Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
Water SA
versión On-line ISSN 1816-7950
versión impresa ISSN 0378-4738
Water SA vol.38 no.5 Pretoria ene. 2012
Sorptive removal of ciprofloxacin hydrochloride from simulated wastewater using sawdust: Kinetic study and effect of pH
Sunil Kumar BajpaiI; Manjula BajpaiI, *; Neelam RaiII
IDepartment of Chemistry, Polymer Research Laboratory, Government Model Science College (Autonomous), Jabalpur (Madhya Pradesh) - 482001, India
IIDepartment of Chemistry, Global Institute of Engineering and Management, Jabalpur (Madhya Pradesh) - 482001, India
ABSTRACT
The present work describes dynamic uptake of the antibiotic drug ciprofloxacin hydrochloride (CH), by using a cost-effective agricultural by-product - sawdust (SD). The sawdust was characterised by FTIR and SEM analysis. The sorbent particles were highly porous with average pore diameter of nearly 10 μm. The optimum pH and solid/liquid ratio for sorption of CH were found to be 5.8 and 2.0, respectively. The dynamic drug uptake data was applied to various kinetic models and their order of fitness was found to be pseudo second order > Elovich equation > power function model, as indicated by their regression values. The experimental equilibrium uptake values (q ) were in close agreement with those evaluated from the pseudo second order equation for initial sorbate concentrations of 10 and 20 mg-l-1 at 33°C. The drug uptake mechanism was found to be attractive non-electrostatic interactions, involving H-bonding interactions between H atoms and other electronegative species such as F, O and N of the drug molecule. The mechanism is discussed on the basis of pHpzc of sawdust and zwitterionic nature of drug CH. Mass transfer analysis was carried out using the drug uptake data obtained with sorbate concentrations of 10 and 20 mg-l-1. The used sorbent could be regenerated using 1.0 moll-1 HCl solution with a regeneration efficiency of nearly 85%.
Keywords: sawdust, antibiotic drug, pseudo second order, intra-particle diffusion, mass transfer analysis
Introduction
Groundwater contamination by pharmaceutical ingredients (analgesics, antidepressants, contraceptives, antibiotics. etc.) has become an environmental problem of widespread concern (Ghauch, 2008; Robinson et al., 2007; Zhou et al., 2006). Antibiotics are probably the most successful family of drugs so far developed to improve human health. Besides this fundamental application, antibiotics have also been used for preventing and treating animal and plants infection as well as for promoting growth in animal farming (Cabello, 2006; Martinez, 2009). All of these applications cause antibiotic drugs to be released in large quantities to natural ecosystems. As micro-contaminants, antibiotics in the aquatic environment may persist and be transported to reservoirs, supply sources and drinking water treatment plants (Ye et al., 2007). The potential presence of antibiotics in drinking water sources is of major concern due to the unknown health effects of chronic low-level exposure to antibiotics over a lifetime, if the antibiotics survive the water treatment process and persist in consumers' drinking water. In addition, these drugs cause unpleasant odours and skin disorders, and may cause microbial resistance among pathogen organisms or the death of microorganisms which are effective in wastewater treatment (Budyanto et al., 2008). The resistant bacteria may also cause disease that cannot be treated by conventional antibiotics (Andersons, 2003). For these reasons, antibiotic contamination of drinking water needs to be eliminated or minimised.
Many methods have been attempted, in the recent past, for the removal of antibiotic drugs from different water sources. These include coagulation and sedimentation (Boyd et al. 2003), biodegradation (Kimura and Hara, 2005), photo-transformation (Pereira et al., 2007), chlorination (Boyd and Zhang, 2005), ozonation (Ternes et al., 2003), nanofiltration through membranes (Koyuncu et al. 2008), and adsorption (Cahskan and Gokturk, 2010; Cuerda-Correa et al., 2010; Gauch et al., 2009; Putra et al., 2009; Reverra-Utrilla et al., 2009;). Out of these, adsorption processes have proved to be an effective technique because of major advantages such as applicability over a large concentration range of sorbate, effective removal efficiency, low instrumentation cost, and the presence of many rate-controllable parameters (Bajpai and Bhowmik, 2010). Recently, clays and oxides have been exploited for the removal of antibiotic drugs using adsorption technology (Chang et al., 2009; Li. et al., 2010). However, in the majority of the studies involving sorptive removal of antibiotics, activated carbon has been employed as a potential sorbent material (Cahskan and Gokturk, 2010; Cuerda-Correa et al., 2010; Putra et al., 2009; Reverra-Utrilla et al., 2009). The relatively higher production cost of activated carbon places a question mark on its large-scale application. Environmental chemists have therefore focused their attention on employing agricultural wastes as sorbents.
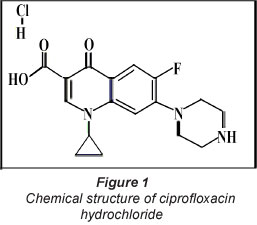
Ciprofloxacin hydrochloride (Fig. 1) is a wide-spectrum antibiotic that is active against Gram-positive and Gram-negative bacteria. Its presence in drinking water may cause headaches, diarrhoea, nervousness, tremors, nausea, vomiting, etc. (Eiselt et al., 2010). If this drug is present in relatively higher concentrations in drinking water then it may cause serious adverse effects including acute renal failure, elevation of liver enzymes, thrombocytopenia, eosinophilia and leucopenia, etc. (Baek et al., 2010). CH was once considered as an antibiotic of last resort for particular infections, but is now one of the most widely-distributed antibiotics in the United States and Europe (Bhandary et al., 2008). Although there have not been many studies reported in the literature regarding the removal of ciprofloxacin from aquatic systems, few studies, carried out in the recent past, may be mentioned here. Recently, Jiang et al. (2012) reported that potassium ferrate (lV), a strong oxidant, could be successfully employed for the removal of ciprofloxacin (CIP) from simulated wastewater. It was found that ferrate could remove at least 60% of CIP from model wastewater, even at very low ferrate doses (<0.3 mg-l-1). The initial pH of the CIP model wastewater sample had no influence on CIP removal. CIP removal efficiency by ferrate decreased significantly when final pH of the adsorption system was greater than the pKa of CIP (i.e. pH>8). In another study by Li et al. (2011), the interactions between CIP and kaolinite in aqueous solutions were investigated by batch experiment, XRD and FTIR analysis.
Quantitative correlations between the exchangable cations desorbed and CIP adsorbed confirmed experimentally that cation exchange was the dominant mechanism of CIP adsorption on kaolinite. Fitting of experimental data to the cation exchange model resulted in a selectivity coefficient of 27, suggesting a strong affinity of CIP for the negatively-charged kaolinite surface. In a similar study by Wu et al. (2010), the adsorptive removal of CIP by sodium montmorillonite (MMT) was reported in batch tests. The adsorption of CIP on MMT was instantaneous, with a large rate constant and a high initial rate. Higher CIP adsorption was achieved when solution pH was lower than the pKa value of CIP, above which the adsorption coefficient decreased significantly.
In order to make the overall process cost-effective and applicable at an industrial scale, it is essential to exploit cheap and easily available adsorbents, such as agricultural by-products. A thorough survey of the literature revealed that agricultural waste such as sawdust has not been employed for the removal of ciprofloxacin in the past. This study may therefore open new possibilities for removing ciprofloxacin-like antibiotic drugs by using highly cost-effective sorbents such as sawdust.
Experimental
Materials
Sawdust (SD) was collected from a local saw-mill (Prahlad Timber Merchant, MP, India). Ciprofloxacin hydrochloride (C17 H18FN3O3-HCl-H 2O, mol. wt. 385.82) with purity 99.8% was purchased from a local medical store. The structural formula of CH is given in Fig. 1. Other chemicals were obtained from Hi Media Chemicals, Mumbai, India and were of analytical grade. Double-distilled water was used throughout the investigation.
Preparation of sorbent
In order to make the overall sorption process more cost-effective, no chemical treatment of sawdust was done. It was dispersed in double-distilled water for a period of 7 days to remove all colouring materials present. The water was changed every 24 h. Sawdust was allowed to dry in a dust-free chamber at 50°C till it attained a constant weight. Finally, it was ground and passed through standard sieves to obtain particles with geometrical mean diameter of 250 μm. All experiments were performed using these sorbent particles.
Characterisation of sawdust (SD)
Fourier transform infrared (FTIR) spectroscopy analysis was carried out with Shimadzu FTIR Spectrophotometer (8400S, Shimadzu, Japan) using the KBr pelleting method. The physico-chemical parameters of sawdust were determined using n-heptane as a non-polar solvent and the method described by Eiselt et al. (2010). The chemical analysis of sawdust was performed at the Indian Institute of Technology, Mumbai, India. In order to investigate surface morphology, SEM images were recorded using a LEO 435 VP scanning electron microscope (LEO Electron Microscopy Ltd., Cambridge, England) operating at 15 kV. The samples were placed on a conducting carbon tape above the metal stub and coated with a thin layer of gold for charge dissipation during SEM imaging.
Determination of pHpzc
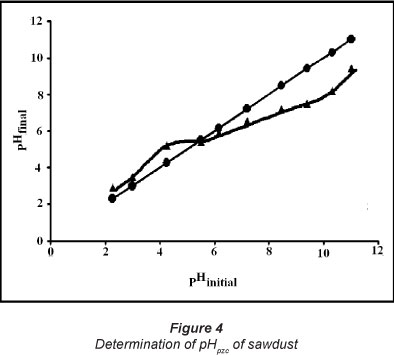
The point of zero charge (pHpzc) was determined using the method described by Mall et al. (2006). A series of 0.01 molt-1 KNO3 solutions (50 mt each) were prepared and their pH was adjusted in the range of 1.0 to 12.0 by addition of 0.1 molt-1 HCl and NaOH. To each solution, 1 g of adsorbent sawdust was added and the solution was kept for a period of 48 h. The final pH of the solution was recorded and a graph was plotted between pHinitial and pHfinal. The point of intersection of this curve with the pHinitial = pHfinal linear plot yielded point of zero charge.
Determination of active sites
Acidic and basic sites on sawdust were determined using the acid-base titration method proposed by Boehm (1994). The acidic sites were neutralised using 0.1 molt-1 NaOH, while the basic sites were neutralised with 0.1 molt-1 HCl. The acidic and basic sites were determined by leaving 50 mt of 0.1 molt-1 titration solution and 0.2 g of the sawdust for 5 days at room temperature with occasional shaking, before titrating a 10 mt sample with 0.1 molt-1 HCL or NaOH solution.
Sorption experiments
The sorbent to sorbate ratio plays a key role in obtaining the maximum per cent sorption. To investigate this, various quantities of sorbent SD (mg) were agitated with 20 mt of sorbate solutions with initial concentration of 40 mg-l-1, at 33ºC for a period of 1 h (this was considered as sufficient contact time to reach equilibrium).
For the kinetic studies, sorption experiments were carried out by agitating 50 mg of sorbent SD with 20 mt of aqueous solutions of drug CH with concentrations of 10 and 20 mg-l-1 in 125 mt Erlenmeyer flasks under constant stirring in a flask shaker equipped with a thermostat (Rivotek, India).
The adsorbate solution was taken out at different time intervals, centrifuged at 120 r-min-1 and the supernatant analysed spectrophotometrically at 277 nm to determine residual concentration of drug in the solution. In preliminary experiments the detection limit of the drug using a spectrophotometer was found to be 4 mg-l-1. The amount of drug sorbed, in mg per gram of sorbent (i.e. qt or x/m), and per cent sorption were calculated using the following expression (Baek et al., 2010):
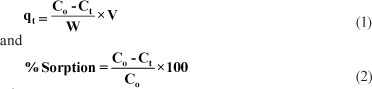
where:
C0 is the initial concentration of drug solution (mg-l-1)
Ct is concentration of drug solution (mg-l-1) after time t of contact with SD
V is volume of solution (l) taken for sorption experiment
W is the amount of sorbent (g) taken in the flask
All of the sorption experiments were performed in triplicate and average values reported. Similar procedures were applied to perform adsorption experiments at constant CH concentration and varying pH, by agitating the solution for 1 h. The pH of the drug solution was adjusted to the required value by adding 0.1 mol£-1 HCl or NaOH.
Results and discussion
Characterisation of sorbent
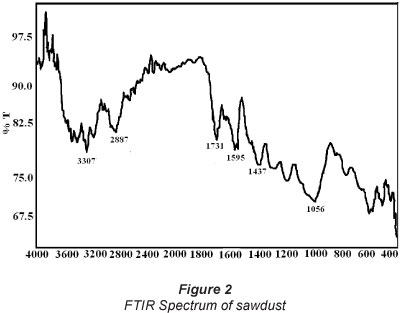
The FTIR spectrum of plain sawdust is shown in Fig. 2. The absorption band, appearing around 3 614 cm-1, indicates the existence of free and intermolecular bonded -OH groups. A broad band, at 3 440-3 460 cm-1, is attributed to the sum of contributions from water; -OH groups can undergo protonation-deprotonation reactions (phenolic and alcoholic) (Anirudhan et al., 1998). The band appearing at 2 880-2 900 cm-1 corresponds to CH stretching vibrations from -CH2 group. Likewise, the band present at around 1 731 cm-1 could be assigned to -C=O stretching attributed to lignin aromatic groups. The presence of a band at around 1 600 cm-1 may be due to amide (N-H) groups in sawdust. Moreover, the band at 1 437 cm-1 corresponds to -OH deformation. Finally, additional peaks at 532 and 451 cm-1 can be assigned to bending vibration modes of aromatic compounds (Bansal et al., 2009).
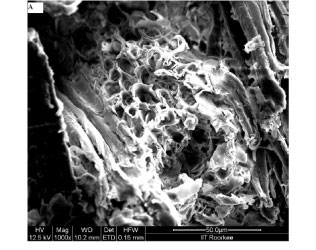
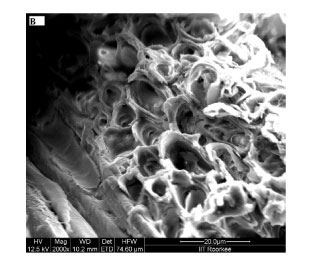
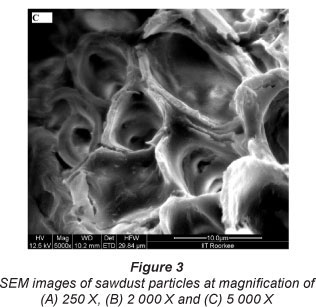
The physicochemical characterisation of SD, given in Table 1, indicates that sorbent sawdust is fairly porous in nature. In order to investigate the surface morphology of the sawdust, its SEM images were recorded. These images are shown in Fig. 3. The surface of sawdust particles appears to be quite rough as seen at 250 X magnification, shown in Fig. 3(A). Further magnification (2 000 X), as shown in Fig. 3(B), clearly shows the presence of pores on the surface. The average size of the pores, as evaluated using the 5 000 X magnified image shown in Fig. 3 (C), was found to be nearly 10 μιη. Therefore, it may be inferred that the sorbent used in this study is quite porous in nature. The high value of per cent porosity (i.e. 95%), as shown in Table 1, supports this observation.
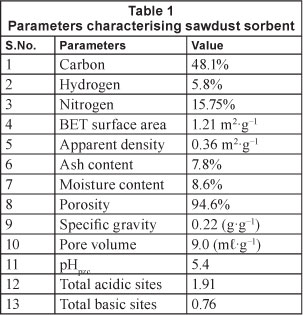
The point of zero charge, i.e. pHpzc, as determined using the point of intersection of the experimental curve with the theoretical linear plot (see Fig. 4) was found to be around 5.4. This indicates that below this pH the sawdust particles acquire positive charge due to protonation of R-OH groups into R-OH2+ groups, whereas above this pH a negative charge exists due to ionisation of -COOH groups into -COO- groups. The value of pHpzc is towards the lower or acidic end of the pH scale; this observation was also supported by the observed concentrations of active acidic and basic sites, of 1.81 and 0.76 mmolg-1, respectively.
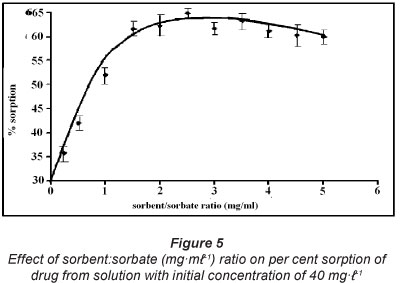
Effect of sorbent:sorbate (mg-ml-1) ratio on drug uptake
The per cent drug sorption was plotted against sorbent:sorbate (mgml-1) ratios as shown in Fig. 5. It is clear that per cent sorption increases with sorbent:sorbate ratio and finally attains an almost constant value of 64% when the ratio becomes 2.0 (i.e. 40 mg of SD in 20 ml of CH solution). The percentage sorption remains almost constant even when the sorbent:sorbate ratio is increased beyond 2.0. Therefore, in all future experiments the sorption studies were carried out with sorbent:sorbate ratio of 2.0. This finding may be attributed to the fact that the increase in sorbent dose causes an increase in the number of active sites available for drug uptake. However, when all of the sites have been fully occupied by drug molecules, further increase in sorbent dose does not cause an increase in drug uptake, and the ratio of concentration of CH in equilibrium to CH sorbed is determined by the partition coefficient of the drug molecule, between the sorbent and sorbate phase. Therefore, further increase in ratio does not cause any more increase in drug uptake. Similar results have also been reported elsewhere (Chakravarty et al., 2008).
Effect of pH on drug uptake
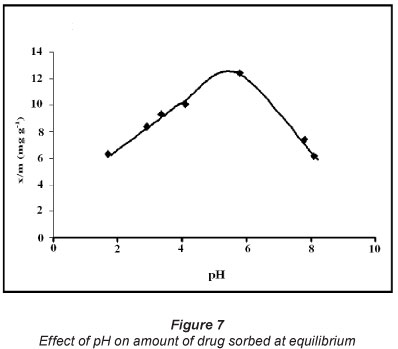
The results for studies of pH-effect, as shown in Fig. 7, reveal that drug adsorption is fairly low at low pH and then continues to increase as the solution pH is raised. The maximum uptake of 11.6 mg.g1 is observed at pH 5.8, and thereafter uptake begins to decrease with further increase in solution pH. The pH of the sorbent/sorbate adsorption system plays a significant role in governing the amount of sorbed CH. This parameter becomes especially important when sorbent or sorbate both carry groups which may be protonated /deprotonated with change in pH of the sorption system. The pKa1 and pKa2 values of ciprofloxacin (CH) are 5.5 and 7.7, respectively (Zhang and Huang, 2007). The cationic form, CH+, exists due to protonation of the amine group in the piperazine moiety (Fig. 1) when solution pH is below 6.1. As the solution pH is above 8.7, the anionic form, CH-, prevails, due to ionisation of carboxylic groups. When solution pH is between 5.5 and 7.7 the zwitterionic form, CH±, is the dominant species, which results from the charge balance between the above two groups (see Fig. 6).
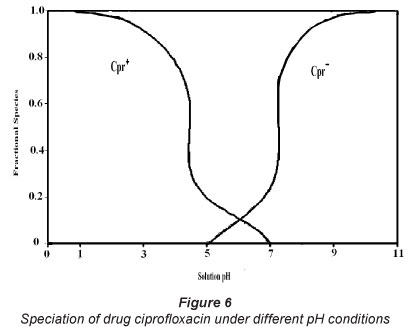
The adsorbent SD has a point of zero charge value (i.e. pHpzc) of 5.4. Below this pH the adsorbent possesses positive charge, while it acquires a negative charge above pH 5.4.
In the light of the facts mentioned above, the observed findings may be explained as follows. Initially, when pH of the adsorption system is fairly low, the sawdust/adsorbent particles carry positive charge and the CH molecules also have a positive charge. Therefore, the electrostatic repulsion between them does not favour adsorption and less drug uptake occurs. When solution pH is around 5.8, the adsorbent particles exhibit near-neutrality (pHpzc=5.4) and the ciprofloxacin molecules also exist as zwitterionic species, thus carrying no charge. In this condition, the electrostatic repulsion forces are not operative and the maximum uptake of 11.6 mgg-1 is obtained, due to important non-electrostatic attractive interactions which also operate in the sorption process for CH, such as H-bonding interactions between electronegative moieties, such as N, F and O, present in the ciprofloxacin molecule and -OH of cellulosic sawdust. A low adsorption capacity also supports the argument proposing the absence of favourable electrostatic interactions. Therefore, in the pH range of 5.5 to 7.7, the Van der Waals forces can be said to be responsible for observed drug uptake. However, when pH of the adsorbate solution is further increased beyond 7.7, the sawdust particles exhibit negative charge and the ciprofloxacin molecules exist as CH- moieties. Hence sorption is not favoured due to repulsion among similar charges.
Therefore, it may be inferred that drug uptake is maximum, although not appreciable, in the vicinity of pH 5.8, and decreases on either side due to the existence of repulsive forces among similar charges. Hence a non-exchange adsorption mechanism prevailed in this study. It should be noted that, though the solubility of ciprofloxacin is pH-dependent, ranging from 6 190 mgl-1 at pH 5.0 to 150 mgl-1 at pH 7 at 37ºC (Li et al., 2007), the concentrations used in the pH-effect and other sorption experiments were far below these solubility limits. Therefore, pH variation did not alter the initial concentrations of drug solutions in these investigations.
Kinetic drug uptake studies
Effect of initial drug concentration and contact time
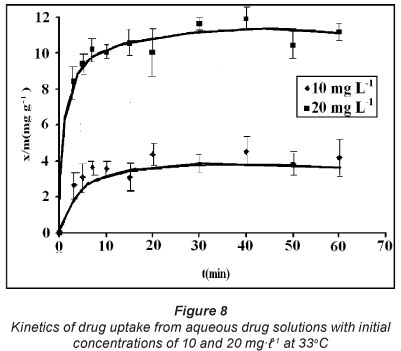
The contact time necessary to reach equilibrium depends upon the initial sorbate concentration (Dogan et al., 2006). The effect of contact time on the adsorption of CH on the SD at different initial drug concentrations is shown in Fig. 8. It is clear that the amount of drug sorbed per unit mass of sorbent (i.e. x/m) increases with initial drug concentrations. The amount of drug sorbed at equilibrium increased from 4.39 to 11.91 mgg-1 as the initial concentration was raised from 10 to 20 mgl-1. The initial concentration provides an important driving force to overcome all mass transfer resistances of drug molecules between the solution and solid phases. Hence, higher drug concentration enhances the sorption process. It could be said that the higher the sorbate concentration, the faster will be diffusion of sorbate molecules from the sorbent surface into the micropores. A close look at the dynamic uptake profiles reveals some interesting facts. The drug sorption process appears to be very fast and nearby 80% of the total amount of the drug is sorbed in the first 5 min. However, later on, the process becomes slow and finally equilibrium is attained within 40 to 60 min. The fast uptake of CH molecules is traceable to solute transfer, as there are only sorbate and sorbent interactions with negligible interference from solute-solute interactions (Baek et al., 2010). The initial rate of sorption was therefore greater for higher initial sorbate concentration; the resistance to the CH uptake diminished as the mass transfer driving force increased.
Kinetic models
The main issue when searching for an appropriate sorption mechanism is to select a mathematical model that not only fits the experimental data with satisfactory accuracy but also complies with a reasonable sorption mechanism. Generally, several steps are involved during the sorption process by porous sorbent particles:
bulk diffusion
external mass transfer (boundary layer or film diffusion) between the external surface of the sorbent particles and the surrounding fluid phase
intra-particle transport within the particle
reaction kinetics at phase boundaries.
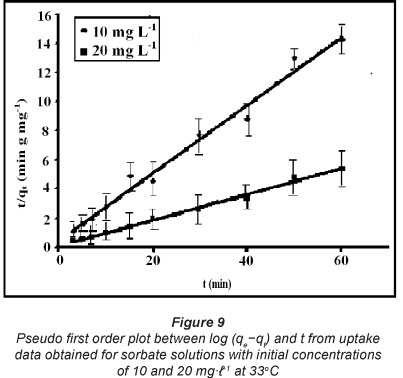
However, in the majority of the research work conducted it has been observed that sorbate uptake is usually governed by two mechanisms: external and internal diffusion (Hamdaoni and Chiha, 2007). In the case of chemisorption the rate of sorption is usually controlled by the kinetics of bond formation. The kinetic drug uptake data, shown in Fig. 8, was analysed using various kinetic models, namely, pseudo first order, pseudo second order, simple Elovich equation and power function model. The pseudo first order equation of Lagergren (Lagegren, 1898) is given, in its most popular logarithmic form, as:

where:
k1 is the first-order rate constant for adsorption
qt and qe are amount of drug sorbed per g of sorbent (i.e. x/m)) at time t and at equilibrium, respectively.
The slope and intercept of ln(qe-qt) versus t plots can be used to determine k1 and qe. The pseudo second order model (Ho and McKay, 2000) can be represented in the following form:

where k2 is the second-order rate constant (gmg-1min-1). After integrating with the initial condition, Eq. (4) takes the form:

The linear plot, obtained between 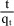 and t may be used to determine rate constant k2 and drug uptake at equilibrium qe.
and t may be used to determine rate constant k2 and drug uptake at equilibrium qe.
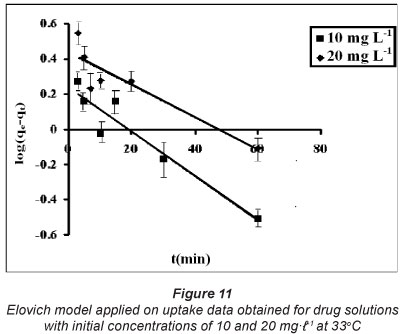
The Elovich model is used to describe second-order kinetics, assuming that the actual solid surface is energetically heterogeneous (Razmovsla and Sciban, 2008). This model is usually expressed as:

In addition, the initial sorption rate (h) may be given as:

where:
qt is the amount sorbed (mgg-1) at time t
α and β are constants during any one experiment.
The constant α can be regarded as the initial rate since 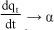 as qt 0. Integration of Eq. (6), assuming the initial boundary conditions qt = 0 at t = 0, yields:
as qt 0. Integration of Eq. (6), assuming the initial boundary conditions qt = 0 at t = 0, yields:

To simplify the Elovich equation assuming α β t >>>1 and applying the boundary condition qt = 0 at t = 0 and qt = qt at t = t, Eq. (8) can be written as:

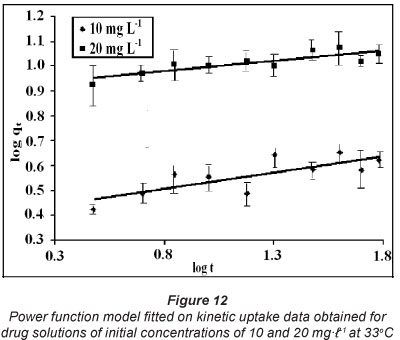
Hence, constants α and β can be obtained from the slope and intercept of the linearized plot of qt against ln t. Finally, the power function model (Srihari and Das, 2008) is given as:

A linear plot between log qt and log t gives the constants a and b of the power function model. The constant a represents the initial rate and refers to the y-intercept of the straight-line plot. The constant b is the slope of the linear plot and measures the rate constant of the uptake process.
These kinetic models were applied on the dynamic sorption data obtained for drug solutions with initial concentrations of 10 and 20 mgt-1 at 33°C. The linear plots, obtained for the pseudo first order, pseudo second order, Elovich model and power function model have been illustrated in Figs. 9, 10, 11 and 12, respectively. Based on regression values obtained, the order of fitness of these models was: pseudo second order > Elovich model > power function model > pseudo first order. The various kinetic parameters associated with these models are presented in Table 2. A close look at the values of various parameters displayed reveals that the pseudo second order rate constant k2 decreases with increase in the initial sorbate concentration, while the rate of drug sorption increases. The observed decrease in value of k2 may simply be attributed to the presence of the a 2 term in the denominator 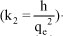
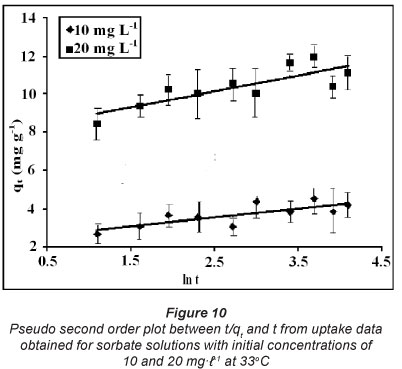
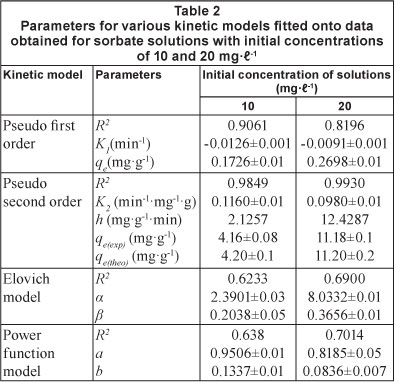
It is interesting to note that for the pseudo second order model, the theoretical equilibrium uptake values (i.e. qe(theo)) are fairly close to the experimental values (i.e. qe()), again confirming the suitability of this model for interpreting kinetic uptake data. Similar results have also been reported for methylene blue adsorption on montmorillonite (Almeida et al., 2009).
External diffusion sorption model
In the present study, bulk diffusion was avoided by proving sufficient agitation during the sorption process. Thus it may be assumed that rate is not limited by mass transfer from the bulk liquid to the surface of the sorbent particles. Under such conditions, diffusion from the film to the surface of the sorbent particles, also known as external diffusion, may govern the sorption process. In the case of strict surface adsorption, the variation in the adsorption rate should be proportional to the first power of sorbate concentration. However, when the pore diffusion limits the uptake process, the relationship between initial solute concentration and rate of adsorption no longer remains linear. The external diffusion model was used to apply the kinetic uptake data obtained using drug solutions with initial concentrations of 10 and 20 mg-l-1 at 33°C (Fig. 4) The expression for the external diffusion model is given as (Hamdaoni and Chiha, 2007):

where:
C0 and Ct are drug solution concentrations (mg-l-1) at time t=0 and at time t, respectively
ks is the external mass transfer coefficient (mg-min-l)
V is volume of equilibrating solution (L)
A indicates the surface area (m2g-1) of sorbent.
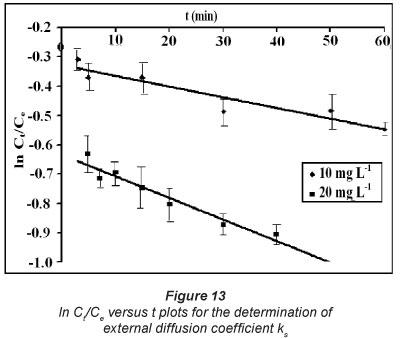
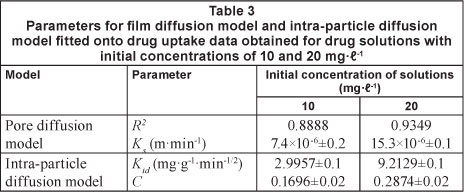
Figure 13 represents  versus t profiles, for kinetic CH drug uptake data. The plots obtained were quite linear with regression values in the range of 0.89 to 0.93. The external mass transfer coefficients, k , calculated using slopes and intercepts, were found to be 7.4 x 10-6 and 15.3 x 10-6 mg-min-1, respectively.
versus t profiles, for kinetic CH drug uptake data. The plots obtained were quite linear with regression values in the range of 0.89 to 0.93. The external mass transfer coefficients, k , calculated using slopes and intercepts, were found to be 7.4 x 10-6 and 15.3 x 10-6 mg-min-1, respectively.
Intra-particle diffusion model
All of the models discussed above are not able to describe the phenomena of pore diffusion and intra-particle diffusion, which are often the rate-limiting steps in a batch reactor system. The possibility of intra-particle diffusion was investigated using the where:
kid is the intra-particle diffusion rate constant (mg-g-1-min-1/2).
Weber Morris equation (Weber and Morris, 1963):

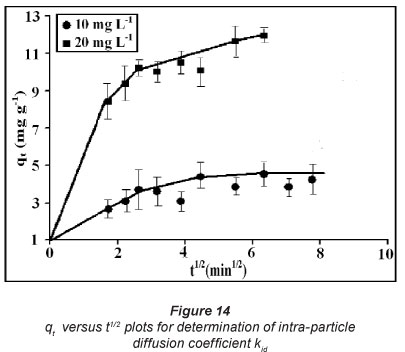
According to Eq. (11), a plot of q versus t1/2 should be a straight line with slope k{A and intercept C. If this linear plot passes through the origin then it indicates that intra-particle diffusion is the rate-controlling step. However, if the straight line does not pass through the origin this indicates that there is a difference between the rates of mass transfer in the initial and final steps of sorption, and that some other mechanism, along with intra-particle diffusion, is involved. To investigate this, q versus t1/2 plots were obtained for dynamic uptake of the drug using the data shown previously (Fig. 14). The results, as shown in Fig. 12, indicate that the plots are bi-phasic in nature, with an initial smooth curve followed by a linear plot. This indicates that diffusion through pores is not the only rate-limiting step. The slope of the linear part yielded values for intra-particle diffusion coefficients, while intercepts give the measure of boundary layer thickness. All of these values are given in Table 3. A close look at these parameters indicates that intraparticle diffusion, kid, and boundary layer thickness, I, increase with initial concentrations of the sorbate solutions.
Mass transfer analysis
The sorptive removal of drug by sawdust may be assumed to occur by the 3-step model given below:
Step 1: Mass transfer of drug molecules from aqueous solution to the adsorbent surface
Step 2: Intra-particle diffusion or migration of drug molecules within pores of the sorbent
Step 3: Adsorption of drug molecules at the interior sites of the sorbent
Mass transfer analysis of CH during the uptake process, involving the above 3 steps, was conducted using the mass transfer diffusion model given below (Mckay et al., 1981):
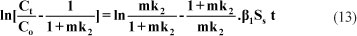
The value of m and Ss were determined using the following equation:

and

where:
m is the mass of sorbent per unit volume of particle free sorbate solution (gl-1)
w is the weight of adsorbent (g)
v is the volume of particle-free adsorbate solution (l)
dF is the particle diameter (cm)
PF is the density of sorbent (gl-1)
φ is the porosity of sorbent particles
Ct is the concentration (mgl-1) of drug solution at time t
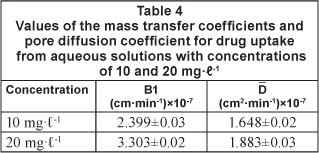
The values of mass transfer coefficient (β1), as determined from the sloe and intercept of the straight line plots obtained between in [ ] and t, are given in Table 4. The low values of the mas s transfer coefficients suggest that the velocity of mass transport of drug molecules from the bulk to the surface of sawdust particles is fairly low. The pore diffusion coefficient for the intra-particle transport of drug molecules was calculated assuming spherical geometry of the sorbent particle using the following equation (Bhattacharya and Venkobechar, 1984):
] and t, are given in Table 4. The low values of the mas s transfer coefficients suggest that the velocity of mass transport of drug molecules from the bulk to the surface of sawdust particles is fairly low. The pore diffusion coefficient for the intra-particle transport of drug molecules was calculated assuming spherical geometry of the sorbent particle using the following equation (Bhattacharya and Venkobechar, 1984):
rQ is the radius of the sorbent,
D is the pore diffusion coefficient (cm2min-1)
t1/2 is the time required for half sorption
The values of D have also been given in Table 4.
Desorption study
The overall cost-effectiveness of a sorbent depends upon its re-usability, which reduces the overall cost of any sorption process. Desorption studies were carried out using HCl solutions of different concentrations, ranging from 0.2 to 1.2 mol-l-1. It was found that per cent desorption of the drug increased with increasing concentration of HCl solution, attaining an optimum value of nearly 85% when HCl concentration exceeded 1.0 mol-l-1. The strong desorption efficiency of HCl may simply be attributed to the fact that in an acidic medium the adsorbent sawdust and CH both acquire a positive charge and the resulting electrostatic repulsion between them results in desorption of drug molecules.
Conclusion
From the above study it may be concluded that sawdust (SD) has great potential for removal of antibiotic drug CH from water. The relatively poor sorption of CH onto sawdust is due to weak Van der Waals forces of attraction between adsorbent and sorbate molecules. Kinetic sorption is optimum at a pH value of 5.8 and follows pseudo second order kinetics. The sorption mechanism involves intra-particle diffusion processes. The sorbent may be regenerated with a fair regeneration efficiency of 85%. This work showed that this agricultural waste could be used as an effective sorbent for antibiotic drug removal, representing an effective and environmentally-clean utilisation of waste material. However, more studies are needed to optimise the system from the point of view of regeneration, to investigate the economic aspects and to confirm the applicability of the sorbent under practical conditions, in batch as well as column sorption systems, using hospital drainage water.
Acknowledgements
The authors are thankful to Dr OP Sharma for providing the experimental facilities.
References
ALMEIDA CAP, DEBACHER NA, DOWNS AJ, COTTER L and MELLO CAD (2009) Removal of methylene blue from colored effluents by adsorption on montmorillonite clay. J. Colloid Interf. Sci. 332 46-53. [ Links ]
ANDERSON DI (2003) Persistence of antibiotic resistant bacteria. Curr. Opin. Microbiol. 6 489-493. [ Links ]
ANIRUDHAN TS and SREEDHAR MK (1998) Adsorption thermodynamics of Co(ll) on polysulfide treated sawdust. Ind. J. Chem. Technol. 5 41-47. [ Links ]
BAEK MI-HWA, IJAGBEMI CO SE-JIN O and DONG-SU K (2010) Removal of Malachite Green from aqueous solution using degreased coffee bean. J. Hazardous Mater. 176 820-828. [ Links ]
BAJPAI SK and BHOWMIC M (2010) Adsorption of diclofenac sodium from aqueous solution using polyaniline as a potential sorbent. Part I: Kinetic studies. J. Appl. Polym. Sci. 117 (6) 3615-3622. [ Links ]
BANSAL M, SINGH D and GARG VK (2009) Chromium(lV) uptake from aqueous solution by adsorption onto timber industry waste. Desalination Water Treat. 12 238-246. [ Links ]
BELLO OS, ADELAIDE OM, HAMMED MA and POPOOLA OAM (2010) Kinetic and equilibrium studies of methylene blue removal from aqueous solution by adsorption on treated sawdust. Macedocian J. Chemical Eng. 29 (1) 77-85. [ Links ]
BHATTACHARYA AK and VENKOBECHAR C (1984) Removal of cadmium (II) by low cost adsorbents. J. Environ. Eng. 110 110-122. [ Links ]
BHANDARI A, CLOSE LI, KIM W, HUNTER RP, KOCH DE and SURAMPALLI RY (2008) Occurrence of ciprofloxacin, sulfo-methoxazole and azithromycin in municipal wastewater treatment plants. Pract. Period. Hazardous Toxic Radioact. Waste 12 275-280. [ Links ]
BOEHM HP (1994) Some aspects of the surface chemistry of carbon blacks and other carbons. Carbon 32 759-769. [ Links ]
BOYD GR, REEMTSMA H, GRIMM DA and MITRA S (2003) Pharmaceuticals and personal care products in surface and treated waters of Louisiana, USA and Ontario. Can. Sci. Total Environ. 311 135-149. [ Links ]
BOYD GR, ZHANG S and GRIMM DA (2005) Naproxen removal from water by chlorination and biofilm processes. Water Res. 39 668-676. [ Links ]
BUDYANTO S, SOEDJONO S, IRAWATI W and INDRASWATI N (2008) Studies of adsorption equilibria and kinetics of amoxicillin from simulated wastewater using activated carbon and natural bentonite. J. Environ. Prot. Sci. 2 72-80. [ Links ]
CABELLO FC (2006) Heavy use of prophylactic antibiotics in aqua- culture: a growing problem for human and animal health and for the environment. Environ. Microbiol. 8 1137-1144. [ Links ]
CAHSKAN E and GOKTURK S (2010) Adsorption characteristics of sulfamethoxazole and metronidazole on activated carbon. Sep. Sci. Technol. 45 (2) 244-255. [ Links ]
CHAKRAVARTY S, PIMPLE S, CHATURVEDI HT, SINGH S and GUPTA KK (2008) Removal of copper from aqueous solution using newspaper pulp as an adsorbent. J. Hazardous Mater. 159 396-413. [ Links ]
CHANG PH, JEAN JS, JIANG WT and LI Z (2007) Mechanism of tetracycline sorption on rectorite. Colloids Surf. A 339 94-99. [ Links ]
CUERDA-CORREA EM, DOMINGUEZ-VARGAS JR, OIVARES-MARIN FJ and BELTRAN DE HEREDIA J (2010) On the use of carbon blacks as potential low-cost adsorbents for the removal of non-steroidal anti-inflammatory drugs from river water. J. Hazardous Mater. 177 1046-1053. [ Links ]
DOGAN M, ALKAN M, DEMIRBAS O, OZDEMIR Y and OZMETIN C (2006) Adsorption kinetics of maxilon blue GRL onto sepiolite from aqueous solutions. Chem. Eng. J. 124 89-101. [ Links ]
EISELT P, YEH J, LATWALA RK, SHEA LD and MOONEY DJ (2010) Porous carriers for biomedical applications based on algi- nate hydrogels. Biomaterials 21 1921-1927. [ Links ]
GHAUCH A (2008) Rapid removal of flutriafol in water by zero-valent iron powder. Chemosphere. 71 816-826. [ Links ]
GHAUCH A, TUQAN A and ABOU ASSI H (2009) Antibiotic removal from water: Elimination of amoxicillin and ampicillin by microscale and nanoscale iron particles. Environ. Pollut. 157 1626-1635. [ Links ]
HAMDAONI O and CHIHA M (2007) Removal of methylene blue from aqueous solution by wheat bran. Acta Chim. Slov. 54 407-418. [ Links ]
HO YS and MCKAY G (2000) The kinetics of sorption of divalent metal ion onto sphagnum moss peat. Water Res. 34 735-742. [ Links ]
JIANG J-Q, ZHOU Z and PAHL O (2012) Preliminary study of ciprofloxacin(cip) removal by potassium ferrate (Vl). Sep. Purif. Technol. 88 95-98. [ Links ]
KIMURA K, HARA H and WATANABE Y (2005) Removal of pharmaceutical components by submerged membrane bioreactors (MBRs). Desalination 178 135-140. [ Links ]
KOYUNCU OA, ARIKAN MR, WIESNER and RICE C (2008) Removal of hormones and antibiotics by nanofiltration membranes. J. Memb. Sci. 309 94-101. [ Links ]
LAGERGREN S (1898) About the theory of so-called adsorption of soluble substances. K. Sven. VetenskapsakadHandl. 24 1-39. [ Links ]
LI X, ZHI F and HU Y (2007) Investigation of excipient and processing on solid phase transformation and dissolution of ciprofloxacin. Int. J. Pharm. 328 177-182. [ Links ]
LI Z, SCHULZ L, ACKLEY C and FENSKE N (2010) Mechanism of tetracycline adsorption on kaolinite with pH-dependent surface charges. J. Colloid Interface Sci. 351 254-260. [ Links ]
LI Z, HONG H, LIAO L, ACKLEY CJ, MACDONALD RA, MIHELICH AL and EMARD SM (2011) A mechanistic study of ciprofloxacin removal by kaolinite. Colloidal Surf. B Biointerfaces 88 (1) 339-344. [ Links ]
MARTINEZ JL (2009) Environmental pollution by antibiotics and by antibiotic resistance determinants. Environ. Pollut. 157 2893-2902. [ Links ]
MALL ID, SHRIVASTAVA VC, KUMAR GVA and MISHRA IM (2006) Characterization and utilization of mesoporous fertilizer plant waste carbon for adsorptive removal of dyes from aqueous solution. Colloids Surf. A Physicochem. Eng. Aspects 278 (1-3) 175-187. [ Links ]
McKAY G, OTTERBURN MS and SWEENY AG (1981) Surface mass transfer process during color removal from effluent using silica. Water Res. 15 327-331. [ Links ]
PEREIRA VJ, LINDANE KG and WEINBERG HS (2007) Evaluation of UV irradiation for photolytic and oxidative degradation of pharmaceutical compounds in water. Water Res. 41 4413-4423. [ Links ]
PUTRA EK, PRANOWO R, SUNARSO J, INDRASWATI N and ISMADJI S (2009) Performance of activated carbon and bento- nite for adsorption of amoxicillin from wastewater: Mechanisms, isotherms and kinetics. Water Res. 43 2419-2430. [ Links ]
RAZMOVSLA R and SCIBAN M (2008) Biosorption of Cr(VI) and Cu(II) by waste tea fungal biomass. Ecol. Eng. 34 179-186. [ Links ]
REVERRA-UTRILLA J, PRADOS-JOYA G, SANCHEZ-POLO M, FERRO-GARCIA MA and BAUTISTA-TOLEDO I (2009) Removal of nitroimidazole antibiotics from aqueous solution by adsorption / bioadsorption on activated carbon. J. Hazardous Mater. 170 298-305. [ Links ]
ROBINSON G, JUNQUA R, COILLIE VAN and THOMAS O (2007) Trends in the detection of pharmaceutical products and their impact and mitigation in water and waste water in North America. Anal. Bioanal. Chem. 387 1143-1150. [ Links ]
SRIHARI V and DAS A (2008) The kinetic and thermodynamic studies of phenol-sorption onto three agro-based carbons. Desalination 225 220-234. [ Links ]
STANKOVIC V, BOZIC D, GORGIEVSKI M and BOYDANOVIC G (2009) Heavy metal ion adsorption from mine waters by sawdust. Chem. Ind. Chem. Eng. Q. 15 (4) 237-249. [ Links ]
TERNES TA, STUBER J, HERRMANN N, McDOWELLl D, RIED A, KAMPMANN M and TEISER B (2003) Ozonation: A tool for removal of pharmaceuticals, contrast media and musk fragrances from wastewater. Water Res. 37 1976-1982. [ Links ]
WEBER WJ and MORRIS JC (1963) Kinetics of adsorption on carbon from solution. J. Sanit. Eng. Div. ASCE. B9 (SA2) 31-39. [ Links ]
WU Q, LI Z, HONG H, YIN L and TIE L (2010) Adsorption and intercalation of ciprofloxacin on montmorillonite. Appl. Clay Sci. 50 (2) 204-211. [ Links ]
YE Z, WEINBERG HS and MEYER MT (2007) Occurrence of antibiotics in drinking water. Anal. Bioanal. Chem. 387 1365 -1377. [ Links ]
ZHANG H and HUANG CH (2007) Adsorption and oxidation of fluoroquinolone antibacterial agents and structurally related amines with goethite. Chemosphere 66 1502-1512. [ Links ]
ZHOU P, SU C, LI B and QIAN Y (2006) Treatment of high-strength pharmaceutical wastewater and removal of antibiotics in anaerobic and aerobic biological treatment process. J. Environ. Eng. Sci. 132 129-136. [ Links ]
Received 19 November 2011; accepted in revised form 11 October 2012.
* To whom all correspondence should be addressed. +91 9993220651; e-mail: sunil.mnlbpi@gmail.com