Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Water SA
On-line version ISSN 1816-7950
Print version ISSN 0378-4738
Water SA vol.38 n.5 Pretoria Jan. 2012
Aerobic biotransformation of 2, 4, 6-trichlorophenol by Penicillium chrysogenum in aqueous batch culture: degradation and residual phytotoxicity
Nicolás Aranciaga; Ignacio Durruty; Jorge F González; Erika A Wolski*
Grupo de Ingeniería Bioquímica, Fac. Ingeniería, Universidad Nacional de Mar del Plata, J.B. Justo 4302, 7600 Mar del Plata, Buenos Aires, Argentina
ABSTRACT
2,4,6-trichlorophenol (TCP) is a toxic compound widespread in the environment, with numerous applications. There are many fungi capable of degrading it, although little attention has been paid to non wood-degrading species. Penicillium chrysogenum ERK1 was able to degrade 85% of TCP in batch cultures in the presence of sodium acetate. Degradation rate was fitted to a specific first-order kinetic and the growth rate was fitted to a Gompertz model. Hydroquinone and benzoquinone were identified as degradation intermediates. The phytotoxicity of the residues was reduced by half after fungal treatment. These results suggest that Penicillium chrysogenum can be applied successfully to biodegrade TCP.
Keywords: 2, 4, 6-trichlorophenol; Penicillium chrysogenum; biodegradation; phytotoxicity
Introduction
Chlorophenols have been introduced into the environment through their use as biocides and as by-products of chlorine bleaching in the pulp and paper industry. In addition, pentachlorophenol (PCP), trichlorophenol (TCP) and tectrachlorophenol (TeCP) were used historically as fungicides in wood-preservative formulations (Field and Sierra-Alvarez, 2008; McAllister et al., 1996).
Many fungi and yeast are able to co-metabolise or mineralise chlorophenols (Field and Sierra-Alvarez, 2008). Wood-degrading fungi are well established as excellent degraders of chlorophenols (Asgher et al., 2008). However, studies on chlorophenol degradation using fungi which do not belong to the white rot category are scarce.
Penicillium species are commonly found in food, indoor air and soils, and have been shown to be good hydrocarbon assimilators (Leitao, 2009; Samson et al., 2004). Several authors have reported on their ability to degrade phenol and chlorophenols. For example: P. chrysogenum CLONA 2 degrades phenol (Leitao et al., 2007); P. simplicissimun SK9117 (Marr et al., 1989) and Penicillium strain Bi/72 (Hofrichter et al., 1992) degrade mono-chlorophenols; and Penicillium camemberti degrades pentachlorophenol (Taseli and Gokcay, 2005). Nevertheless, these strains were not able to use those chlorophenols as growth substrates. In addition, degradation of trichlorophenol has not been reported for these fungi (Leitao, 2009).
Several studies have been conducted on the biotransformation and degradation of chlorophenols from the water-soil environment. However, most of these did not analyse the toxicity of the final residues. Thus, it is also relevant to assess the phytotoxicity of these wastes before and after degradation (Osma et al., 2010).
In this work, both the degradation of TCP by a soil isolate of P. chrysogenum and the analysis of the toxicity of TCP residues on wheat seeds are reported. In addition, a growth model was selected and experimental data were fitted to it. Finally, metabolic products were identified by HPLC.
Experimental
All of the reagents used were analytical grade, except for 2, 4, 6-trichlorophenol (TCP) which was of chromatographic grade (purity 99%), from Sigma-Aldrich (St. Louis, USA). HPLC acetonitrile was obtained from Sintorgan (Buenos Aires, Argentina).
A Penicillium chrysogenum ERK 1 isolate (GenBank, accession numbers HQ336382 and HQ336383) was maintained in potato dextrose agar (PDA, Gibco) at room temperature for 14 days (without TCP). This fungus was isolated from commercial crop soils from Balcarce, Buenos Aires province, Argentina, as described by Wolski et al. (2010).
For the degradation assays the fungus was inoculated directly from the PDA plate into an Erlenmeyer flask of 250 ml with 150 ml of liquid mineral salt medium (LMS) containing: 1 000 ml deionised water, 1 g MgSO47H2O 0.1 g, K2HPO4 0.1 g, NH4NO3 and 0.1 g KCl and 25 μΐ of trace element solution (in mg-l-1: MnSO4 15.4, FeCl3 40, ZnSO4-7H 2O 6.3, CuSO4-5H2O 2.5, (NH4)6-Mo7-O24-4H2O 0.5), and supplemented with 10 mg-l-1 of TCP alone, or with 2 g-l-1 of sodium acetate or 2 g-l-1 of glucose, depending on the assay. The TCP concentration was selected to avoid the substrate inhibition: in preliminary experiments run with 25 mg-l-1 and 50 mg-l-1 no degradation was observed. The pH was previously adjusted to 6.0. Each flask was inoculated with 4 PDA agar discs of 4 mm containing the fungal mycelium. The cultures were incubated at 30°C for 30 days, in a shaker at 80 r-min-1 and operating in the dark in order to avoid photo-degradation of TCP. Non-inoculated flasks with LMS supplemented with TCP were used as controls. All experiments were carried out in triplicate, and the results show the mean value of 3 independent experiments.
At different times after inoculation the mycelium from each flask was filtered and the dry weight of the mycelia was measured. The TCP content of the liquid medium was measured in the filtrates after removing the mycelia. The fungal mycelium was filtered onto a Whatman GF/A filter, rinsed twice with distilled water and dried at 100°C until constant weight. Biomass was calculated as mg of dry weight per volume of reactor (l).
The concentration of TCP was estimated by HPLC. A Waters HPLC system (Millipore, Waters Division, Milford, Massachusetts, USA) consisting of a Model 590 pump, equipped with a UV detector Model 484 variable-wavelength detector set at 310 nm was used.
The separation was achieved with a Water Spherisorb ODS2 C18 (5 μιη) 4.6 x 250 mm analytical column (Millipore Corporation, Milford, MA, USA). A mixture of 7 mM phosphoric acid: acetonitrile (50:50, vol/vol) isocratic system was used as solvent and the flow rate was maintained at 1 ml-min-1. The compounds were identified by comparing their retention time with those similarly treated, and by co-chromatography. Under the above conditions, the retention times of the external standards were: TCP - 8.73 min, hydroquinone - 3.32 min, benzoquinone - 3.78 min, 2,4,6-trichloroanisole (TCA) - 4.77 min.
The amount of TCP adsorbed to the fungal mycelium was measured as described by Leontievsky et al. (2000a). The fungus was grown in 150 ml of LMS supplemented with 10 mg-l-1 of TCP and with 2 g-l-1 of acetate. The mycelia were filtered from the liquid culture, washed 3 times with distilled water, suspended in 2 ml of distilled water and homogenised with a IKA T18B Ultra-Turrax homogeniser (by IKA Gmbh, Staufer in Breigan, Germany). The mycelium extract was collected and centrifuge at 12 000 g for 15 min and the supernatant recovered was used to determinate TCP content by HPLC. Three independent samples were analysed for TCP adsorption. Additional HPLC runs were carried out with the water phase from the washing process.
Qualitative assays were performed on agar plates to study the enzymatic activity involved in phenol degradation. The fungus was inoculated in LMS medium supplemented with 2% agar, 1% glucose and different substrates. Medium and substrate solutions were autoclaved separately and mixed after cooling down to around 50°C. The test fungus was centrally inoculated and incubated at 25°C for 8 days.
Laccase activity was assayed using 2,2'-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) as substrate (0.02%); the formation of a green halo indicates laccase activity (Rubilar Araneda, 2007).
Poly R-478 was used as substrate (0.02%) to determinate peroxidase activity (manganese peroxidases and lignin peroxidases) by decolouration of Poly R-478 from violet to yellow (Levin et al., 2004; Rubilar Araneda, 2007).
The toxicity of the TCP and its degradation products was assessed by measuring the phytotoxic effect of LMS supplemented with TCP 10 mg-l-1, and the residue of TCP degradation (1.43 mg-l-1), on the germination of wheat (Triticum aestivum) seeds, according to Zucconi et al. (1985) and Osma et al. (2010). LMS was used as control. Five replicates of 10 seeds each were used for each treatment. The seeds were submerged in LMS plus TCP 10 mg-l-1 and the residue of TCP degradation. After 5 days of incubation in the dark at 25°C, the seed germination and root length of seeds immersed in the solutions mentioned before were measured. The germination index (GI) was calculated as follows: GI=GP x La/Lc, where GP is the number of germinated seeds expressed as a percentage of control values (LMS). La is the average root length in the TCP solutions or TCP residues and Lc is the average root length in the control.
The fungal growth shows a phase in which the specific growth rate is initially very slow; after a lag period (λ) it accelerates to a maximal value (μmax). In addition, the growth curves contain a final phase in which the rate decreases and finally reaches zero, so that an asymptote (A) of the growth curve is reached (Zwietering et al., 1990). When the growth curve is shown as the logarithm of the number of organisms plotted against time, these growth rate changes result in a sigmoid curve with a lag phase just after t=0, followed by an exponential phase and then a stationary phase. Different models were used to model fungal growth (Amrane et al., 2005; Hamidi-Esfahani et al., 2007). In this work, the Gompertz growth model was used to fit the biomass growth curve (Eq. (1)), by the least squares method. In addition, degradation substrate was fitted to a specific first-order kinetic (Eq. (2)).
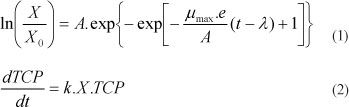
where:
TCP is 2, 4, 6-trichlorophenol concentration in mg-l-1 t is time in days
X is biomass expressed as mg of dry weight per litre of reactor volume
The parameters A, μmax and λ are as described above and k is the first-order specific constant for TCP degradation. The agreement of the model with the data was analysed by ANOVA test. The growth rate (rX) and degradation rate (rS) were determined as first derivatives of concentration, (Eqs. (3) and (4)), while specific degradation rates were calculated as in Eq. (5):

Results and discussion
When degradation of TCP was evaluated in the absence of a carbon source other than TCP itself, P. chrysogenum was not able to degrade TCP as a single substrate (data not shown). Therefore, different ancillary carbon sources were added, and 85% of degradation of 10 mg-l-1 of TCP was achieved in 26 days in presence of sodium acetate (Fig. 1), but no degradation of TCP was observed in presence of 2 g-l-1 of glucose. These results agree with those reported by Hofritcher et al. (1992) and Marr et al. (1989), where other Penicillium isolates degraded mono-chlorophenols only in the presence of a co-substrate. Degradation of TCP has been well studied for the fungus Phanerochaete chrysosporium, which was able to degrade 58% of TCP in 30 days with 2% of glucose as a co-substrate (Reddy et al., 1998). Therefore, P. chrysogenum shows good degradation potential taking into account that this fungus had not been acclimated.
No sorption of TCP was detected in the fungal mycelium or in the water phase from the mycelium washing process. This result confirms that the decrease of TCP in the liquid culture was due to its biotransformation and not adsorption to the fungal mycelium. Therefore, P. chrysogenum degraded TCP effectively.
Biomass growth is shown in Fig. 1. The fungus started to grow immediately, without a lag phase, and grew exponentially until Day 15, when an evident deceleration was observed showing the transition between the exponential and stationary growth phase. This behaviour has been commonly observed in bacterial growth (Zwietering et al., 1990), and has also been studied and modelled in fungal growth (Amrane et al., 2005; Hamidi-Esfahani et al., 2007). The biomass growth was fitted to a Gompertz sigmoid curve (Eq. (1)) which takes into account the three growth phases: lag phase, exponential phase and stationary phase. The lag time parameter (λ) obtained was 0, indicating that a lag phase was not appreciable. However, the maximum specific growth rate (μmax) value obtained was approx. 0.163 ± 0.015 d-1, which represents an active exponential growth phase. The maximum asymptotic value (A=ln (Xoo/X0)) was 2.557±0.155, indicating that, in the stationary phase, the final biomass was almost 13 times higher than the initial biomass. The fitting of the model to experimental data is shown in Fig. 1. The ANOVA analysis did not show significant differences at 95% confidence intervals and the adjusted regression coefficient was 0.92, demonstrating a good fit of the model to the experimental data.
The yield coefficient for the fungus with acetate as substrate, in the presence of TCP, was about 0.048 (expressed as mg of mycelium dry weight per mg of acetate consumed). As a control, the fungi was grown in LMS supplemented only with 2 g-l-1 of acetate and the yield coefficient was 0.178, showing that TCP inhibited the mycelium growth by 73%. In addition, when the fungus was grown in 25 g-l-1 of TCP, no growth was observed. Similar results were reported for Coriolus versicolor, where 25 g-l-1 of TCP inhibited mycelia growth almost completely, and 15 g-l-1 resulted in a marginally toxic concentration which still permitted some biomass growth (Leontievsky et al., 2000a).
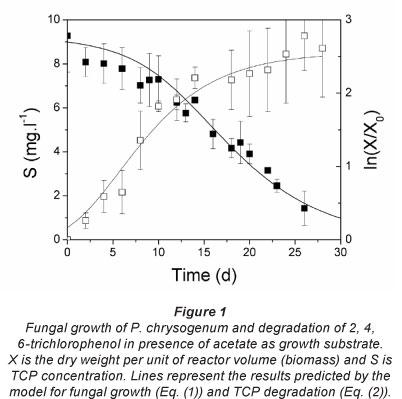
Figure 1 also shows that the TCP degradation started from time zero and that the degradation rate increases as the biomass grows (Fig. 2), indicating that the degradation is proportional to biomass. Furthermore, at the end of the test, when the biomass was almost constant, the degradation rate decreased as the concentration of TCP decreased (Fig. 2), indicating that the degradation rate is also proportional to TCP concentration. For this reason, the TCP degradation was fitted to a specific first-order kinetic (Eq. (2)), with a constant of 1.17 x 10-3 ±0.06 x 10-3 £-d-1-mg-1. The fitting of the model to experimental data is also shown in Fig. 1. The ANOVA analysis did not shown significant differences at 95% confidence intervals and the adjusted regression coefficient was 0.98. This indicates a good fit from model to experimental data.
Within the range studied, P. chrysogenum showed an average specific degradation rate of 8.39 x 10-3 d-1, while Penicillium strain Bi 7/2 showed specific degradation rates of 8.16 x 10-3 d-1, 1.56 x 10-2 d-1 and 3.70 x 10-2 d-1 for 2-chlorophenol, 3-chlorophenol and 4-chlorophenol, respectively, for an initial concentration of 50 mg-£-1 (Hofrichter et al., 1992). Therefore, P. chrysogenum showed good degradation potential taking into account that TCP is more toxic than mono-chlorophenols (Field and Sierra-Alvarez, 2008; Leontievsky et al., 2000b).
Figure 2 shows that the biomass growth rate reached a maximum of 4.86 mg-l-1-d-1 on Day 12, before the onset of degradation of TCP, which reached a maximum of 0.044 mg-l-1-d-1 on Day 15. These results suggest that the fungus needs to initially grow at the expense of acetate and then at the expense of TCP, at a lower growth rate.
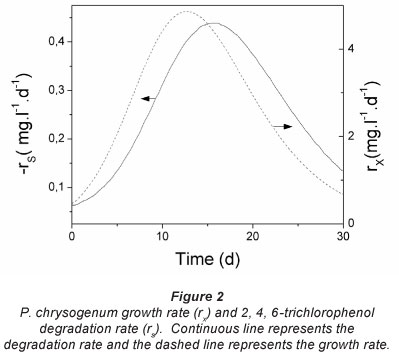
The TCP concentration was reduced by 85% at Day 26, and the appearance of new peaks in the HPLC chromatograms was observed, indicating the active biotransformation of TCP (Fig. 3). Identification of the intermediate products of TCP degradation were made by comparing the chromatographic characteristics of the peaks with those of pure compounds. Under co-metabolic conditions some TCP sub-products were putatively identified: two of the compounds detected have the same retention times (rt) as the external standards hydroquinone (3.32 min) and benzoquinone (3.78 min), and these identifications were confirmed by co-chromatography of the sample and the internal standards. The TCP degradation intermediates identified are the same as those previously described for white rot fungi (Field and Sierra-Alvarez, 2008). In addition, other minority compounds were observed at very low concentrations, but these were not identified.
It is widely reported by several authors that filamentous fungi, including several Penicillium species like P. chrysogenum, can metabolise 2,4,6-TCP to 2,4,6-trichloroani-sole (TCA). (Alvarez-Rodríguez et al., 2002; Fontana and Altamirano, 2010; Prak et al., 2007). However, the retention time of the external standard TCA and the sample, and the co-chromatography results for these, were not in agreement. Future studies, using HPLC-MS and GC-MS need to be conducted to determine the chemical structures of the intermediate metabolites in TCP transformation.
Laccases and peroxidases are two groups of enzymes involved in lignin and hydrocarbon degradation by fungi. White-rot fungi are responsible for lignin degradation in wood. However, most C turnover from plant-residue lignin in soil cannot be attributed only to white-rot fungi (Rodríguez et al., 1996). However, neither laccases nor peroxidase activity (manganese and lignin peroxidases) was detected in P. chrysogenum ERK1. In a previous report, Rodriguez et al (1996) detected an extracellular laccase capable of oxidising ABTS in ligninolytic cultures of Penicillium chrysogenum, but lignin peroxidase, manganese-dependent peroxidase or aryl-alcohol oxidase was not detected in the same culture. It is well known that ligninolytic fungi from various ecological niches have very different enzyme activity patterns. On the other hand, Szewczyk and Dlugonski (2009) have reported that the biodegradation of PCP by filamentous fungi is mediated by cytochrome P450 monooxygenases rather than ligninolytic enzymes (laccases and peroxidases), also producing quinones as biodegradative intermediates. Therefore, further research is necessary to identify more intermediate metabolites and to elucidate the enzymatic pathways used in this biodegradation process.
The phytotoxicity of plant growing media based on the germination index (GI) of seeds was evaluated as described by Zucconi et al. (1985). This is one of the most common phytotoxicity assays used in the literature. The GI combines measurements of relative seed germination and relative root elongation that are both sensitive to the presence of phytotoxic compounds. Several species have been traditionally used for evaluating phytotoxicity. However there are no standardised seed species in use worldwide (Osma et al., 2010; Warman, 1999).
Wheat (Triticum aestivum) was used for this assay, because it is a common crop in Argentinean fields. Seed germination was 100% for wheat in LMS as a control, 73.11% for seeds treated with the products of TCP degradation and 40.22% for 10 mg-l-1 of TCP; root elongation was 14.73, 11.08 and 9.41 mm, respectively. Therefore, GI values were: 55.26% for seeds treated with TCP residues and 25.88% for TCP 10 mg-l-1, showing that fungal treatment reduced the phytoxicity of TCP by approximately 50%. According to Zucconi et al. (1985), values for GI lower than 50% indicate high phytotoxicity, values between 50% and 80% indicate moderate phytotoxicity and values over 80% indicate that the material presents no phytotoxicity. Therefore, from the phytotoxicity assays of products of TCP transformation, we can conclude that these products are moderately phytotoxic for wheat. Further studies are required; for example, using mixed cultures to improve the reduction of the toxicity of TCP transformation products.
Conclusions
Degradation of TCP by Penicillium chrysogenum has been demonstrated successfully at laboratory scale. The biomass growth and degradation were fitted to a Gompertz sigmoid curve and to a specific first-order kinetic, respectively. Both models fit very well. Hydroquinone and benzoquinone were identified as degradation intermediates, although more studies need to be done to elucidate the enzymatic pathways and metabolites involved in the degradation of TCP. In addition, the TCP degradation products were moderately phytotoxic for wheat, while TCP was highly phytotoxic. This is the first work to report on the degradation potential of P. chrysogenum for 2, 4, 6-trichlorophenol.
Acknowledgements
This research was supported by the Universidad Nacional de Mar del Plata, the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) 2010-2011 PIP 00082, the Agencia Nacional de Promoción Científica y Tecnológica (ANPCYT) PICT 08-00528, and MAPFRE foundation. We would also like to thank Julian Paolini from CIATI, Rio Negro Argentina for his advice and help, and Hector L Villar for providing the wheat seeds.
References
ALVAREZ-RODRÍGUEZ M, LÓPEZ-OCANA L, LÓPEZ-CORO-NADO J, RODRÍGUEZ E, MARTÍNEZ M, LARRIBA G and COQUE J (2002) Cork taint of wines: Role of the filamentous fungi isolated from cork in the formation of 2,4,6-trichloroanisole by O methylation of 2,4,6-trichlorophenol. Appl. Environ. Microbiol. 68 5860-5869. [ Links ]
AMRANE A, ADOUR L and COURIOL C (2005) An unstructured model for the diauxic growth of Penicillium camembertii on glucose and arginine. Biochem. Eng. J. 24 125-133. [ Links ]
ASGHER M, BHATTI H, ASHRAF M and LEGGE R (2008) Recent developments in biodegradation of industrial pollutants by white rot fungi and their enzyme system. Biodegradation 19 771-783. [ Links ]
FIELD J and SIERRA-ALVAREZ R (2008) Microbial degradation of chlorinated phenols. Rev. Environ. Sci. Biotechnol. 7 211-241. [ Links ]
FONTANA AR and ALTAMIRANO JC (2010) Sensitive determination of 2,4,6-trichloroanisole in water samples by ultrasound assisted emulsification microextraction prior to gas chromatography-tandem mass spectrometry analysis. Talanta 81 1536-1541. [ Links ]
HAMIDI-ESFAHANI Z, HEJAZI P, SHOJAOSADATI SA, HOOGSCHAGEN M, VASHEGHANI-FARAHANI E and RINZEMA A (2007) A two-phase kinetic model for fungal growth in solid-state cultivation. Biochem. Eng. J. 36 100-107. [ Links ]
HOFRICHTER M, GÜNTHER T and FRITSCHE W (1992) Metabolism of phenol, chloro- and nitrophenols by the Penicillium strain Bi 7/2 isolated from a contaminated soil. Biodegradation 3 415-421. [ Links ]
LEITAO A, DUARTE M and OLIVEIRA J (2007) Degradation of phenol by a halotolerant strain of Penicillium chrysogenum. Int. Biodeterioration Biodegradation 59 220-225. [ Links ]
LEITAO AL (2009) Potential of Penicillium Species in the bioremediation field. Int. J. Environ. Res. Public Health 6 1393-1417. [ Links ]
LEONTIEVSKY A, MYASOEDOVA N, BASKUNOV B, EVANS C and GOLOVLEVA L (2000a) Transformation of 2,4,6-trichloro-phenol by the white rot fungi Panus tigrinus and Coriolus versicolor. Biodegradation 11 331-340. [ Links ]
LEVIN L, PAPINUTTI L and FORCHIASSIN F (2004) Evaluation of Argentinean white rot fungi for their ability to produce ligninmodifying enzymes and decolorize industrial dyes. Bioresour. Technol. 94 169-176. [ Links ]
MARR J, KREMER S, STERNER O and ANKE H (1989) Transformation and mineralization of halophenols by Penicillium simplicissimum SK9117. Biodegradation 7 165-171. [ Links ]
McALLISTER KA, LEE H and TREVORS JT (1996) Microbial degradation of pentachlorophenol. Biodegradation 7 1-40. [ Links ]
OSMA J, TOCA-HERRERA J and RODRIGUEZ-COUTO S (2010) Transformation pathway of Remazol Brilliant Blue R by immobilised laccase. Bioresour. Technol. 101 8509-8514. [ Links ]
PRAK S, GUNATA Z, GUIRAUD J-P and SCHORR-GALINDO S (2007) Fungal strains isolated from cork stoppers and the formation of 2,4,6-trichloroanisole involved in the cork taint of wine. Food Microbiol. 24 271-280. [ Links ]
REDDY VBG, SOLLEWIJN GELPKE MD and GOLD MH (1998) Degradation of 2,4,6-trichlorophenol by Phanerochaete chrysosporium: Involvement of reductive dechlorination. J. Bact. 180 5159-5164. [ Links ]
RODRIGUEZ A, FALCÓN M, CARNICERO A, PERESTELO F, DE LA FUENTE G and TROJANOWSKI J (1996) Laccase activities of Penicillium chrysogenum in relation to lignin degradation. Appl. Microbiol. Biotechnol. 45 399-403. [ Links ]
RUBILAR ARANEDA O (2007) Biorremediación de suelos contaminados con pentaclorofenol (PCF) por hongos de pudrición blanca. Universidad de la Frontera. 30 pp. [ Links ]
SAMSON R, SEIFERT K, KUIJPERS A, HOUBRAKEN J and FRISVAD J (2004) Phylogenetic analysis of Penicillium subgenus Penicillium using partial β-tubulin sequences. Stud. Mycol. 49 175-200.
SZEWCZYK R and DLUGONSKI J (2009) Pentachlorophenol and spent engine oil degradation by Mucor ramosissimus. Int. Biodeterioration Biodegradation 63 123-129. [ Links ]
TASELI BK and GOKCAY CF (2005) Degradation of chlorinated compounds by Penicillium camemberti in batch and up-flow column reactors. Process Biochem. 40 917-923. [ Links ]
WARMAN P (1999) Evaluation of seed germination and growth tests for assessing compost maturity. Compost Sci. Util. 7 33-37. [ Links ]
WOLSKI E, BARRERA V, CASTELLARI C and GONZALEZ J (2010) Biodegradation of phenol by a soil fungus: Identification and degradation potential. In: BMS-IMA (ed.) The 9th International Mycological Congress (IMC9: the Biology of Fungi), Edinburgh. Elsevier, Oxford, UK. 2-101. [ Links ]
ZUCCONI F, MONACO A, FORTE M and DE-BERTOLDI M (1985) Phytotoxins during the stabilization of organic matter. In: Gasser JCECe (ed.) Composting of Agricultural and Other Wastes. Elsevier Applied Science Publishers, London. 73-86. [ Links ]
ZWIETERING M, JONGENBURGER I, ROMBOUTS F and VAN 'T RIET K (1990) Modeling of the Bacterial Growth Curve. Appl. Environ. Microbiol. 56 1875-1881. [ Links ]
Received 25 October 2011; accepted in revised form 2 October 2012.
* To whom all correspondence should be addressed. +54 223 4816600; fax: +54 223 4810046; e-mail: ewolski@mdp.edu.ar














