Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Water SA
On-line version ISSN 1816-7950
Print version ISSN 0378-4738
Water SA vol.39 n.1 Pretoria Jan. 2013
SHORT COMMUNICATION
The effect of ultrasound at 256 KHz on Microcystis aeruginosa, with and without gas vacuoles
Silke JachlewskiI; Marelize BotesII, *; T Eugene CloeteII
IDepartment of Microbiology and Plant Pathology, University of Pretoria, 0011, Pretoria, South Africa
IIDepartment of Microbiology, Stellenbosch University, 7600, Stellenbosch, South Africa
ABSTRACT
The effect of ultrasound on the growth of M. aeruginosa confirmed to contain gas vacuoles and on a laboratory culture with no gas vacuoles was investigated. Both cultures were treated continuously for 9 d with an ultrasonic flow device. To evaluate the influence of ultrasound during the treatment, the chlorophyll-a concentration was measured daily. Furthermore, changes in culture characteristics, e.g., flotation and gas vesicle formation, were determined. The results showed that, in contrast to the control, both ultrasonic-treated cultures had a lower chlorophyll-a concentration and cell aggregates were disrupted. Transmission electron microscopy confirmed a collapse of gas vacuoles in the environmental culture, while the laboratory culture, which did not contain gas vacuoles, showed many membrane-damaged cells. It was concluded that ultrasonic treatment of M. aeruginosa caused the disruption of gas vacuoles and destruction of cell membranes.
Keywords: Ultrasound, cyanobacteria, Microcystis aeruginosa, chlorophyll, gas vacuoles
Introduction
Recent studies have shown that the application of ultrasound to a sample of cyanobacteria resulted in a reduction of chlorophyll-a concentration and cell number, due to the breaking up of cyanobacterial gas vacuoles, thus disabling the cyanobacteria and preventing their floating to the surface to absorb light-energy needed for photosynthesis (Zhang et al., 2006).
An increase in power input from 32 W to 80 W resulted in an increase in the release of microcystin; however, use of different ultrasound frequencies, of 20 kHz, 80 kHz and 150 kHz, showed limited impact on microcystin release. A lower energy consumption of 0.134 kWh was needed to remove 90% cells at 80 W, in comparison to 0.175 kWh at 32 W. A big disadvantage, despite the possible release of toxins, is the fast regeneration of cells after interruption of sonication. This occurrence is also influenced by frequency, power input and duration of treatment. It was shown that ultrasonic irradiation with 1.7 MHz for 5 min inhibits the growth of the cyanobacterium Spirulina platensis for 3 d, while a sonication with 20 kHz showed no constant effect (Hao et al., 2004).
Several mechanisms were discovered by which ultrasonic treatment affects the cells, of which the collapsing of gas vacu-oles during cavitation was considered to be the main mechanism. The presence of gas vacuoles leads to a decrease in cell density, making the cell less dense than water. As a result the cell can migrate vertically and reach upper water layers with better light conditions, thus leading to a higher rate of photosynthesis (Oliver, 1981). Other mechanisms by which ultrasonic treatment affects cyanobacterial cells are the inhibition of cell division, damage to photosynthetic activities and cell lysis (Zhang et al., 2006).
The objective of this study was to investigate the effect of ultrasound on two different cultures of Microcystis aeruginosa, a laboratory culture lacking gas vacuoles and an environmental culture containing gas vacuoles.
Materials and methods
Cultivation of Microcystis aeruginosa
The laboratory culture of M. aeruginosa was obtained from the University of Pretoria culture collection and the environmental culture was collected in sterile Falcon tubes at the Hartbeespoort Dam (HB Dam), Pretoria, and immediately taken back to the laboratory to be cultured. Both cultures were cultivated in a 5 l Erlenmeyer flask containing 3 l of BG 11 culture medium in an incubator (continuous light of 2 000 lux provided by 18 W cool white fluorescent lamp, 24 ± 2°C). Before the start of the ultrasound experiment 500 ml of this culture was transferred into bigger plastic basins, each containing 35 l BG 11, and cultivated for a further 4 d in a greenhouse with an average temperature of 26°C and environmental light conditions (sunrise: approximately 06:55, sunset: approximately 17:30). During these 4 days the medium was stirred twice a day, manually. Immediately before the onset of an experiment the chlorophyll-a concentration was measured and adjusted to be the same in both basins.
Ultrasonic treatment
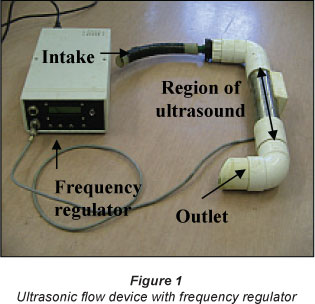
For the ultrasonic treatment, an ultrasonic flow device was used (Fig. 1). The water was circulated through it at a constant flow rate of 15.4 l/min. The frequency used was 256 KHz. For both cultures, a control culture was prepared. All basins were manually stirred twice a day to detach cells attached to surfaces.
Evaluation of cyanobacteria properties: Floating ability
To determine whether the cultures were capable of floating a small amount of sample was transferred into a 30 ml glass flask containing BG 11 medium. After 15 min, the position of the cells was visually recorded. The floating ability was measured before the treatment, after 9 d and after 14 d.
Transmission electron microscopy analysis
A transmission electron microscope (TEM; Philips EM301, Netherlands) was used to assess the presence or absence of gas vacuoles before and during the treatment. A standard technique was used for preparing samples and doing electron microscopy (Wagner, 2001).
Results
Determination of cyanobacterial properties: Floating ability
When the cyanobacteria were transferred into McCartney bottles, the M. aeruginosa laboratory culture settled to the bottom within a few minutes, while the culture from the HB Dam continued floating, except for a few single cells that settled out. The HB Dam culture did however settle to the bottom after 9 d and 14 d. Figure 2a shows gas vacuoles in the M. aeruginosa HB Dam culture. In contrast to this, Fig. 2b shows a cell of the laboratory culture that did not contain any gas vacuoles. Several additional cells of both cultures were investigated and showed the same structural characteristics. In addition the HB Dam cells showed several granules, most likely phosphate granules and crystalline bodies, while the laboratory culture cell showed no granules.
Effect of ultrasound on the M. aeruginosa cultures
To examine the influence of ultrasound on the M. aeruginosa HB Dam culture, one basin was treated with ultrasound continuously for 9 d. During the experiment the cyanobacteria of the control, which was not treated with ultrasound, were floating on the water surface. From 6 d onwards some cyanobacteria in the control settled to the bottom. Their colour started to change from deep green to brown, which indicated a lack of chlorophyll-a. In the ultrasonic-treated basin the cellular aggregates, which were observed before the start of the experiment, disappeared after 1 day and no floating was noticeable in this basin anymore (Fig. 3). The experiment was repeated with the M. aeruginosa laboratory culture. During the experiment no cells were buoyant.

Chlorophyll-a concentration before and after ultrasonic treatment
The chlorophyll-a concentration was measured to determine the photosynthetic activity of M. aeruginosa. The concentration of chlorophyll-a in the control HB Dam culture increased continuously from about 60 ng/l at the start of the experiment to about 350 ng/l at Day 9 (Fig. 4). In the treated basin a small decrease of chlorophyll-a was observed during the first day in the treated HB Dam culture, which was followed by an increase to about 107 ng/l after 4 d. During the remaining days the concentration remained nearly constant. It was therefore concluded that the ultrasonic treatment had an influence on M. aeruginosa, resulting in inhibition of chlorophyll-a formation and thereby also growth of the M. aeruginosa culture. The chlorophyll-a concentration of the laboratory culture control increased during the experiment, from 75.78 (ig/l on Day 1 to 560 (ig/l after 9 d (Fig. 4). When the laboratory culture was treated with ultrasound the chlorophyll-a concentration decreased from 107.36 (ig/l to 69.47 (ig/l over the experimental period. This demonstrated that ultrasonic treatment also had an effect on cells that did not contain gas vacuoles. The collapse of gas vacuoles was therefore not the only cause of inhibition of M. aeruginosa growth under laboratory conditions. After 9 d the ultrasonic treatment was terminated and the M. aeruginosa cultures were tested for 4 d to determine regeneration of the cultures. The chlorophyll-a concentration of the laboratory culture increased from 69.47 (ig/l to 189.45 (ig/l after 4 d, and the HB Dam culture from 107.36 ig/l to 249.44 ig/l (Fig. 4).

Effect of ultrasound on inner cell structures
After ultrasonic treatment for 9 d, samples of the control and of the treated samples were prepared for transmission electron microscopy (TEM). In contrast to the control the cells of the treated HB Dam sample showed no gas vacuoles (Fig. 5a). Instead, long, thin, dark, membrane-like structures could be observed, which may represent membranes of collapsed gas vacuoles (Fig. 5b). The transmission electron microscopy (TEM) images for the laboratory culture showed that cell membranes were ruptured after the treatment (Figs. 6a and 6b). This was not observed in the HB Dam culture.
Discussion
Ultrasound treatment is thought to mainly disrupt gas vacuoles, but could have further influences on cyanobacteria. Therefore the laboratory and environmental cultures of M. aeruginosa, which differ in the presence versus absence of gas vacuoles, were ideally suitable for testing the efficiency of ultrasound treatment.
TEM analysis of the two cultures confirmed that the HB Dam culture contained gas vacuoles and the laboratory culture did not. This can be explained by the different origins of the samples. The environmental culture of M. aeruginosa was sampled from the HB Dam, which has an average depth of 9.6 m. Due to this, non-buoyant cyanobacteria, which would settle to the bottom of the lake, would receive insufficient light for photosynthesis. Therefore, the formation of gas vacuoles is a required adaptation of cyanobacteria to environmental conditions. In contrast, the laboratory culture was stored in an Erlenmeyer flask filled with BG 11 medium in an incubator and received all growth requirements. These cells, therefore, did not need to adapt to special environmental conditions such as the necessity to float.
Compared with the respective control, after 9 d of treatment the HB Dam culture showed a 3.3 times lower chlorophyll-a concentration, while the laboratory culture showed a 8.2 times lower concentration. This showed that the efficiency of ultrasound was 2.5 times higher for the laboratory culture than for the HB Dam culture. Cyanobacterial cells were, however, able to regenerate after ultrasound treatment was terminated. The chlorophyll-a concentrations in both treated samples increased similarly to their respective controls during the first days of the experiment. After the treatment with ultrasound the HB Dam culture lost the gas vacuoles. This confirms the results of other studies (Ahn et al., 2003; Hao et al., 2004) which have indicated that ultrasonic treatment caused a collapse of gas vacuoles. Instead of gas vacuoles, dark membrane-like structures were observed in the treated samples and these were suspected to be the vestiges of the collapsed gas vacuoles.
The treated sample of the laboratory culture showed a high incidence of membrane-damaged cells, which was not observed in the treated Dam culture. This confirmed that ultrasonic treatment can cause damage to the outer cell membrane. Damage to the outer cell membrane as a result of ultrasound treatment has been reported before (Ahn et al., 2003). However, no prior study evaluated the influence on a culture of M. aeruginosa without gas vacuoles. Therefore, this study highlights that the range of application of ultrasonic treatment extends beyond cyanobacteria with gas vacuoles. Cell lysis caused a decrease of the chloro-phyll-a concentration within the first day.
Conclusion
In conclusion, ultrasound was effective against algae without gas vacuoles. Outer membrane damage was more obvious in the laboratory culture, which did not contain gas vacuoles, than in the HB Dam cultures containing gas vacuoles. Moreover the laboratory culture, despite growing better under experimental conditions than the HB Dam culture, was more sensitive to ultrasound than the natural HB Dam culture.
Acknowledgements
Special thanks are extended to Allan Hall and Chris van der Merwe for helping to obtain the microscopy pictures.
References
AHN C-Y, PARK M-H, JOUNG S-H, KIM H-S, JANG K-Y and OH H-M (2003) Growth inhibition of cyanobacteria by ultrasonic radiation: Laboratory and enclosure studies. Environ. Sci. Techno!. 37 (13) 3031-3037. [ Links ]
HAO H, WU M, CHEN Y, TANG J and WU Q (2004) Cyanobacterial bloom control by ultrasonic irradiation at 20 kHz and 1.7 MHz. J. Environ. Sci. Health 39 (6) 1435-1446. [ Links ]
OLIVER RL, KINNEAR AJ and GANF GG (1981) Measurements of cell density of three freshwater phytoplankter by density gradient centrifugation. Limnol. Oceanogr. 26 (2) 285-294. [ Links ]
WAGNER AM (2001) Morphological and molecular identification of filamentous microorganisms associated with bulking and foaming activated sludge. M.Sc. thesis, University of Pretoria, South Africa. [ Links ]
ZHANG G, ZHANG P, WANG B and LIU H (2006) Ultrasonic frequency effects on the removal of Microcystis aeruginosa. Ultrasonics Sonochem. 13 (5) 446-450. [ Links ]
Received 19 January 2012;
Accepted in revised form 31 October 2012.
* To whom all correspondence should be addressed. ffi +27 21 808 2708; fax: +27 21 808 3680; e-mail: mbr@sun.ac.za














