Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
Water SA
versão On-line ISSN 1816-7950
versão impressa ISSN 0378-4738
Water SA vol.38 no.4 Pretoria Jan. 2012
Comparative study of EVA-Cloisite® 20A and heat-treated EVA-Cloisite® 20A on heavy-metal adsorption properties
Derrick S Dlamini; Ajay K Mishra*; Bhekie B Mamba
Department of Applied Chemistry, University of Johannesburg, PO Box 17011, Doornfontein 2028, Johannesburg, South Africa
ABSTRACT
Ethylene vinyl acetate (EVA)/ Cloisite® 20A (C20A) composite fabricated via the melt-blending method was used for the development of a heavy-metal adsorbent through acid and heat treatment. Heat-treated composites were produced at 400°C to 1 000°C in air and N2 atmospheres. The materials were characterised through TGA, FT-IR, contact angle and Zetasizer. Treating EVA/C20A composites with H2SO, at 130°C reduced the contact angle from 99.73° to 30.40°. The acid-function-alised composite was tested for the removal of Pb2+ and an adsorption capacity of 49 mg-g-1 was recorded while the heat-treated composite exhibited an adsorption capacity of 153 mg-g-1.
Keywords: ash, EVA, bentonite, activation, adsorption
Introduction
Heavy-metal pollution is known to cause instability, disorder, harm or discomfort to living organisms (Al-Attar, 2011). Several studies have shown that heavy metals such as lead, zinc, cadmium, chromium and copper can be very toxic even at low concentrations (Periasamy and Namasivayam, 1996). Industrial and agricultural activities are a major source of heavy-metal pollution worldwide. In South Africa, for instance, industrial, mining and agricultural activities are considered as the driving force of the country's economy. Due to the rapid development of agriculture, industrial and traffic activities large amounts of heavy-metal pollutants are discharged to the local environment (Bai et al., 2011; Liua et al., 2011. The aggressive development of agriculture and industrial sectors makes it difficult to combat heavy-metal pollution. It has been reported that landfill is the best available technology especially for developing countries for the disposal of solid waste; however, heavy-metal pollution by landfill leachate is still possible (Longa et al., 2011). Heavy metals may also end up in sewage effluents (Bystrzejewski et al., 2011).
Removal of heavy-metal ions from sewage effluents and other water resources is essential to ensure environmental and human safety. Several techniques like reverse osmosis, nano-filtration, ion exchange and adsorption have been used in the removal of heavy metals from water. The use of activated carbon (AC) as an adsorbent is a simple and economically viable method of pollutant removal (Vargas et al., 2011). According to Tongpoothorn et al. (2011) activated carbon is a widely-used adsorbent because of its extremely high surface areas, micro-pore volumes, large adsorption capacities, fast adsorption kinetics, and relative ease of regeneration.
In this study, a heavy-metal adsorbent was derived from EVA/C20A composites that had been synthesised via the melt-blending method. The melt-blending method has been gaining significant attention in the fabrication of polymeric composites with improved mechanical properties, relative to those produced by alternative composite fabrication strategies (solution blending and in situ polymerisation). Polymer/ clay composites have been the centre of research over the past decade because of their potentially large application area. To ensure environmental safety, the materials should be discarded safely after use. Experiments in our laboratory have shown that EVA/C20A composites synthesised via the melt-blending method are hydrophobic and non-biodegradable in garden soil and compost. Therefore, we have derived a heavy-metal adsorbent from the EVA/C20A composite through acid and heat treatments of the non-biodegradable composites. The treatment methods were compared. Both procedures were kept as simple as possible to minimise costs. Bench-scale experiments on the removal of Pb2+ from aqueous solution were undertaken to test the potential application of the novel adsorbent in heavy-metal removal from water. Equilibrium and kinetic models were extensively applied on the adsorption results in order to establish whether the adsorbent is suitable for heavy-metal removal. The results indicate that composites used for different purposes may still be reused in adsorption technology after acid or heat treatment.
Experimental
Materials
Ethylene vinyl acetate (EVA) with 9% vinyl acetate (VA) was supplied by Plastamid, South Africa. The density of EVA was 0.930 g-cm-3 and the melting point was 95°C. Cloisite® 20A (C20A), a natural montmorillonite clay modified with dimethyl dihydrogenated to allow quaternary ammonium salt - CEC = 95 meq-100 g-1, was obtained from Southern Clay Products, Texas, USA. Concentrated acids (HCl and H2SO4) and KOH were sourced from Aldrich Chemicals, South Africa.
Preparation of adsorbent
The EVA/C20A was fabricated and characterised for thermal and morphological properties as discussed in our work previously published (Dlamini et al., 2011a; b). The adsorbent was derived through acid and heat treatments. To prepare the acid-treated adsorbent, a composite strip was oxidised with concentrated H2S04 at a ratio of 1:2 (weight) and heated at 130°C in an oven for 24 h. The dry curing was meant to catalyse the oxidation process. The acidified composite was allowed to cool and thereafter the free acid was removed by rinsing the composite material with deionised water until the filtrate reached a pH of between 6.5 and 7.5, after which it was then dried in an oven at 100°C for 24 h. Heat-treated composite adsorbents were derived from the ΕVA/C20A composite strip at different temperatures: 400°C, 500°C, 600°C, 700°C, 800°C, 900°C, and 1 000°C, fora period of 15 min in air andN2 atmospheres.
Characterisation of adsorbent
Thermogravimetric analysis (TGA)
Thermogravimetric analyses were performed to approximate the EVA residue after heating. The analyses were done in a Perkin Elmer TGA 4000 Analyzer equipped with PyrisTM Software. The sample mass was 8-10 mg and the temperature ranged from 100 °C to 600°C at a heating rate of 15°C-min-1. The analyses were performed under nitrogen atmosphere at a flow rate of 15 mf-min-1.
Contact angle measurements
The hydrophilic/hydrophobic nature of the composite strips, before and after acid treatment, was examined by using contact angle (CA) measurements using the sessile-drop method. The measurements were done on a Dataphysics Optical Contact Angle SCA20 at a dosing volume of 12 μ£. The values are given from an average of 6 measurements made at different locations on the specimen surface.
Zetasizer analysis
Zeta potential and particle size were investigated using a Zetasizer Nano ZS from Malvern Instruments. The dispersant was water with a pH of 5.5.
ATR-FT-IR analysis
The functional properties were measured directly by Bruker Tensor 27 FT-IR spectrometer and analysed with OPUS software. With this ATR-FT-IR model (attenuated total reflectance (ATR) Fourier transform infrared (FT-IR) model) the samples were analysed as they were. A small piece of sample was sliced from the polymeric composite material and was analysed. Prior to analysis, the samples were dried in an oven overnight.
Batch adsorption studies
The batch experiment technique was adopted for bench-scale adsorption experiments to study the heavy-metal adsorption capabilities of the composites using Pb2+ as an analyte. The experiments were carried out in 25 ml stopper reagent bottles. A stock solution of Pb2+ solution (200 mg-f-1) was prepared by dissolving 0.319 g of lead nitrate salt in deionised water. Batch adsorption experiments were conducted to establish the optimum pH, contact time and adsorbent dose, and the effect of temperature and initial concentration on Pb2+ adsorption. The adsorbent weight was 20 mg and the volume of the analyte solutions was 20 mf, unless otherwise specified. Initial pH of the solution was adjusted using 0.1M KOH or HCl. After adsorption the solutions were analysed for the remaining Pb2+ concentration using atomic adsorption spectroscopy (AAS).
Adsorption isotherms and kinetic models
Methods and supporting information used for the analysis of adsorption isotherms and kinetics are described in Appendix 1 (Langmuir, Freundlich, pseudo-first-order, pseudo-second- order, Natarajan and Khalaf, and Elovich models).
Results and discussion
TGA
The thermal degradation mechanism in char-forming polymers like EVA may be described as a generalised chemical bond scission process consisting of primary and secondary decomposition events (Bahramian et al., 2008). Generally, the degradation of EVA occurs via a 2-step mechanism with the loss of acetic acid during the first step (300°C to 400°C) and random chain scission of the remaining material in the second step, to form unsaturated vapour species (= 430°C), such as butene and ethylene (Hull et al., 2003). The thermographs of the composite and neat EVA are shown in Fig. 1.
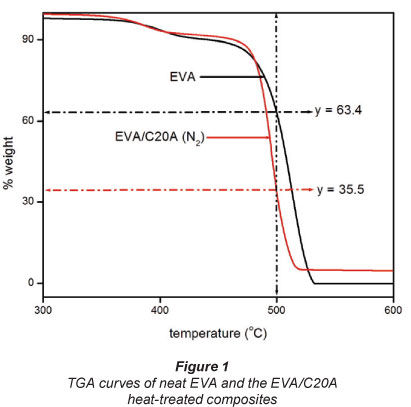
The catalytic effect of layered silicates on crosslinking/ charring reactions derives mainly from the acid sites formed on silicates due to the degradation of the organic treatment of the clay (Kiliaris and Papaspyrides, 2010). The degradation of C20A is discussed in our previous work published on EVA/ C20A composites (Dlamini et al., 2011a; b).
The percentage weight loss in each TGA curve clearly suggests that the clay residue after thermal degradation corresponds to the 5% clay dosage. Of most interest to this study was the residue at 500°C marked with the horizontal arrows in each TGA curve. It can be seen that about 35.5% (weight) EVA residue remains at 500°C. The EVA residue is essential in the sense that it suggests that we still have a composite.
FT-IR analysis
The FT-IR results of the EVA/C20A composite and the func-tionalised EVA/C20A composite are shown in Fig. 2. The peaks located at 2 917 cm-1 and 2 849 cm-1 were attributed to v(CH) and v(CH2) groups, respectively, (Dlamini et al., 2011a) and the broad peak located at 3 351 cm-1 was assigned to the v(OH) group.
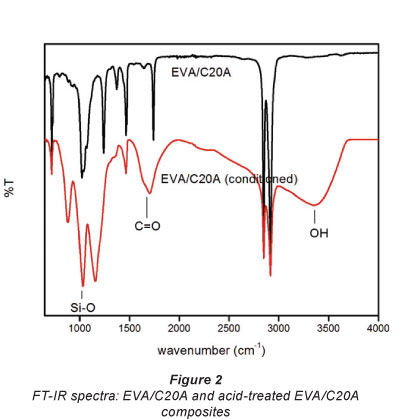
In the carbonyl region (1 600-1 to 1 800 cm-1) there is a peak at 1 704 cm-1 attributed to v(C=O). Noteworthy, the v(C=O) peak in the un-functionalised composite is shifted about 35 cm-1 higher indicating that the carbonyl bond is shorter in C=O, most likely because it belongs to the COOC component whereas in the oxidised composite it belongs to the COOH component, which may have resulted from the elimination of the acetate group in EVA. The band at 1 439 cm-1 resulted from the C-O stretching of the carboxylate anion in carboxylic groups and the band at 1 654 cm-1 was attributed to C=O stretching vibration modes in carbonyl groups (Han et al., 2011; Wang et al., 2011; Wei et al., 2011). The spectra show a broad and strong peak at 1 020 cm-1 which was confidently assigned to Si-O adsorption.
The FT-IR results of the heat-treated composites are shown in Fig. 3. The heat-treated composites were obtained by heating the acid-functionalised composites at different temperatures ranging from 400°C to 1 000°C.
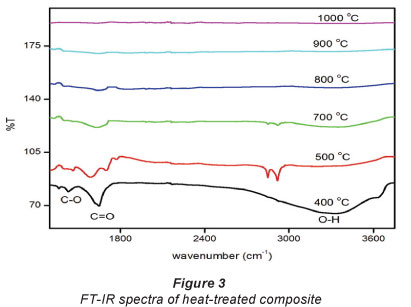
The results indicate that the heating at higher temperatures resulted in the degradadation of the functional groups introduced during the heat treatment.
Contact angle
Contact angles provide useful information regarding surface hydrophilicity. The rate at which water wets the composite determines how easily the water can penetrate the composite assemblage. Mixing EVA with the clay increased the contact angle from 95.60° to 99.73° and the polar component consequently decreased from 19.18 mJ-nf2 to 10.31 mJ-nf2. This is understandable because both materials are hydrophobic. After oxidation, the contact angle of the composite substantially decreased from 99.73° to 30.40° with a polar component of 44.86 mJ-nf2. The reductions in the polar components suggest a decrease in the hydrophobic character (Bessadok et al., 2007).
Zetasizer analysis
The zeta potential results are summarised in Table 1. For the heat-treated composites, the composites derived at 500°C were used.
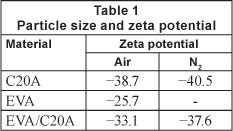
Visual comparison of the composite produced under the different atmospheres showed that the composite derived in air was more like ash. The argument that the heat-treated composite was composed of EVA residue was justified by heating pure EVA as a reference experiment. Zeta potential results show that all the materials are negatively charged. The nucleophilic nature of the adsorbents suggests that the composites will interact chemically with Pb2+.
Heavy-metal adsorption
Effect of heating temperature
The purpose of acid/heat treatment of the composites was to improve the surface area and provide physicochemical alterations in the structure of the clay. The effect of heating temperature (in air) on the modified composite and its adsorption capacity for Pb2+ was investigated. The uptake of Pb2+ was calculated by using the following equation:

where:
Co and Ct (mg-f-1) are the highest initial Pb2+ concentration and remaining concentration at time (t), respectively.
The temperatures selected were 400°C, 500°C, 600°C, 700°C,
800°C, 900°C and 1 000°C. The results, given in Fig. 4, show a sharp increase from 400°C to 500°C and thereafter a substantial decrease from 500°C to 900°C in per cent Pb2+ uptake.
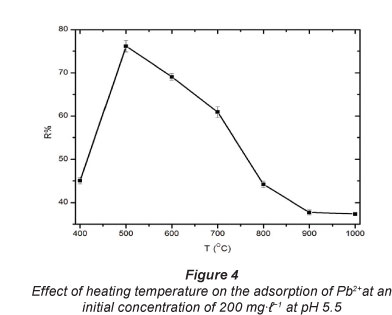
Koyuncu (2008) activated bentonite at 600°C. In the present work, 500°C was found to be the optimum temperature for heat treatment of carbonised EVA/C20A composites for the adsorption of Pb2+. This can be attributed to the destruction of the adsorption active sites (-COOH) as shown in FT-IR at temperatures higher than 500°C leaving only silica and alumina behind.
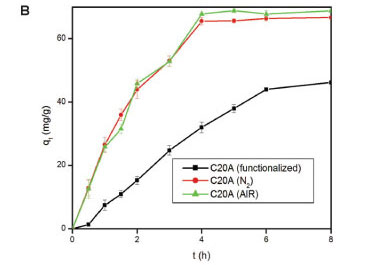
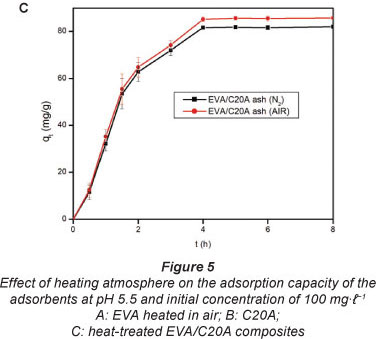
Effect of heating atmosphere
The atmospheres used were air and N The results are shown in Fig. 5. The effect of heating atmosphere on the adsorption properties of the adsorbents was tested on EVA (Fig. 5A), C20A (Fig. 5B), and on the EVA/C20A composite (Fig. 5C). Under N2 atmosphere, EVA formed a gel which solidified after cooling.
Apparently, there is little change in the adsorption properties of the adsorbent thermally treated in either air or N2. The EVA residue had the lowest adsorption capacity, probably because of the absence of silicates. The results are consistent with the data obtained from Zetasizer analyses. Based o these results, the composite derived under air conditions was used forthwith for all adsorptions with heat-treated composites.
Effect of pH
Optimisation of pH for sorption medium plays an important role in sorption studies (Hosseini-Bandegharaei et al., 2011). This is because the hydronium ions are strong competing adsorbate ions and partly due to the fact that the pH of a solution influences the chemical speciation of the metal ions in solution. The amount of Pb2+ adsorbed to the heat-treated (in air) composite, expressed in terms of adsorption efficiency as a function of pH, is shown in Fig. 6.
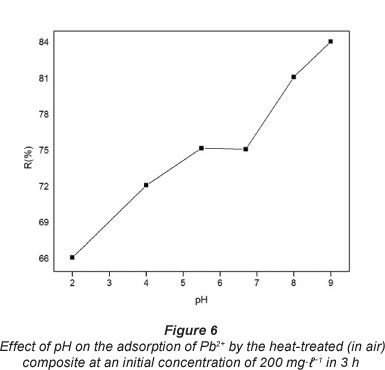
The adsorption increased with increase in the pH of the solution until pH 5.5. The low uptakes at low pH were attributed to a competitive adsorption as a result of high concentrations of hydronium ions (H3O+) which compete for adsorption sites with Pb2+. The observed adsorption increased with increasing pH and this was attributed to an increase in the concentration of Pb(OH)+ resulting in the decline in the hydronium ion concentration. For pH values >7.0, a new increase in Pb2+ uptake is observed as a result of the chemical precipitation of the metal in the form of hydroxide. Therefore, the Pb2+ adsorption phenomenon in aqueous solution at 100 mg-l-1 occurs at pH below 6.0.
Adsorption time profile: Acid vs. heat treatment
The adsorbents prepared by acid treatment were compared to the adsorbents prepared via heat treatment in terms of adsorption capacity (qt). The sorption capacity is one of the most important parameters of the sorbent characteristics in the sense that it determines how much of the heavy-metal pollutant can be removed from the aqueous solution by a unit mass of the sorbent (Bystrzejewski et al., 2011). The adsorption capacity (qt) was calculated using the following equation:

where:
Co and Ct represent the initial concentration and the remaining concentration at different time intervals ν is the volume of the solution ws is the weight of the composite
Figure 7 depicts the adsorption capacity of untreated EVA/ C20A composite. The plots given in Fig. 8 show the adsorption kinetics of Pb2+ adsorbed onto the acid-treated (A) and heat-treated in air (B) composite. It can be seen that the adsorption of Pb2+ on the composite increased steadily until equilibrium was attained, i.e, after about 6 h for acid-treated and 4 h for heat-treated composites, respectively. The maximum adsorption capacities were 153 mg-g-1 for heat-treated and 49 mg-g-1 for acid-treated composites, respectively, from an initial concentration of 200 mg-f-1. At an initial Pb2+ concentration of 100 mg-f-1, the heat-treated (in air) composite had an adsorption capacity of 80 mg-f-1. This is a significant increase from the 6 mg-g-1 recorded with untreated EVA/C20A (Fig. 7).
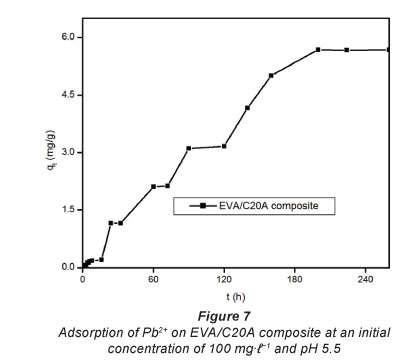
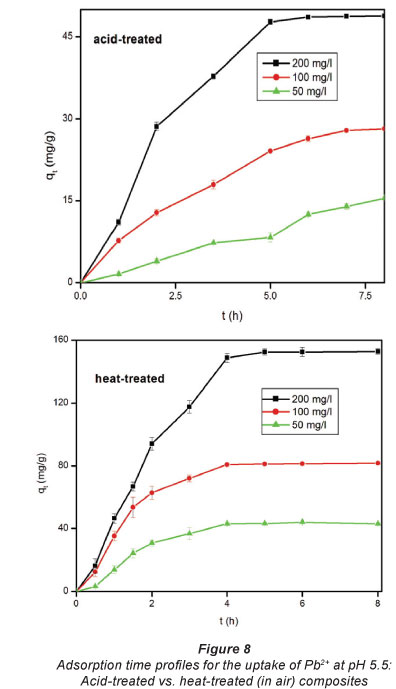
The steep increase in the adsorption capacity during the early stages of the adsorption may be attributed to the abundance of adsorption sites available for binding. The increase in adsorption capacity with an increase in initial concentration can be attributed to the higher concentration of Pb2+ ions vying for adsorption sites, resulting in a higher driving mass force. This effect can be attributed to adsorbate-adsorbent ratios at low initial concentrations which imply that metal ion adsorption involves higher energy sites (Naiya et al., 2009).
Effect of adsorbent dose and temperature
The effect of adsorbent quantity and temperature on the adsorption of Pb2+ on the composites heat-treated in air was investigated. Fig. 9 shows how the adsorption capacity changed with an increase in composite dose.
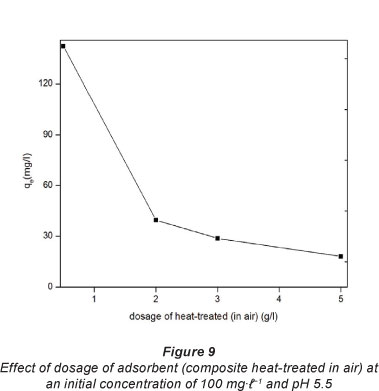
The adsorption capacity of Pb2+ decreased as the dosage of the composite increased from 0.5 to 2.0 g-f-1. This may be attributed to the heavy metal ions competing for limited adsorption sites at lower composite dosage. At higher composite dosage, there would be more sites available for adsorption meaning that there would be not competition for by the Pb2+ for active sites. A similar observation was reported by Zhi-rong et al. (2010) on the removal of Cu2+ and Ni2+ using bentonite clay.
The results showing the effect of temperature on the extent of Pb2+ adsorption onto the heat-treated composite are shown in Fig. 10. The temperatures used here are 303, 313 and 323 K.
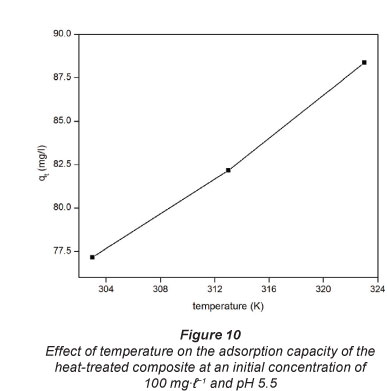
The results indicate that in the temperature range used in this work the adsorption of Pb2+ onto the composites increased with an increase in temperature. The observed increase in adsorption efficiency can be attributed to an increase in the movement of the ions with an increase in temperature as the density of the solution becomes lower. Adsorption is based on the collision between the adsorbate and the adsorbent.
Adsorption isotherms
The Langmuir and Freundlich mathematical models were applied to define and analyse the adsorption isotherm and equilibrium properties of the adsorption of Pb2+ onto the composites (Han et al., 2011). The equilibrium adsorption capacity (qe) was calculated from the equilibrium concentration (Ce) using the following equation:

The results are shown in Fig. 11. The correlation coefficients appear to suggest that both models may be suitable to describe the adsorption behaviour of Pb2+ onto the composites under conditions used in this study.
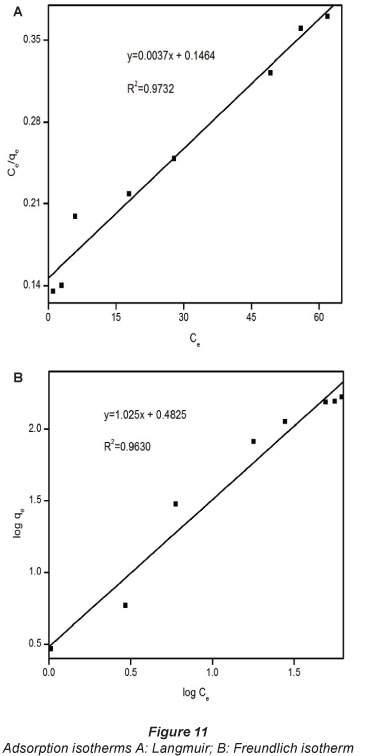
The dimensionless constant RL was calculated from the Langmuir isotherm parameters and was found to be 0.1016. It has been widely reported that an RL value between 0 and 1 indicates favourable adsorption (Bello et al., 2010; Hosseini-Bandegharaei et al., 2011; Tofighy and Mohammadi, 2011). The constant, l/n from the Freundlich isotherm is the heterogeneity coefficient and the heavy-metal removal is usually associated with chemical adsorption in cases where n<\. In this work, η was found to be 0.9757, suggesting a chemical interaction between Pb2+ and the composite. Chemical binding was hypothesised considering the nucleophilic nature of the composite and the pro tic strength and electrophonic nature of the Pb2+.
Adsorption kinetics
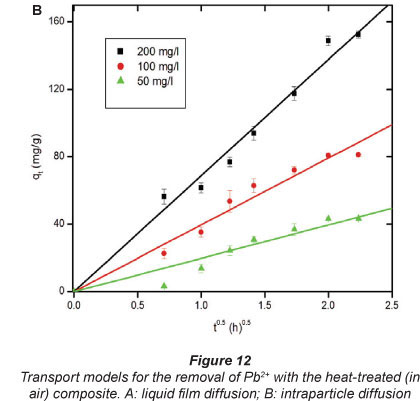
The adsorption process occurs in at least 4 stages that can have different kinetic rates (Bystrzejewski et al., 2011). Firstly, the transport of the analyte will involve the migration of Pb2+ from the bulk liquid to the composite particles. Since the composite particles would be enveloped by liquid film, the second stage of the adsorption process for Pb2+ involves penetration through the film to gain contact with the composite particles. In the next step, there will be intraparticle diffusion of the Pb2+ to reach the active sites of the composite. Lastly, adsorption will occur. It is imperative to identify the dominant (the slowest) step because it may control the kinetics of the adsorption process and therefore determine the removal rate of Pb2+ from the aqueous solution.
However, since the adsorption experiments were done under vigorous shaking, the migration of the Pb2+ from the liquid phase to the surface of the composite particles cannot be the rate-determining step. The interaction (binding) of Pb2+ with the composite may not be the rate-limiting step because the adsorbate and adsorbent are hypothesised to bind instantly due to the opposite charges. Therefore, the rate-determining step could either be the migration of Pb2+ through the ash pores (intraparticle diffusion) or the liquid-film-diffusion process. According to Alemayehua et al. (2011) the following equation, known as the liquid-film model, can be applied to predict whether liquid-film diffusion is the rate-determining step in an adsorption process:

where:
F is a constant (F = q/q)
kfd is the adsorption rate constant
t is time (h)
A linear plot of -ln(1-F) against tpassing through the origin (0,0) indicates that the liquid film diffusion is the rate-determining step of the kinetics of the adsorption process. According to Wang et al. (2011) and Li et al. (2011) the following equation can be applied to predict whether intraparticle diffusion is the rate-limiting step in an adsorption process:

where:
Kp is the intraparticle diffusion rate constant, in mg-g-1h1/2
When the plot -ln(1-F)against f1'2 is linear and passes through the origin (0, 0) then the intraparticle diffusion process is the primary limiting mechanism (Tofighy and Mohammadi, 2011). Figures 12A and 12B illustrate the results for liquid-film diffusion and intraparticle diffusion transport models, respectively.
Figure 12B shows that intraparticle diffusion was the rate-limiting stage. It is not surprising that intraparticle diffusion can be the primary rate-determining step in adsorption on the ash particle. The powder adsorbents may agglomerate significantly to larger particles when in contact with polar solvents like aquatic solutions of Pb2+ (Bystrzejewski and Pyrzyn'Ska, 2011).
A study on adsorption kinetics does not rest only on the determination of the rate-limiting step, but also encompasses investigations of how different experimental conditions may influence the speed of an adsorption process, which can be understood by monitoring kinetic parameters. In the present work, batch adsorption kinetics of Pb2+ with the composite particles derived from the EVA/C20A (in air) were investigated in terms of pseudo-first-order kinetics, pseudo-second-order kinetics, and as Natarajan, Khalaf and Elovich models. These models are described in the supporting information provided. The linear plots (shown in Fig. 13, next page) with good correlation coefficients (>0.900) imply that the adsorption of Pb2+ under conditions used in this study conforms to the selected kinetic models.
Adsorption is expected to follow either pseudo-first-order or pseudo-second-order models. The correlation coefficients of the linear plots of these models may be used to predict the applicability of the models to fit the adsorption data. However, it is possible to obtain good correlation coefficients (>0.900) for both models as shown in the present work. Therefore, to identify the suitable model to describe the adsorption of the adsorbate onto the adsorbent, the kinetic constants (ki, i=1 or 2) and, more importantly, the calculated equilibrium adsorption capacity (qe cal) are compared. The k1, k2 and qe cal for the adsorption of Pb2+ onto the ash were calculated and are given in Table 2. The results show lower kinetic constant values for the pseudo-first-order model compared to the pseudo-second-order model. The qe cal values for the pseudo-second-order model were closer to the experimental values (mean qe exp) when compared to those of the pseudo-first-order model at the initial concentrations studied.
The validity of the pseudo first-order model and pseudo second-order model was tested by a normalised standard deviation, Δq (%), which was calculated using the following equation (Hosseini-Bandegharaei, 2011):
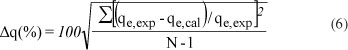
where:
qeexp and qe,cal , are experimental and calculated amounts Je,exp Je,cal r of Pb2+ adsorbed onto the composites at time t; N is the number of measurements made.
It was established that the pseudo-second-order model gave better standard deviations than the pseudo-first-order model. The calculated values from the pseudo-first-order model were found to be 29.72%, 25.66% and 32.23%, corresponding to 200 mg-l-1, 100 mg-l-1 and 50 mg-l-1, respectively. On the other hand, the normalised standard deviations calculated from the pseudo-second-order model were as follows: 16.85% (200 mg-ℓ-1), 24.27% (100 mg-ℓ-1) and 26.44% (50 mg-ℓ-1). These values confirm that the adsorption of Pb2+ onto the heat-treated (in air) composites may best be described by pseudo-second-order kinetics.
The results presented in Table 2 show that reliable conclusions can be drawn from the Elovich parameters. The higher values of α compared to β indicate that there was more adsorption than desorption during the experiment. This confirms that the adsorption of Pb2+ was through chemical adsorption. It is widely accepted that chemical adsorption is an irreversible process. It can be deduced from α values that Pb2+ removal is faster at the higher initial concentrations during the early stages of adsorption. This observation can be explained by the fact that there are more Pb2+ competing for the active sites in the composites. This was further confirmed by the Natarajan and Khalaf model kinetic constants (KN), which are seen to increase with a decrease in initial concentration.
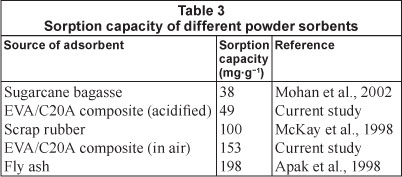
The effectiveness of the composites in removing heavy metals from water, especially in terms of adsorption capacity, can be shown by comparing the experimental values obtained with a few values for sorption capacities reported in the literature, as shown in Table 3. The sorbents summarised in Table 3 have been described as good adsorbents elsewhere. The heat-treated composites showed superior adsorption capabilities compared to EVA/C20A and acid-treated EVA/C20A composites.
Conclusion
The results presented in this study demonstrate that the non-biodegradable and hydrophobic EVA/C20A composites could be suitable heavy-metal adsorbents after acid- and heat-treatments. With an adsorption capacity of 153 mg-g-1, the heat-treated composite was found to be more effective in the removal of Pb2+ from an initial concentration of 200 mg-l-1, when compared to 49 mg-g-1 observed for the acid-treated composite. The adsorption data for heat-treated composites were fitted to isotherm and kinetic models. Equilibrium isotherms and Elovich models revealed that the removal of Pb2+ was mostly a result of chemisorption.
Acknowledgements
This study was funded by the National Research Foundation (NRF) and the University of Johannesburg (UJ).
References
ALEMAYEHUA E, THIELE-BRUHNB S and LENNARTZA B (2011) Adsorption behaviour of Cr(VI) onto macro- and micro-vesicular volcanic rocks from water. Sep. Purif. Technol. 78 (1) 55-61. [ Links ]
AL-ATTAR AM (2011) Antioxidant effect of vitamin E treatment on some heavy metals-induced renal and testicular injuries in male mice. Saudi J. Biol. Sci. 18 (1) 63-72. [ Links ]
APAK RE, HUGUL M and HIZAL J (1998)hieavy metal cation retention by unconventional sorbents (red muds and fly ashes). Water Res. 32 (2) 430-440. [ Links ]
BAHRAMIAN AR, KOKABI M, FAMILI MHN and BEHESHTY MH (2008) High temperature ablation of kaolinite layered silicate/ phenolic resin/asbestos cloth nanocomposite. J. Hazard. Mater. 150 (1) 136-145. [ Links ]
BAI J, XIAO R, CUI B, ZHANG K, WANG X, LIU Q, GAO H and HUANG L (2011) Assessment of heavy metal pollution in wetland soils from the young and old reclaimed regions in the Pearl River Estuary, South China. Environ. Pollut. 159 (3) 817-824. [ Links ]
BELLO OS, ADELAIDE OM, ABDUL HAMMED M and ABDUL MUIZ POPOOLA O (2010) Kinetic and equilibrium studies of methylene blue removal from aqueous solution by adsorption on treated sawdust. Macedonian J. Chem. Chem. Eng. 29 (1) 77-85. [ Links ]
BESSADOK A, MARAIS S, GOUANVE* F, COLASSE L, ZIMMERLIN I, ROUDESLI S and ME'TAYER M (2007) Effect of chemical treatments of Alfa (Stipa tenacissima) fibres on water-sorption properties. Comp. Sci. Technol. 67 (3-4) 685-697. [ Links ]
BYSTRZEJEWSKI M and PYRZYN'SKA K (2011) Kinetics of copper ions sorption onto activated carbon, carbon nanotubes and carbon-encapsulated magnetic nanoparticles. Coll. Surf. A: Physicochem. Eng. Aspects 377 (1-3) 402-408. [ Links ]
DLAMINI DS, MISHRA SB, MISHRA AK and MAMBA BB (2011a) Ethylene vinyl acetate and polycaprolactone-organoclay nanocom-posite: thermal, mechanical and morphological properties. J. Inorg. Organomet. Polym. 21 (2) 229-236. [ Links ]
DLAMINI DS, MISHRA SB, MISHRA AK and MAMBA BB (2011b) Comparative studies of the morphological and thermal properties of clay/polymer nanocomposites synthesized via melt blending and modified solution blending methods. J. Comp. Mater. 45 (21) 2211-2216. [ Links ]
HAN J, DAI J and GUO R (2011) Highly efficient adsorbents of poly(o-phenylenediamine) solid and hollow sub-microspheres towards lead ions: A comparative study. J. Colloid Interface. Sci. 356 (2) 749-756. [ Links ]
HULL TR, PRICE D LIU Y WILLS CL and BRADY J (2003) An investigation into the decomposition and burning behaviour of EVA nanocomposite materials. J. Polym. Deg. Stab. 82 (1) 365-371. [ Links ]
HOSSEINI-BANDEGHARAEI A, HOSSEINI MS, JALALABADI Y, SARWGHADI M, NEDAIE M, AHERIAN A, GHAZNAVI A and EFTEKHARI A (2011) Removal of Hg(II) from aqueous solutions using a novel impregnated resin containing 1-(2-thiazolylazo)-2-naphthol (TAN). Chem. Eng. J. 168 (3) 1163-1173. [ Links ]
KILIARIS P and PAPASPYRIDES CD (2010) Polymer/layered silicate (clay) nanocomposites: An overview of flame retardancy. Prog. Polym. Sci. 35 (7) 902-958. [ Links ]
KOYUNCU H (2008) Adsorption kinetics of 3-hydroxybenzalde-hyde on native and activated bentonite. Appl. Clay Sci. 38 (3-4) 279-287. [ Links ]
LI L, LIU F, JING X, LING P and LI A (2011) Displacement mechanism of binary competitive adsorption for aqueous divalent metal ions onto a novel IDA-chelating resin: Isotherm and kinetic modelling. Water Res. 45 (3) 1177-1188. [ Links ]
LIUA M, LIUA X, DINGB W and WUA L (2011) Monitoring stress levels on rice with heavy metal pollution from hyperspectral reflectance data using wavelet-fractal analysis, Intern. J. Appl. Earth Obs. Geoinf. 13 (2) 246-255. [ Links ]
LONGA Y-Y, SHENA D-S, WANGB H-T, LUB W-J and ZHAOB Y (2011) Heavy metal source analysis in municipal solid waste (MSW): Case study on Cu and Zn. J. Hazard. Mater. 186 (2-3) 1082-1087. [ Links ]
McKAY G, VONG B and PORTER JF (1998) Isotherm studies for the sorption of metal ions onto peat. Adsorp. Sci. Technol. 16 (1) 51-66. [ Links ]
MOHAN D and SINGH KP (2002) Single- and multi-component adsorption of cadmium and zinc using activated carbon derived from bagasse - an agricultural waste, Water Res. 36 (9) 2304-2318. [ Links ]
NAIYA TK, BHATTACHARYA AK and DAS SK (2009) Adsorption of Cd(II) and Pb(II) from aqueous solutions on activated alumina. J. Coll. Inter. Sci. 333 (1) 14-26. [ Links ]
PERIASAMY K and NAMASIVAYAM C (1996) Removal of cop- per(11) by adsorption onto peanut hull carbon from water and copper plating industry wastewater. Chemosphere 32 (4) 769-789. [ Links ]
TOFIGHY MA and MOHAMMADI T (2011) Adsorption of divalent heavy metal ions from water using carbon nanotube sheets. J. Hazard. Mater. 185 (1)140-147. [ Links ]
TONGPOOTHORN W, SRIUTTHA M, HOMCHAN P, CHANTHAI S and RUANGVIRIYACHAI C (2011) Preparation of activated carbon derived from Jatropha curcas fruit shell by simple thermo-chemical activation and characterization of their physico-chemical properties. Chem. Eng. Res. Des. 89 (3) 335-340. [ Links ]
VARGAS AMM, CAZETTA AL, GARCIA CA, MORAES JCG, NOGAMI EM, LENZI E, COSTA WF and ALMEIDA VC (2011) Preparation and characterization of activated carbon from a new raw lignocellulosic material: Flamboyant (Delonix regia) pods. J. Environ. Manage. 92 (1) 178-184. [ Links ]
WANG XS, LU ZP, MIAO HH, HE W and SHEN HL (2011) Kinetics of Pb (II) adsorption on black carbon derived from wheat residue. Chem. Eng. J. 166 (3) 986-993. [ Links ]
WEI Y-T, ZHENG Y-M and CHEN JP (2011) Enhanced adsorption of arsenate onto a natural polymer-based sorbent by surface atom transfer radical polymerization. J. Colloid Interface Sci. 356 (1) 234-239. [ Links ]
ZHI-RONG L and SHAO-QI Z (2010) Adsorption of copper and nickel on Na-bentonite. Proc. Saf. Environ. Prot. 88 62-66. [ Links ]
Received 5 December 2011; accepted in revised form 9 July 2012.
* To whom all correspondence should be addressed. ffi +27 11 559 6180; fax: +27 11 559 6425; e-mail: amishra@uj.ac.za