Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
Water SA
versión On-line ISSN 1816-7950
versión impresa ISSN 0378-4738
Water SA vol.39 no.1 Pretoria ene. 2013
Sorption of toxic metal ions in aqueous environment using electrospun polystyrene fibres incorporating diazole ligands
Godfred DarkoI; Samuel ChigomeI; Stacy LillywhiteII; Zenixole TshentuI; James DarkwaII; Nelson TortoI, *
IDepartment of Chemistry, Rhodes University, PO Box 94, Grahamstown 6140, South Africa
IIDepartment of Chemistry, University of Johannesburg, PO Box 524, Auckland Park 2006, South Africa
ABSTRACT
Electrospun polystyrene fibres incorporating potassium salts of pyrazole-l-carbodithioate and imidazole-l-carbodithioate were employed as sorbents for heavy metals from aqueous environments. The equilibrating time, initial metal concentrations and sorbent mass for optimal adsorption were 40 min, 5 mg/l and 8 mg, respectively. The optimal pH for metal ion uptake was between 6.3 and 9.0 and was found to be dependent on the basicity of the ligands. Protonation constants for the ligands in aqueous solutions were determined potentiometrically; pK of the imidazole was 6.82 while that of the pyrazole was 3.36. The efficiencies of adsorption and desorption of metals on the imidazolyl-incorporated sorbents were more than 95%, up to the fifth cycle of usage. The limits of quantification were < 0.0145 mg/l for all the metals. Accuracy of the determinations, expressed as relative error between the certified and observed values of certified reference groundwater samples was < 0.2% with relative standard deviations < 3%. Electrospun polystyrene fibres incorporating imidazoles proved to be efficient sorbents for divalent heavy metal ions in aqueous environments as their efficiencies exceeded those of chitosan microspheres, ion-imprinted composites, amino-functionalised mesoporous materials and most of the biomass-based sorbents previously reported on.
Keywords: electrospinning, polystyrene, heavy metals, diazole
Introduction
Heavy metal ions are widespread in the human environment and very often occur, at low concentrations, in surface waters (Akoto et al., 2008). Although their concentrations in environmental samples are low, heavy metal ions tend to bio-accumulate through the food chain (Gundacker, 2000; Pourang, 1995) and exert various health effects in humans and animals. The direct determination of these metal ions in water samples has proven to be a challenge as their concentrations are usually too low for accurate detection on many analytical instruments (Mohammadi et al., 2010). There is therefore the need for a pre-concentration step to bring the concentrations of the ions to detectable levels for accurate measurements. The current practice of acid-digesting water samples prior to analysis is however cumbersome and renders the samples susceptible to cross-contamination, due to the multiple steps they are taken through. Therefore, a sample-handling methodology that allows for on-site sampling as well as enrichment of the analyte is preferred. Such a method reduces the number of analytical procedures associated with the handling of aqueous samples. For example, there will be no need for transporting large volumes of water samples from the sampling site (typically, 1 l per site) to the laboratory. The acid-digesting step will also be eliminated.
The ability of resins (Zhang et al., 2009; Vasiliev et al., 2009), porous materials (Heidari et al., 2009; Aguado et al., 2009), bentonite (Mishra and Patel, 2009), alumina (Ezoddin et al., 2010), biomass (Gundogdu et al., 2009; Hamissa et al., 2010; Mapolelo et al., 2005; Miretzky et al., 2010; Sari and Tuzen 2009; Tang et al., 2010), sediments (Oh et al., 2009), substituted naphthalene (Rezaei et al., 2009), metal hydroxides (Bologo et al., 2012) and natural clinoptilolites (Mamba et al., 2009) to adsorb heavy metal ions from aqueous solutions has been reported. Most of the reported sorbents exhibited low loading capacities as well as sensitivities. In addition, some of them are not reusable (Zaman et al., 2009).
Sorbents developed from electrospun fibres have the potential to overcome these limitations as the fibres could be functionalised or incorporate moieties that can enhance their sorption abilities (Anderson and Long 2010; Zhang et al., 2009; Zhao et al., 2006). Electrospun polymer fibres with incorporated carbodithioate ligands, for example, can exhibit excellent adsorption capabilities owing to the strong affinity of their het-eroatoms for metal ions (Change and Chen 2005; Rashchi et al., 2004; Qu et al., 2005; Samal et al., 2000). As bidentate ligands, the carbodithioates coordinate strongly with metal ions through their nitrogen and sulphur atoms (Bogdanovic et al., 2005). They could also act as bridging polydentate ligands when they have donor groups (Mukhopadhyay et al., 2001; Koysal et al., 2005; Szécsényi et al., 2006). Moreover, the benzene ring of the polymer can hold the ligand in place through hydrogen bonds (Tretinnikov, 2000; Vogler, 1998).
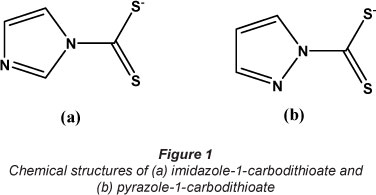
By virtue of their large specific surface areas and high porosities, the electrospun fibres have higher loading capacities for metal ions (Teo and Ramakrishna, 2006). The metal ions adsorbed could be released for quantification by placing the sorbent in an acidic solution. The desorbed sorbent could be generated for reuse after washing in ultrapure water and drying. The carbodithioates are stable in both acidic and basic conditions (Anderson and Long, 2010). The activity of the ligand could therefore be preserved during use. Electrospun polymer fibres incorporating carbodithioate ligands are, therefore, seen as a potential sorbent for sorption of metal ions in aqueous environments prior to their determination. In this study, polystyrene fibres incorporating pyrazole-l-carbo-dithioate and imidazole-l-carbodithioate were employed as sorbents for removal of heavy metal ions in aqueous environments. The sorbents were also assessed for their abilities to desorb the adsorbed metals back into solutions with the view of using them (as sorbents) for sample preparation prior to metal analysis. To the best of our knowledge, this is the first time that electrospun polymer fibres incorporating carbodithioates have been employed as a sorbent for adsorptive enrichment of heavy metals. Fig. 1 shows the chemical structures of (a) imidazole-l-carbodithioate and (b) pyrazole-l-carbodithioate.
Experimental
Materials
Polystyrene (Mw = 192 000), nitrate salts of Cu(II), Ni(II), Co(II), Cd(II) and Pb(II), all of purity more than 99.0%, were purchased from Sigma-Aldrich (St. Louis, USA). Tetrahydrofuran (THF, 98%) and N,N-dimethylformamide (DMF, 99%) were purchased from Merck Chemicals (Wadesville, South Africa). All the chemicals were of analytical grade and were used without any further purification. All glassware was soaked overnight in 4 M HNO3 solution prior to use. Standard solutions were freshly prepared using ultrapure water generated from MilliQ systems (Massachusetts, USA). All solutions used in the potentiometric titrations were prepared using freshly-boiled, degassed ultrapure water to ensure the removal of carbon dioxide and oxygen.
Instrumentation
Infrared spectra (400-4 000 cm-1) were recorded on a PerkinElmer FT 100 spectrometer (Massachusetts, USA), equipped with a universal ATR sampling accessory. 1H and 13C NMR spectra of the ligands were recorded on a Bruker AMX 400 NMR MHz spectrometer (Zurich, Switzerland) and reported relative to tetramethylsilane (δ=0.00). Microanalyses of the ligands were carried out using a Vario Elementar Microcube ELIII (New Jersey, USA). The morphology of the fibres was studied using the Tescan (TS5136ML) scanning electron microscope (Brno, Czech Republic), operating at an accelerated voltage of 20 kV after gold sputter coating. The pH of the solutions was determined using the Jenway (3510) pH meter (Essex, UK). Concentrations of metals were determined using an iCAP 6000 series inductively coupled plasma-optical emission spectrometer (ICP-OES) from Thermo Electron Corporation (Cheshire, United Kingdom). Potentiomentric acid-base titrations for the determination of protonation constants of the diazole ligands were carried out on a Metrohm 794 Titrino (Herisau, Switzerland), that was equipped with a macro glass electrode. The pyrazole-l-carbodithioate and imidazole-l-carbodithioate ligand were synthesised and characterised as reported elsewhere (Anderson and Long, 2010).
Brunauer-Emmet-Teller (BET) analysis
Surface areas and pore characteristics of the fibres were determined using the Brunauer-Emmet-Teller (BET) isotherms obtained from nitrogen adsorption on an Accelerated Surface Area and Porosimetry System (ASAP™ 2020), Micromeritics (Bedfordshire, England). Prior to analysis, 0.3 g portions of samples were degassed overnight at 105°C in N2 environment using a Micromeritics SmartVac degassing system. The pore size distribution and specific surface areas were determined via N2 adsorption/desorption isotherms obtained at -196°C. Analyses were repeated, at least twice, for all samples, and the measurements were in good agreement.
Polymer solution preparation and electrospinning
Polystyrene solution (25%, w/v) was prepared by dissolving 2.5 g in a 10 ml DMF:THF (4:1 v/v) solvent system. The mixture was stirred continuously for 12 h using a magnetic stirrer to obtain a consistent solution. An optimised mass of 1 mg ligand was then added to the solution and stirred continuously overnight to obtain a thoroughly mixed solution. The viscous polystyrene solution formed was then loaded into a 10 ml glass syringe from Poulten GmbH (Berlin, Germany), and mounted on a New Era, NE-1000 programmable syringe pump (New York, USA). The solution was pumped at a rate of 1.0 ml/h through a steel needle of 0.58 mm internal diameter. The distance between the needle tip and the collector was kept at 12 cm. The needle tip and the collector were held at optimised voltages of +15 kV and -5 kV respectively. The fibre diameter was evaluated through the distance transform approach using the Scandium software (Ziabari et al., 2009).
Metal adsorption and desorption studies
The influence of the initial concentrations of the metal ions was investigated for a range (0-10 mg/l) of standard solutions. To vials containing 10 ml aliquots of metal solutions of known concentrations, 10 mg of electrospun fibres were added and the solutions stirred for 2 h. The fibres were filtered off through a 0.45 µπι sintered filter using suction. The concentration of metal ions left in solution was then determined using the ICP-OES. The concentration of metal ions adsorbed was taken as the difference between the initial and the final concentrations of the solution. This was confirmed through desorption experiments. The effect of fibre dose on the uptake of metals was investigated for fibre masses ranging from 2-20 mg. Portions of the fibres (with masses ranging from 2-20 mg) were stirred for 2 h, in 10 ml portions of 5 mg/l metal solutions. The loaded sorbent was then filtered off, washed 3 times with 5 ml portions of ultrapure water and dried on the filter using vacuum suction. The dried fibres were placed in 10 ml aliquot of 0.10 M HNO3 solution and stirred for 2 min in order to desorb the metal ions enriched on the fibres. To investigate the optimal pH for metal ion enrichment, adsorption experiments were carried out in 5 mg/l standard solutions of the metals, buffered to the desired pH values ranging from 2 to 12. The extent to which metal ions were enriched was then determined using the ICP-OES. The effect of contact time on the uptake of metal ions was also investigated in 5 mg/l metal ion solutions in batch experiments. In order to avoid precipitation at higher pH, the solutions were kept at the optimal pH of the metal under study using an ammonia buffer (Sun et al., 2006).
Analytical quality control procedure
A custom-made certified reference material for groundwater (SEP-3), purchased from Inorganic Ventures (Christiansburg, USA), was used to validate the analytical procedure. Analytical calibrations, based on the recommended concentration points and emission lines of each element, were carried out in aqueous standard solutions (US-EPA, 2001). Adsorption and desorption experiments were carried out using 10 mg of the imidazolyl-incorporated fibre sorbent in 10 ml portions of the certified r ference groundwater. Repeatability of the method was evaluated by comparing the signals obtained from 5 determinations of the reference material. The limits of detection (LOD) and quantification (LOQ) were evaluated as 3 and 10 times the estimated regression standard deviation, respectively, based on 5 replicate determinations.
Potentiometric measurements
The protonation constants of the ligands in aqueous solutions were determined by potentiometric titration at 25.00±1.00ºC in a double-walled titration cell in an inert, nitrogen environment. Titrations were performed over a pH range of 2 to 11 using 0.10 M HCl and 0.10 M tetramethylammonium hydroxide. Titrations were controlled using the Tiamo software. The glass electrode was calibrated for a strong acid-strong base reaction by the Gran method (Gran, 1952) and E ° value of the reaction was obtained using the GLEE software (Gans and O'Sullivan, 2000). The pKw value of 13.83±0.01, obtained in 0.1 M tetra-methylammonium chloride (TMACl) as an ionic medium, was used for all of the computations (Bazzicalupi, 2009).
The HYPERQUAD program (2008 version) was used for computation of protonation constants in equilibrium state (Gans et al., 1996). Data points (450) emanating from 3 independent titrations were used in calculating the protonation constants of the ligands. The statistical error (σ) was below 0.03 for all of the refinements.
Results and discussion
Electrospinning of the fibres
Preliminary experiments showed that a mixture of DMF:THF (4:1 v/v) was a suitable solvent system for dissolving polystyrene into solution. The high conductivity of DMF and the volatility of THF both favoured formation of smooth fibres during electrospinning (Lee et al., 2003). By varying the solution concentration, applied voltage, feed rate and distance between the syringe needle and the collector, it was determined that 25% polystyrene gives smooth, bead-free fibres (see Fig. 2) when it is electrospun at a feed rate of 1.0 ml/h through electric field strength of 1.67 kV/cm. Under these optimised conditions, fibres with average diameter of 2±0.1 µιη were obtained. Fibres with smooth morphologies and small diameters present higher surface areas and are, therefore, the option of choice for adsorption applications.
No observable differences were found between the fibres generated from the pristine polymer solution and those from the solutions dosed with the ligand, in terms of morphology and diameter. The small mass of the ligand added (1 mg) could not significantly alter the solution characteristics or the electro-spinning parameters. The effect of polymer concentration on the formation and morphology of the fibre is illustrated in Fig. 2. Different concentrations of polystyrene (10-35 wt%) were electrospun at: +25 kV, -5 kV, 12 cm gap. Only a concentration range of 20-30 wt% could give smooth and bead-free fibres. At low polymer concentrations, the high surface tension due to the solvents leads to formation of beads. At high concentrations, effects such as tip drying and blockages can also result in formation of beads.
Fibre characterisation
Figure 3 shows the relationship between polymer concentrations, BET surface areas and average pore width of polystyrene nanofibres. A linear relationship was observed between the polymer concentration (20-35 wt%) and the BET surface area of the nanofibres formed. The BET surface area decreased linearly from 14.08 m2/g for nanofibres generated from 20 wt% concentration polymer to 4.95 m2/g for nanofibres generated from 35 wt% concentration. Increasing the polymer concentration leads to formation of nanofibres with larger diameters and hence smaller specific surface area (Patanaik et al., 2010). The pore size did not follow the linear trends observed for BET surface area. Pore size showed more of a logarithm relationship to polymer concentration.
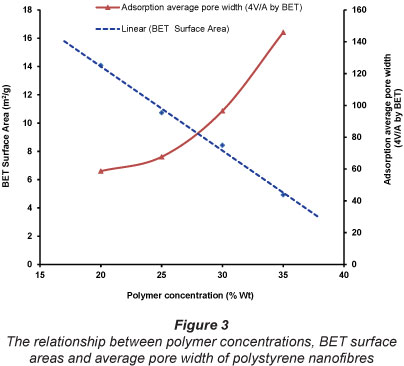
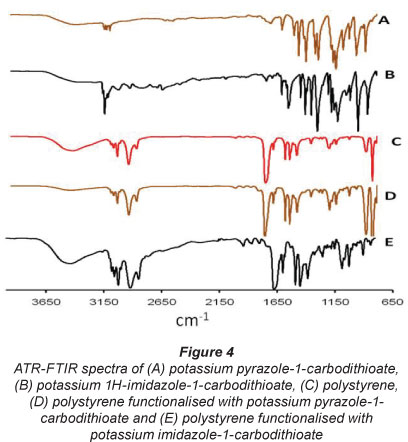
Figure 4 shows the ATR-FTIR spectra of the pristine and functionalised polystyrene fibres as well as those of pyrazole-l-carbodithioate and imidazole-l-carbodithioate in the 4 000-650 cm-1 range. The characteristic C=S, C=N and C-N peaks of the diazole ligands were present in both pyrazole-l-carbodithioate and imidazole-l-carbodithioate (Troitskaya et al., 1974). Similar peaks were observed in the IR spectra of the diazole-incorporated polystyrene but not in the spectra of the pristine polystyrene.
pH dependence
Because H+ ions compete with metal ions in solution for binding sites on sorbents, pH becomes a very important parameter in binding studies. The concentration of H+ in an acidic solution is relatively high and H+ ions tend to fill up the binding sites on the sorbent. The presence of H+ ions on the sorbent surface creates an electrostatic repulsion for the metal cations. Sorption of metal ions onto the sorbent is therefore expected to be low in highly acidic solutions (pH less than 4). Metal uptake is, however, favoured at higher pH values where H+ ion concentrations and, consequently, electrostatic repulsions are low.
Figure 4 shows the sorption profile of metal ions on the electrospun fibre sorbent at various pH values. The imidazolyl-incorporated fibres did not adsorb any of the metals studied when the pH of solution was less than 3. The optimal pH for adsorption of Cu(II), Ni(II) and Pb(II) was 6.3, 7.4, and 8.3, respectively. In increasing order of their optimal pH for adsorption, these metals are arranged as Cu(II)<Ni(II)<Pb(II). This order suggests that pH for metal uptake is determined, somehow, by thermodynamic factors, such as electronegativity, acidity and ionic radius of the metal ion. That is, metal ions of higher electronegativity (higher acidity or lower ionic size) are better adsorbed in the acidic range. The optimal pH for enriching the metal ions on the pyrazolyl-incorporated fibres followed the same order (Cu = 8.3; Ni = 8.6 and Pb = 9.0) as for the imidazole. There was, however, a significant sorption at lower pH values. For example, at a pH of 2 there were 16, 22 and 27% adsorptions for Cu(II), Ni(II) and Pb(II), respectively. These relatively high sorption levels at lower pH values may be attributed to the low pK of the pyrazolyl nitrogen (Chen et al., 1991; Trofimov, 1992).
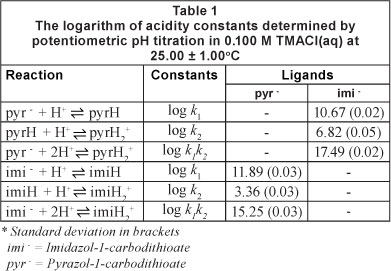
Protonation constants
Table 1 shows the stepwise protonation constants (the pK values) of the ligands. Both ligands exhibited 2 protonation processes in the pH range between 2 and 11. Log K values of the imidazole ligand were higher than those of the pyrazole. The pK of the imidazole nitrogen was 6.82 while that of pyrazole was 3.36. This implies that at pH of 3.36 about 50% of the binding sites on the pyrazole nitrogen were still free for binding. This gives credence to the relatively high adsorptions that occurred on the pyrazole at lower pH values (less than 3).
Effect of contact time
The rate at which metal ions were adsorbed on the diazole-incorporated sorbents as well as on the pristine polystyrene sorbent is profiled in Fig. 5. The pristine fibres and the imi-dazole-incorporated sorbents exhibited only minimal levels of adsorption (< 10%) when solution pH was less than 4. The pyrazole-incorporated sorbents, however, showed a significant level of adsorption (~ 20°%) in acidic solutions (pH < 4). At all pH levels, the diazole-incorporated sorbents adsorbed better than the pristine sorbent. Incorporation of the diazole ligands therefore enhanced adsorption on the sorbents.
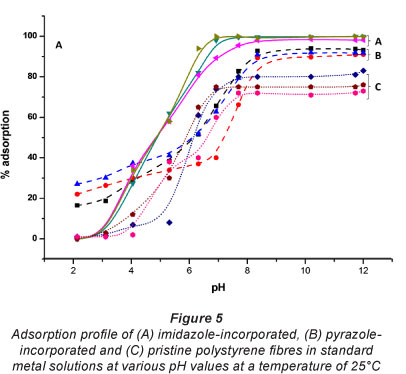
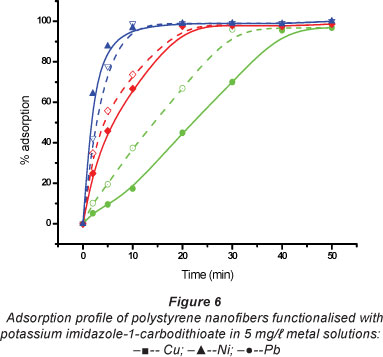
The adsorption profile (Fig. 5) showed that the metal ions were initially adsorbed rapidly, until the sorbent got saturated. Equilibration times for maximum sorption of Cu(II) and Ni(II) were 10 min and 18 min, respectively. These times were roughly the same for the two kinds of sorbents. The longest time recorded for equilibration was 42 min, for the uptake of Pb(II) on imida-zolyl-incorporated fibres. Thi s equilibration time is shorter than the 3-h period recorded for functionalised chitosan sorbents (Justi et al., 2005) and the 8 hours recorded for thiourea-modified magnetic chitosan microspheres (Zhou et al., 2009).
Effect of sorbent dosage
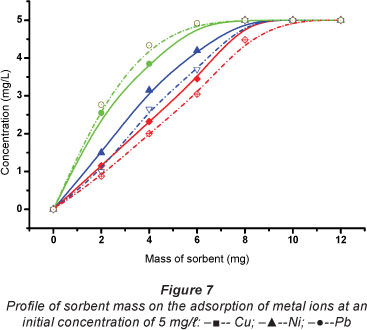
The effect of the mass of sorbent used on the sorption of metal ions in solution was studied (Fig. 6). Sorbent dosage is an important parameter because it determines the capacity of the sorbent at the initial concentration. Adsorptions of all metal ions increased significantly with an increase in the mass of sorbent up to 8 mg, and then levelled off. For ease of handling, a reasonably large amount of sorbent, 10 mg sorbent mass, was used in the experiments. Standard solutions were consequently spiked to relatively high concentrations (5 mg/t) because of the sorbent's high adsorption capacity.
Desorption of metal ions and regeneration of sorbent
Sorbent reusability is largely determined by the extent to which the adsorbed ions are desorbed. Because the pyrazolyl-incorpo-rated sorbent could not desorb effectively in the first instance of use, it could not be regenerated for further usage. The efficiencies of adsorption and desorption for all of the metal ions on the imidazolyl-incorporated sorbent ranged between 99 and 95% up to the 4th cycle of usage (Fig. 7). There was an overall drop of 3.49% in adsorption and 5.07% in desorption up to the 5th cycle of sorbent usage. This decrease in efficiency could be attributed to the loss of the ligand and/or small quantities of the sorbent during use. The regeneration of sorbent showed that the adsorption-desorption process was reversible for the imidazolyl-incorporated sorbent.
Interference study
The interfering effect of the metal ions on one another was investigated. Results are presented in Table 2. An ion was said to be interfering if it caused more than 5% reduction in the uptake of another ion under investigation. Based on the criteria for interference set, it was inferred that sorption of Ni(II) and Cu(II) were not affected by the presence of any of the metals studied. Sorption of Cd(II) was somehow suppressed by all of the other metals. At higher concentrations, Cu(II) and Ni(II) interfered with the uptake of each other. Co(II), at higher concentrations, interfered with the uptake of both Cu(II) and Ni(II). SorptionofPb(II)was affectedbythepresenceof Cd(II).
Analytical quality control
Table 3 gives the quality control parameters for the determination of metal concentrations in aqueous solutions. Accuracy of the determinations, expressed as relative error between the certified and observed values of the reference material, was £ 0.2%. The precision of these measurements, expressed as relative standard deviation on 5 independent determinations, was also satisfactory, being lower than 3% in all cases (Huber, 2003). The LOD of the metals ranged from 0.0004±0.0001 mg/l for Cd to 0.0045±0.0001 mg/l for Pb. These LODs achieved with the imidazolyl-incorporated sorbent were comparable to 0.0015 mg/l. Cd and 0.0017 mg/l recorded using nano-alumina sorbents (Ezoddin et al., 2010), and were lower than 1.0 mg/l. Cu achieved using substituted naphthalene (Rezaei et al, 2009). The LOQ was lower than 0.0145 mglt for all of the metals studied.
Adsorption in real aqueous environments
The capacity of the imidazolyl-incorporated sorbent to enrich metal ions in the natural aqueous environment was evaluated using river, sea, tap, treated and untreated sewage water samples. The pH, background concentrations of the metal ions, and recovery efficiencies of the sorbent in 5 mg/l. spiked natural water samples are recorded in Table 4.
The average pH ranged from 6.51 (in untreated sewage) to 8.13 (in tap water). The sorbent was able to quantitatively ad sorb the trace concentrations of metal ions (< 0.15 mg/l) originally present in all of the water types. Because the background concentrations of the metals were low compared to the spiking concentrations, and the sorbent could adsorb them quantitatively, they were ignored. The recoveries in the 5 mg/t fortified samples ranged from 71.06-78.28% for Ni(II), 84.4097.57% for Cu(II), 82.21-99.00% for Pb(II), 73.68-98.90% for Cd(II) and 68.54-77.48% for Co(II). These levels of recoveries translate to loading capacities of between 45-50 mg/g of the sorbent, which is comparable to 57.74 mg/g reported for amino-functionalised-nanoporous silica (Heidari et al., 2009). The loading capacities achieved with the imidazolyl-incorporated sorbent were higher than 12.36 mg/t recorded for amino-functionalised mesoporous silica (Aguado et al., 2009) and the range of 27-44 mg/g recorded for biomass-based sorbents (Hamissa et al., 2010; Miretzky et al., 2010; Sari and Tuzen 2009; Tang et al., 2010). The imidazolyl-incorporated sorbent was found to be more sensitive to Pb(II), Cu(II) and Cd(II) than to Ni(II) and Co(II).
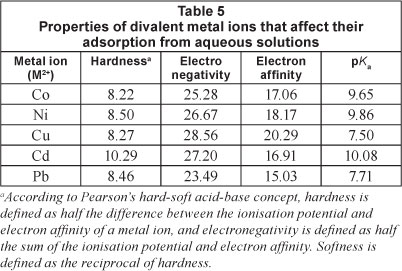
Table 5 gives the main characteristics (hardness, electronegativity, electron affinity, pKa) of metal ions that determine their binding to a ligand. The order of adsorption efficiencies, Co(II)<Ni(II)<Cd(II)<Cu(II)<Pb(II), obtained in this work, does not wholly fit the order of any of the parameters outlined. For the first row transition metal ions, however, the order of adsorption followed the order of electron affinity, Co(II)<Ni(II)<Cu(II), as espoused by Martin (1998).
Conclusions
Potassium salts of pyrazole-carbodithioate and imidazole-l-carbodithioat were incorporated in polystyrene, which was successfully electrospun into fibres. The fibres were found to have optimal adsorption of metal ions around the natural pH of the water types sampled. While the sorbent incorporated with the imidazole ligand could be regenerated and reused, the pyrazolyl-incorporated fibres displayed poor desorption properties. The sorbent exhibited fast adsorption kinetics and high loading capacities for metal ions. These qualities, coupled with the sorbent's tunability for easy desorption of the metal ions, make electrospun polystyrene fibres incorporating imidazole-l-carbodithioate excellent sorbents for the enrichment of heavy metal ions in aqueous environments.
Acknowledgements
The authors gratefully acknowledge the financial support from the African Network of Analytical Chemists (SEANAC).
References
AGUADO J, ARSUAGA JM, ARENCIBIA A, LINDO M, GASCON V (2009) Aqueous heavy metals removal by adsorption on amine-functionalized mesoporous silica. J. Hazard. Mater. 163 213-221. [ Links ]
AKOTO O, BRUCE TN and DARKO G (2008) Heavy metals pollution profiles in streams serving the Owabi reservoir. Afr. J. Environ. Sci. Tech. 2 354-359. [ Links ]
ANDERSON EB and LONG TE (2010) Imidazole- and imidazolium-containing polymers for biology and material science applications. Polymer 51 (12) 2447-2454. [ Links ]
BAZZICALUPI C, BENCINI A, BIANCHI A, DANESI A, GIORGI C and VALTANCOLI B (2009) Anion binding by protonated forms of the tripodal ligand. Trends Inorg. Chem. 48 (6) 2391-2398. [ Links ]
BOGDANOVIC GA, JACIMOVIC ZK and LEOVAC VM (2005) Transition metal complexes with pyrazole-based ligands. Acta Crystallogr. C. 61 (8) 376-379. [ Links ]
BOLOGO V, MAREE JP and CARLSSON F (2012) Application of magnesium hydroxide and barium hydroxide for the removal of metals and sulphate from mine water. Water SA 38 (1) 23-28. [ Links ]
CHANG YC and CHEN DH (2005) Preparation and adsorption properties of monodisperse chitosan-bound Fe3O4 magnetic nano-particles for removal of Cu(II) ions. J. Colloid Interface Sci. 283 (2) 446-451. [ Links ]
CHEN LZ, FLAMMANG R, MAQUESTIAU A, TAFT RW, CATALAN J, CABILDO P, CLARAMUNT RM and ELGUERO J (1991) Thermodynamic basicity vs. kinetic basicity of diazoles (imidazoles and pyrazoles). J. Org. Chem. 56 (1) 179-183. [ Links ]
EZODDIN M, SHEMIRANI F, ABDI KH, SAGHEZCHI MK and JAMALI MR (2010) Application of modified nano-alumina as a solid phase extraction sorbent for the preconcentration of Cd and Pb in water and herbal samples prior to flame atomic absorption spectrometry determination. J. Hazard. Mater. 178 (1-3) 900-905. [ Links ]
GANS P and O'SULLIVAN B (2000) GLEE, a new computer program for glass electrode calibration. Talanta 51 (1) 33-37. [ Links ]
GANS P, SABATINI A and VACCA A (1996) Investigation of equilibria in solution. Determination of equilibrium constants with the HYPERQUAD suite of programs. Talanta 43 (10) 1739-1753. [ Links ]
GRAN G (1952) Determination of the equivalence point in potentio-metric titrations. Part II. Analyst 77 661-671. [ Links ]
GUNDACKER C (2000) Comparison of heavy metal bioaccumulation in freshwater molluscs of urban river habitats in Vienna. Environ. Pollut. 110 61-71. [ Links ]
GUNDOGDU A, OZDES D, DURAN C, BULUT VN, SOYLAK M and SENTURK HB (2009) Biosorption of Pb(II) ions from aqueous solution by pine bark (Pinus brutia Ten.). Chem. Eng. J. 153 (1-3) 62-69. [ Links ]
HAMISSA AMB, LODI A, SEFFEN M, FINOCCHIO E, BOTTER R and CONVERTI A (2010) Sorption of Cd(II) and Pb(II) from aqueous solutions onto Agave americana fibres. Chem. Eng. J. 159 (1-3) 67-74. [ Links ]
HEIDARI A, YOUNESI H and MEHRABAN Z (2009) Removal of Ni(II), Cd(II), and Pb(II) from a ternary aqueous solution by amino functionalized mesoporous and nano mesoporous silica. Chem. Eng. J. 153 (1-3) 70-79. [ Links ]
HUBER W (2003) Basic calculations about the limit of detection and its optimal determination. Accredit. Qual. Assur. 8 (5) 213-217. [ Links ]
JUSTI KC, FÁVERE VT, LARANJEIRA MCM, NEVES A and PERALTA RA (2005) Kinetics and equilibrium adsorption of Cu(II), Cd(II), and Ni(II) ions by chitosan functionalized with 2[-bis-(pyridylmethyl)aminomethyl]-4-methyl-6-formylphenol. J. Colloid Interface Sci. 291 (2) 369-374. [ Links ]
KOYSAL Y, ISIK S, SAHIN G and PALASKA E (2005) Two N-substituted 3,5-diphenyl-2-pyrazoline-l-thiocarboxamides. Acta Crystallogr. C. 61 (9) 542-544. [ Links ]
LEE KH, KIM HY, RA YM and LEE DR (2003) Characterization of nano-structured poly(e-caprolactone) nonwoven mats via electro-spinning. Polymer 44 (4) 1287-1294. [ Links ]
MAMBA BB, NYEMBE DW and MULABA-BAFUBIANDI AF (2009) Removal of copper and cobalt from aqueous solutions using natural clinoptilolite. Water SA 35 (3) 307-314. [ Links ]
MAPOLELO M, TORTO N and PRIOR B (2005) Evaluation of yeast strains as possible agents for trace enrichment of metal ions in aquatic environments. Talanta 65 (4) 930-937. [ Links ]
MARTIN RB (1998) Metal ion stabilities correlate with electron affinity rather than hardness or softness. Inorg. Chim. Acta 283 (1) 30-36. [ Links ]
MIRETZKY P, MUNOZ C and CARRILLO-CHAVEZ A (2010) Cd (II) removal from aqueous solution by Eleocharis acicularis biomass, equilibrium and kinetic studies. Bioresour. Technol. 101 (8) 2637-2642. [ Links ]
MISHRA PC and PATEL RK (2009) Removal of lead and zinc ions from water by low cost adsorbents. J. Hazard. Mater. 168 (1) 319-325. [ Links ]
MOHAMMADI SZ, AFZALI D and POURTALEBI D (2010) Flame atomic absorption spectrometric determination of trace amounts of lead, cadmium and nickel in different matrixes after solid phase extraction on modified multiwalled carbon nanotubes. Cent. Eur. J. Chem. 8 (3) 662-668. [ Links ]
MUKHOPADHYAY S, MUKHOPADHYAY U, MAK TCW and RAY D (2001) Bis(3,5-dimethylpyrazole-l-carbodithioato) nickel(II) and its transformation to a dinuclear complex: Crystal structure of [Ni29u-3,5-Me2Pz)2(L1)2] (L1 = 3,5-dimethylpyrazole-l-carbodith-ioate). Inorg. Chem. 40 (5) 1057-1059. [ Links ]
OH S, KWAK MY and SHIN WS (2009) Competitive sorption of lead and cadmium onto sediments. Chem. Eng. J. 152 (2-3) 376-388. [ Links ]
PATANAIK A, JACOBS V and ANANDJIWALA RD (2010) Performance evaluation of electrospun nanofibrous membrane. J. Membr. Sci. 352 136-142. [ Links ]
POURANG N (1995) Heavy metal bioaccumulation in different tissues of two fish species with regards to their feeding habits and trophic levels. Environ. Monit. Assess. 35 207-219. [ Links ]
QU R, WANG C, JI C, SUN C, SUN X and CHENG G (2005) Preparation, characterization, and metal binding behavior of novel chelating resins containing sulfur and polyamine. J. Appl. Polym. Sci. 95 (6) 1558-1565. [ Links ]
RASHCHI F, FINCH JA and SUI C (2004) Action of DETA, dextrin and carbonate on lead-contaminated sphalerite. Colloids Surf. A. 245 (1-3) 21-27. [ Links ]
REZAEI B, SADEGHI E and MEGHDADI S (2009) Nano-level determination of copper with atomic absorption spectrometry after pre-concentration on N,N-(4-methyl-1,2-phenylene)diquinoline-2-carboxamide-naphthalene. J. Hazard. Mater. 168 (2-3) 787-792. [ Links ]
SAMAL S, DAS RR, DEY RK and ACHARYA S (2000) Chelating resins VI: Chelating resins of formaldehyde condensed phenolic schiff bases derived from 4,4*-diaminodiphenyl ether with hydroxybenzaldehydes - synthesis, characterization, and metal ion adsorption studies. J. Appl. Polym. Sci. 77 967-981. [ Links ]
SARI A and TUZEN M (2009) Kinetic and equilibrium studies of biosorption of Pb(II) and Cd(II) from aqueous solution by macro-fungus (Amanita rubescens) biomass. J. Hazard. Mater. 164 (2-3) 1004 -1011. [ Links ]
SUN CM, QU RJ, JI CN, WANG CH, SUN YZ, YUE ZW and CHANG GX (2006) Preparation and adsorption properties of crosslinked polystyrene-supported low-generation diethanolamine-typed dendrimer for metal ions. Talanta 70 (1) 14-19. [ Links ]
SZÉCSÉNYI KM, LEOVAC VM, KOVÁCS A, POKOL G and JACMOVIC ZK (2006) Transition metal complexes with pyrazole- based ligands. J. Therm. Anal. Calorim. 85 (2) 289-293. [ Links ]
TANG Q, TANG X, HU M, LI Z, CHEN Y and LOU P (2010) Removal of Cd(II) from aqueous solution with activated Firmiana Simplex Leaf: Behaviors and affecting factors. J. Hazard. Mater. 179 (1-3) 95-103. [ Links ]
TEO WE and RAMAKRISHNA S (2006) A review on electrospinning design and nanofibre assemblies. Nanotechnology 17 (14) 89-106. [ Links ]
TRETINNIKOV ON (2000) Hydrophilic (hydrogen-bonding) polystyrene surface by substrate-induced surface segregation of benzene groups. Langmuir 16 (6) 2751-2755. [ Links ]
TROFIMOV BA (1992) The Chemistry of Heterocyclic Compounds, Pyrroles. Wiley, New York. [ Links ]
TROITSKAYA VS, KONEVSKAYA ND, VINOKUROV VG and TYULIN VI (1974) Effect of hydrogen bonding on the frequency of the out-of-plane deformation vibrations of the NH bonds in pyra-zole and imidazole molecules. Chem. Heterocycl. Compd. 10 471. [ Links ]
US-EPA (UNITED STATES ENVIRONMENTAL PROTECTION AGENCY) (2001) EPA-821-R-01-010. Method 200.7. US EPA, Washington D.C. [ Links ]
VASILIEV AN, GOLOVKO LV, TRACHEVSKY VV, HALL GS, and KHINAST JG (2009) Adsorption of heavy metal cations by organic ligands grafted on porous materials. Microporous Mesoporous Mater. 118 251-257. [ Links ]
VOGLER EA (1998) Structure and reactivity of water at biomaterial surfaces. Adv. Colloid Interface Sci. 74 (1-3) 69-117. [ Links ]
ZAMAN MI, MUSTAFA S, KHAN S and XING B (2009) Heavy metal desorption kinetic as affected by of anions complexation onto manganese dioxide surfaces. Chemosphere 77 (6) 747-755. [ Links ]
ZHANG Y, QU R, SUN C, CHEN H, WANG C, JI C, YIN P, SUN Y, ZHANG H and NIU Y (2009) Comparison of synthesis of chelating resin silica-gel-supported diethylenetriamine and its removal properties for transition metal ions. J. Hazard. Mater. 163 127-135. [ Links ]
ZHANG Y, QU R, SUN C, CHEN H, WANG C, JI C, YIN P, SUN Y, ZHANG H and NIU Y (2009) Chemical modification of silica-gel with diethylenetriamine via an end-group protection approach for adsorption to Hg(II). J. Hazard. Mater. 255 (11) 5818-5826. [ Links ]
ZHAO XS, BAO XY, GUO W and LEE FY (2006) Immobilizing catalysts on porous materials. Mater. Today 9 (3) 32-39. [ Links ]
ZHOU L, WANG Y, LIU Z and HUANG Q (2009) Characteristics of equilibrium, kinetics studies for adsorption of Hg(II), Cu(II), and Ni(II) ions by thiourea-modified magnetic chitosan microspheres. J. Hazard. Mater. 161 (2-3) 995-1002. [ Links ]
ZIABARI M, MOTTAGHITALAB V and HAGHI AK (2009) Application of direct tracking method for measuring electrospun nanofibre diameter. Brazilian J. Chem. Eng. 26 (1) 53-62. [ Links ]
Received 8March 2012;
Accepted in revised form 22 November 2012.
* To whom all correspondence should be addressed. ffi +27 46 603 8924; fax: +27 46 622 8254; e-mail: n.torto@ru.ac.za














