutralise acidity, remove metals and is locally abundant. Batch tests involved the leaching of the materials in deionised water to determine the leachable component of each reactive material and the pH that each material could achieve in deionised water. The materials were also tested in acidic water (pH 5.54) to determine the effectiveness of each reactive material in removing contaminants. In terms of the ability to increase the pH, the top-performing reactive materials were limestone and fly ash as they both achieved a pH above 11. Limestone, concrete, fly ash and dolomite successfully removed at least 99% of the iron (Fe) from the mine water. Limestone and fly ash removed at least 99% of manganese (Mn) and magnesium (Mg) from the mine water. While the other reactive materials were ineffective in removing sulphate (SO42-), limestone and fly ash, respectively, removed 72% and 99.9 % of SO42- from the mine water. The study of 3 reactive material mixtures, namely: (i) limestone-wood chips-concrete, (ii) limestone-fly ash, and (iii) fly ash-concrete, showed that all 3 systems were effective in removing heavy metals present in the mine water. All of the mixtures increased the pH to above 11, increased the alkalinity and decreased Fe, Mn, and Mg concentrations to below the prevailing South African discharge criteria of wastewater into a water resource. Reactive Mixtures 2 and 3 successfully removed 99% of SO42- within 14 days. This study found that the most suitable reactive material for remediating acid mine groundwater was fly ash because it was able to neutralise acidity and remove Fe, Mn, and Mg and SO42-.
Keywords: acid mine drainage, permeable reactive barrier, batch tests, remediation
INTRODUCTION
The formation of acid mine drainage (AMD) and the contaminants associated with it have been described as the largest environmental problem facing the mining industry. Commonly referred to as acid rock drainage (ARD) or AMD, acid drainage from mine waste rock, tailings, and mine structures such as pits and underground workings is primarily a function of the mineralogy of the rock material and the availability of water and oxygen (USEPA, 1998). It is a naturally occurring process of weathering and erosion of sulphur and iron (Fe) bearing material, as well as other metallic sulphidic materials. It occurs when groundwater comes into contact with remnant coal and rock rich in sulphide. These sulphide minerals oxidise in the presence of water and oxygen, the by-product being a highly acidic, sulphate (SO42-) rich drainage (Fripp et al., 2000). The resulting drainage has a low pH and high metal concentration.
There are 4 commonly-accepted chemical reactions that represent the chemistry of pyrite weathering to form AMD (Ford, 2003):
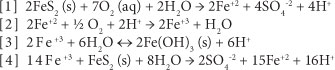
Remediation of acid drainage is difficult and expensive. Treatment falls under two broad categories: active and passive. Active treatment involves physically adding a neutralising agent to the source of the AMD, or directly to the stream that has been impacted. Active treatment can be very successful; however, it necessitates a long-term and continuous commitment to treatment. Weather, equipment failure, and budget reductions can result in lapses in treatment (Fripp et al., 2000).
Passive treatment encompasses a variety of techniques to raise the pH and reduce metal loadings through a constructed treatment or containment project. While initial costs for passive treatment techniques can be higher than active treatment, these systems do not require continuous chemical inputs and provide a controlled environment in which naturally occurring chemical and biological processes help in the treatment of AMD (Skousen, 1990). Permeable reactive barriers (PRBs) are passive, in-situ remediation systems which have seen increased application as means of treating contaminants in groundwater as a result of bacterial sulphate reduction and the subsequent precipitation of sparingly soluble sulphide solids (Waybrant et al., 1998). They have a cost/benefit ratio and the potential to mitigate the spread of contaminants that have proven difficult and expensive to manage with other clean-up methods (EPA, 1998).
Sulphate-reducing bacteria (SRB) convert SO42- to sulphide by catalysing the oxidation of organic carbon with the reduction of SO42-:

The reaction between the SO42- and the organic substrate consumes SO42-, results in the production of H2S and increases bicarbonate alkalinity and the pH. The sulphide produced reacts with dissolved metals (Me2+) and enhances the precipitation of metals as metal sulphides (Ludwig et al., 2002):
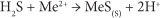
The ability of PRBs to remediate contaminants is dependent on the type of reactive material used. A suitable material must have the following characteristics (Golab et al., 2006; Waybrant et al., 1998; Pagnanelli et al., 2009; Gibert et al., 2004; Cocos et al., 2002):
• Increase the pH of the groundwater to a level that causes metals such as Fe and Al to precipitate out of solution
• Provide reactive sites for the precipitation to take place
• Have a permeability and effective porosity that allows groundwater to pass freely through the barrier
• Longetivity: with time, reactive materials may be consumed by reactions taking place and the reactive sites may become armoured by the precipitates that form
• Environmental compatibility: must not release toxins into the environment
• Must be abundant and low cost
The main contaminants in mine water are acidity, heavy metals and sulphates. This study discusses the laboratory application of locally available reactive materials for use in PRBs to remediate mine drainage. The selection of a suitable reactive material will be based on the results of batch tests (Golab et al., 2006).
Experimental
Mine water sample collection
A water sample was collected from the Black Reef incline (BRI) shaft or 18 winze shafts, near Krugersdorp (South Africa). The area is located near a uranium deposit and has been contaminated with radioactive elements and heavy metals as a result of mining and leaching activities carried out over a long period of time (Krige, 2006). A sample was collected and stored unfiltered in a 2 ℓ high-density polyethylene (HDPE) bottle, which was filled to capacity and capped without any air bubbles remaining in the container. The sample was chilled to between 3 and 5°C and despatched in a portable ice chest ('cooler box') to the laboratory. The pH and electrical conductivity (EC) of the acidic water were measured immediately after sample collection. Samples for metal and anion analysis were filtered into 100 mℓ polyethylene bottles using 0.45 µm syringe filters and stored at below 4°C. Samples for metal analysis were acidified with concentrated nitric acid (HNO3) before storage. This was followed by sample analysis by inductively coupled plasma-mass spectrometry (ICP-MS) and ion chromatography (IC).
Analysis of the reactive materials
The reactive materials analysed in this study included limestone, dolomite, fly ash, wood chips and crushed concrete. Characterisation of each of the materials used as a substrate in the batch studies included the determination of metal composition, pH, and mineral composition. For the analysis of metal composition, a 4-acid digestion method was used.
This method is suited to dissolve certain rock types, soils, and sediments. It uses a combination of nitric, hydrochloric, hydrofluoric and perchloric acid. Thereafter, the samples were analysed by ICP-MS and IC. The solid samples were also analysed by X-ray diffraction (XRD) in order to determine their mineral composition.
Batch Test 1: Reactive materials and deionised water analysis
Following the addition of 100 g of each of the reactive materials into 2-ℓ HDPE bottles, the bottles were filled with deionised (milliQ) water. The pH of the water in each bottle was measured every hour for the first half-day, then once a day for the first week and then after 14, 21 and 28 days. About 100 mℓ of the water sample was filtered into a polyethylene bottle using a 0.45 µm syringe filter after 24 h, 14, 21 and 28 days. One fraction of the water sample was acidified with concentrated nitric acid (HNO3) and analysed by ICP-MS while another fraction was analysed by IC. The results of these tests were used to determine the leachable component of each reactive material in neutral pH, deionised water.
Batch Test 2: Reactive materials and mine water
This test was done to determine the effectiveness of each reactive material in removing contaminants from the mine water collected from the Western basin, Black Reef incline (BRI) shaft or 18 winze shaft, which is affected by acid mine drainage. Into separate 750 ml glass jars, 55 g of wood chips and 125 g of limestone, dolomite, fly ash and concrete were weighed and the jars then filled with mine water. The pH of the water in each jar was measured every hour for the first half day, then once a day for the first week and then after 14, 21 and 28 days. Water samples were extracted from each of the glass jar, after 24 h and then after 14 and 21 days, into 100 mℓ HPDE bottles. One fraction of the sample of water was acidified with concentrated HNO3 and analysed by ICP-MS while another fraction was analysed by IC.
Batch Test 3: Reactive material mixtures and mine water
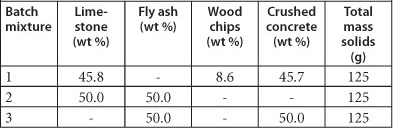
Three reactive mixtures were prepared as shown in Table 1. After the mixtures were added into 750 ml glass jars, the jars were completely filled with mine water and sealed. The pH of the water in each jar was measured every hour for the first half day, then once a day for the first week and then after 14, 21 and 28 days. Water samples were extracted from each of the glass jars, after 24 h and then after 14 and 21 days, into 100 ml HPDE bottles. One fraction of the sample of water was acidified with concentrated HNO3 and analysed by ICP-MS while another fraction was analysed using IC.
TABLE 1
Composition of the three batch reactive mixtures as dry weight percent (wt %)
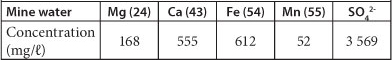
The results of the analysis of the mine water showed that the water contained very high concentrations of magnesium (Mg), calcium (Ca), Fe, manganese (Mn) and SO42-, as can be seen in Table 2. The water also had a pH of 5.54.
TABLE 2
Main contaminants in mine water from the Black reef incline mine shaft. The concentrations represent a single water sample.
Batch Test 1
The limestone and fly ash established a pH in deionised water that is above 11, while crushed concrete and dolomite established a pH that is above 8 (see Fig. 1). Wood chips, on the other hand, fared poorly, with a pH below 6.5 initially, lowering to between pH 5 and pH 4 over the period of observation. Limestone, fly ash and concrete contain calcium hydroxide (Ca(OH)2), calcium carbonate (CaCO3) and other alkaline components, as can be seen in Table 3, which upon dissolution increases the pH.
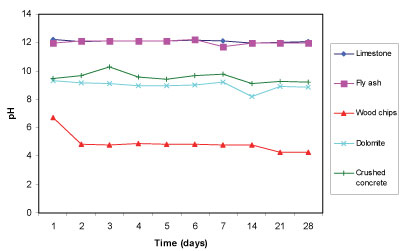
Figure 1
Change in pH with time for the 5 reactive materials in deionised water for a total of 28 days
TABLE 3
XRD analysis of 5 samples (expressed in wt %)
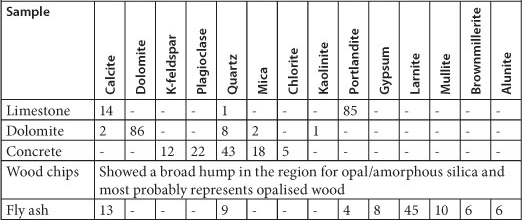
Limestone and fly ash released considerable amounts of Ca, sodium (Na), potassium (K) and strontium (Sr), as well as trace amounts of barium (Ba) and rubidium (Rb). Limestone is made up mostly of the mineral calcite (CaCO3) and portlandite, which is a form of calcium hydroxide (Ca(OH)2). Fly ash is composed of 45% larnite (2CaO-SiO2) as well as traces of calcite and portlandite (Table 3). Therefore, both materials have considerable amounts of Ca, which explains the elevated Ca concentration. Fly ash also released high levels of Al, K and Ba, which are typically found in fly ash. Potassium was possibly leached from K-feldspars and allunite (KAl3(SO4)2(OH)6) found in concrete and fly ash, respectively, while Na, Ba and Rb came from micas and Sr from carbonates. Dolomite released high quantities of Mg and Ca as it is a carbonate mineral composed of calcium magnesium carbonate CaMg(CO3)2. Out of all the reactive materials, the wood chips released the highest concentrations of Mg, Ca, Fe, Mn and SO42-.
Batch Test 2
The pH of the mine water was measured to be 5.54. Figure 2 displays the pH of the supernatant for 5 reactive materials 28 days after the commencement of the batch test aimed at determining how well each of the reactive materials can increase the pH of the mine water. Limestone and fly ash successfully increased the pH of the mine water to above 11. The high pH is likely to be caused by the lime content present in both limestone and fly ash. Limestone is also dominated by calcite, which has been found to be effective in raising pH. Dolomite raised the pH of the water to 6.66. Wood chips and crushed concrete performed poorly in terms of raising the pH as they dropped the pH values to 4.5 and 3.5, respectively.
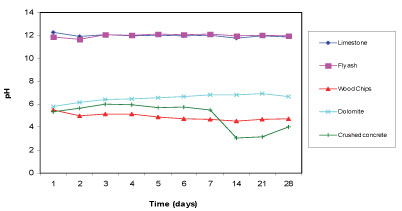
Figure 2
Change in pH with time for the 5 reactive materials in mine water for a total of 28 days
The batch tests, aimed at determining how well each of the reactive materials can remove the elements of concern in the mine water, showed that limestone, concrete, fly ash and dolomite successfully removed at least 99% of the Fe from the mine water. Limestone and fly ash removed at least 99% of Mn and Mg from the mine water. While the other reactive materials were ineffective in removing SO42-; limestone and fly ash, respectively, removed 72% and 99.9% of SO42- from the mine water. Similar to the results of the leachate test, some of the elements were elevated in the batch test. Na and Ca were elevated in tests with all the reactive materials. K, Ba and Sr were elevated in the limestone and fly ash tests. Mg was elevated in the concrete and dolomite tests, while Al was increased slightly in the concrete and fly ash tests. A possible explanation for the elevated elements in the water is that some of them are leached from the reactive materials. Materials made up of ferromagnesian minerals are characterised by high Mg, those from feldspars by K and Ba, and those from carbonates by Ca, Mg and Sr. Al is a key component of both concrete and fly ash. The wood chips failed to remove any elements and instead contributed to their increased concentrations. It is therefore not suitable for use as a reactive material. Most of the elements were measured at concentrations below the limits applicable to discharge of wastewater into a water resource (Table 4). Figure 3 displays the concentration of Fe, Mn and SO42- of the supernatant for 5 reactive materials, 21 days after the commencement of the batch test aimed at determining how well each of the reactive materials can increase the pH of the mine water.
TABLE 4
Discharge limits and conditions of wastewater into a water resource
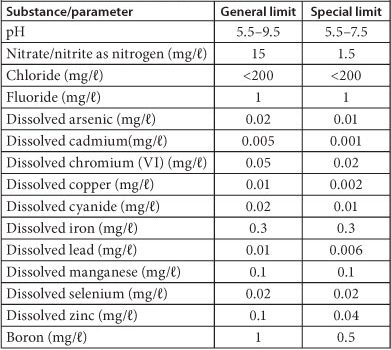
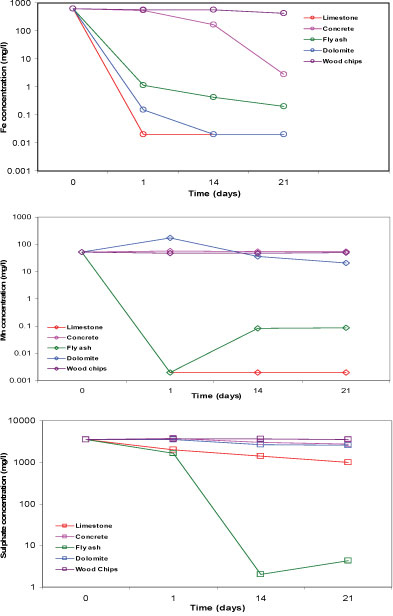
Figure 3
Concentration of iron, manganese and sulphate in the supernatant of 5 reactive materials after 21 days in batch tests using mine water
After the mine water (pH= 5.54) was added to the three reactive mixtures, the pH of the supernatant was measured for 28 days. In terms of ability to increase pH, all three reactive mixtures successfully raised the pH to above 11 (Fig. 4). The three reactive mixtures are dominated by calcite and portlandite, which are assumed to be responsible for increasing alkalinity and raising the pH. Calcite is found in limestone, dolomite and fly ash while portlandite is a constituent of limestone and fly ash.
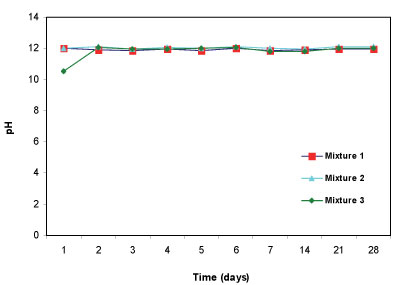
Figure 4
Change in pH with time for 3 batch mixtures in mine water for a total of 28 days
Sulphate-reducing conditions were developed within 14 days in Mixtures 2 and 3, which led to the removal of 99.9% of SO42-. Both mixtures contained fly ash which is assumed to be responsible for the rapid removal of the SO42-. About 63% of SO42- removal was achieved in Mixture 1 containing limestone and crushed concrete, as the SO 2- concentration was decreased from 3 569 mg/£ to 1 288 mg/ℓ. All three mixtures successfully removed more than 99% of Fe, Mn and Mg. On the other hand, an elevation of some of the elements was observed in all three mixtures; Na, K, Ca, Sr and Rb, and Ba, Li and Al were slightly elevated in Mixtures 1 and 3, respectively. This was caused by the leaching out of these elements from the reactive materials. Figures 5, 6 and 7 indicate trends in concentrations of SO42-, Fe, and Mn, respectively, over an observation period of 21 days for the three batch mixtures for mine water. Most of the elements were measured at concentrations below the limitsapplicable to discharge of wastewater into a water resource.
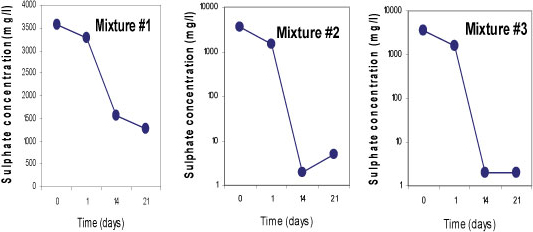
Figure 5
Concentrations of sulphate for 3 batch mixtures in mine water for a total of 21 days
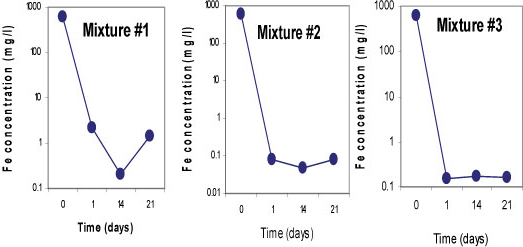
Figure 6
Concentrations of iron for 3 batchmixtures in mine waterfor a total of 21 days
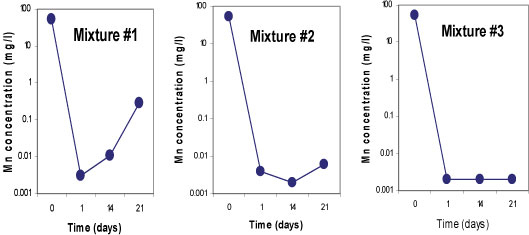
Figure 7
Concentrations of manganese for 3 batch mixtures in mine water for a total of 21 days
CONCLUSION
In this study individual solid reactive materials as well as mixtures of the reactive materials were tested as filling material for biological permeable reactive barriers (PRBs) that can be used in the treatment of heavy metal contamination. The results of the analysis of the mine water collected from Black Reef incline (BRI) shaft near Krugersdorp, South Africa, showed that the water contained very high concentrations of Mg, Ca, Fe, Mn and SO42-. The performance of the reactive materials was determined based on their ability to neutralise acidity and remove metals and SO42- found in the mine water. The batch tests performed on limestone, concrete, fly ash, dolomite and wood chips showed that, in terms of the ability to increase the pH, the top-performing reactive materials were limestone and fly ash, as they both achieved a pH above 11. The mineralogy of both materials is dominated by calcite and calcium hydroxide, which are known to be effective in raising the pH. Limestone, concrete, fly ash and dolomite successfully removed at least 99% of the Fe from the mine water. Limestone and fly ash removed at least 99% of Mn and Mg from the mine water. While the other reactive materials were ineffective in removing SO42-, limestone and fly ash, respectively, removed 72% and 99.9% of SO42- from the mine water. Some of the elements were elevated in the batch tests, for example: Ca and Sr, due to calcium carbonate and calcium hydroxides abundantly available in limestone and fly ash; Mg, from dolomite; K, possibly leached from K-feldspars and allunite found in concrete and fly ash; and Na, Ba and Rb from mica which is a constituent of dolomite and concrete.
Three reactive material mixtures were studied: (i) limestone-wood chips-concrete, (ii) limestone-fly ash, and (iii) fly ash-concrete. The results from the study showed that all three systems were effective in removing heavy metals that were present in the mine water. All the mixtures increased the pH above 11, increased the alkalinity and decreased Fe, Mn, and Mg to below the South African discharge criteria. The SO42- concentration was decreased from 3 569 mg/ℓ to 2 mg/ℓ in Mixtures 2 and 3 within 14 days. Both mixtures contained fly ash which is assumed to be responsible for the rapid removal of the SO42-. The decrease in concentrations of some of the elements was accompanied by an increase in the concentrations of Na, K, Ca, Sr and Ba in all the mixtures, while Al was elevated in Mixture 3. The mineral composition of the reactive materials contributed to an increase of the abovementioned metals.
The worst-performing reactive material was determined to be the material comprising wood chips as it did not neutralise acidity. Beside achieving insufficient removal of Fe, Mn and SO42-, it also released many toxins in the water which led to an increase in the concentration of elements such as B, Mg, K, Ca and Sr. Dolomite achieved varying success. It significantly reduced some of the contaminants such as Fe and Mn but the concentration of SO42- still remained high and did not meet the water quality requirements. Concrete was ineffective in remediating the acidic water with Fe, Mn and SO42- still remaining significantly high.
This study found that the most suitable reactive materials for remediating acid groundwater were fly ash and limestone, because they are:
• Locally abundant
• Capable of neutralising acidity and removing Fe, Mn, and Mg from the groundwater
• Fly ash effectively removed 99.9% of the sulphates from the mine water while limestone achieved 72% removal
Possible problems that could arise from using fly ash and limestone in permeable reactive barriers (PRBs) include the following:
• They are both fine-grained, which could lead to problems with clogging
• Both contain leachable substances which could be released into the environment
• Fly ash tends to harden with time and this would affect the flow through the barrier
More laboratory work is required to determine the performances of the reactive materials over longer periods of time and to monitor the risks associated with leachable toxic substances from the materials which could be released into the environment.
Other possible reactive materials and combinations of the reactive materials need to be studied in order to find the optimal media or materials that could be applied in permeable reactive barriers (PRB) systems for the remediation of contaminants.
After the batch tests are completed, column tests should be conducted, which will simulate the natural flow conditions through the PRB. The result of these tests will assist in the design of the PRB.
Acknowledgements
The author would like to thank the Council for Geoscience in Pretoria, South Africa, for funding the project.
References
CZURDA KA and HAUS R (2000) Reactive barriers with fly ash zeolites for in situ groundwater remediation. Appl. Clay Sci. 21 13-20. [ Links ]
COCOS LA, ZAGURY GJ, CLEMENT B and SAMSON R (2002) Multiple factor design for reactive mixture selection for use in reactive walls in mine drainage treatment. Water Res. 32 167-177. [ Links ]
CLYDE EJ, CHAMPAGNE P and JAMIESON HE (2010) The use of passive treatment alternatives for the mitigation of acidic drainage at the Williams Brother mine, California: Bench-scale study. Appl. Geochem. 25 958-971. [ Links ]
DWAF (DEPARTMENT OF WATER AFFAIRS AND FORESTRY, SOUTH AFRICA) (1999) General Authorisations in terms of Section 39 of the National Water Act, 1998 (Act No. 36 of 1998). Government Gazette No. 20526, 8 October 1999. DWAF, Pretoria. URL: http://www.dwaf.gov.za/Documents/Notices/GenAuthPublished-eng.pdf. (Accessed 09-01-2012). [ Links ]
FRIPP J, SIENKIEWICZ PF and CHARKAVORKI H (2000) Acid mine drainage treatment. EMRRP Technical Notes Collection (ERDC TN-EMRRPSR-14). U.S. Army Engineer Research and Development Center, Vicksburg. [ Links ] FORD KL (2003) Passive treatment systems for acid mine drainage. Technical Note 409, Bureau of Land Management Web based report, Colorado. URL: www.blm.gov/nstc/library/pdf/TN409.PDF (Accessed 19-08-2011). [ Links ]
GIBERT O, DE PABLO J, CORTINA JL and AYORA C (2004) Chemical characteristics of natural organic substrates for biological mitigation of acid mine drainage. Water Res. 38 4186-4196. [ Links ]
GOLAB AN, PETERSON MA and INDRARATNA B (2006) Selection of potential reactive materials for a permeable reactive barrier for remediating acidic groundwater in acid sulphate soil terrains. J. Eng. Geol. Hydrogeol. 39 209-223. [ Links ]
KOMNITSAS K, BARTZAS G and PASPALIARIS L (2004) Efficiency of limestone and red mud barriers: laboratory column studies. Miner. Eng. 17 183-194. [ Links ]
KRIGE WG (2006) Hydrological/chemical aspects of the Tweelopie/Riet/Blaauwbankspruit, with specific reference to the impact water, decanting from the western basin mine void, has on this system. Unpublished report, African Environmental Development. [ Links ]
LUDWIG RD, MCGREGOR RG, BLOWES DW, BENNER SG and MOUNTJOY K (2002) A permeable reactive barrier for treatment of heavy metals. Ground Water 1 (40) 59-66. [ Links ]
PAGNANELLI F, VIGGI CC, MAINELLI S and TORO L (2009) Assessment of solid reactive mixtures for the development of biological permeable reactive barriers. J. Hazardous Mater. 170 998-1005. [ Links ]
RIOS CA, WILLIAMS CD and ROBERTS CL (2008) Removal of heavy metals from acid mine drainage (AMD) using coal fly ash, natural clinker and synthetic zeolites. J. Hazardous Mater. 156 23-35. [ Links ]
SKINNER SJW and SCHUTTE CF (2000) The feasibility of a permeable reactive barrier to treat acidic sulphate and nitrate contaminated groundwater. Water SA 32 (2) 129-135. [ Links ]
SKOUSEN J (1990) Overview of passive systems for treating acid mine drainage. West Virginia University, West Virginia. URL: www.wvu.edu/~agexten/landrec/passtrt/passtrt.htm. (Accessed 22-10-2011). [ Links ]
SCHMIDT KL and SHARPE WE (2002) Passive treatment methods for acid water in Pennsylvania. Penn State College of Agricultural Sciences, Pennsylvania. [ Links ]
USEPA (UNITED STATES ENVIRONMENTAL PROTECTION AGENCY) (1994) Acid mine drainage prediction. USEPA, Washington, DC. URL: http://water.epa.gov/polwaste/nps/upload/amd.pdf (Accessed 15-02-2012). [ Links ]
USEPA (UNITED STATES ENVIRONMENTAL PROTECTION AGENCY) (1998) Permeable reactive barrier technologies for contaminant remediation. USEPA, Washington, DC. 1 pp. [ Links ]
WAYBRANT KR, BLOWES DW and PTACEK CJ (1998) Selection of reactive mixtures for use in permeable reactive walls for treatment of mine drainage. Environ. Sci. Technol. 32 1972-1979. [ Links ]
YOUNGER PL (2000) The adoption and adaptation of passive treatment technologies for mine waters in the United Kingdom. Mine Water Environ. 19 84-97. [ Links ]
Received 30 June 2012
Acepted in revised form 28 March 2013.
* To whom all correspondence should be addressed. +27 11 841 1170; Fax: 0866798441; e-mail: ashabalala@geoscience.org.za














