Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
Water SA
versión On-line ISSN 1816-7950
versión impresa ISSN 0378-4738
Water SA vol.38 no.4 Pretoria ene. 2012
Characterising the reactivity of metallic iron in Fe0/As-rock/H2O systems by long-term column experiments
C Noubactep*
Angewandte Geologie, Universitãt Göttingen, GoldschmidtstraBe 3, D - 37077 Göttingen, Germany
ABSTRACT
The intrinsic reactivity of 4 metallic iron materials (Fe0) was investigated in batch and column experiments. The Fe0 reactivity was characterised by the extent of aqueous fixation of in-situ leached arsenic (As). Air-homogenised batch experiments were conducted for 1 month with 10.0 g/l of an As-bearing rock (ore material) and 0.0 or 5.0 g/l of Fe0. Column experiments were performed for 2 and 3 months. Each dynamic experiment was made up of 2 glass columns in series. The first column contained 2.5 or 5.0 g of the ore material and the second column 0.0 or 5.0 g of a Fe0 material. Results showed no significant reactivity difference in batch studies for all 4 materials; ZVI2 was by far the most reactive material in column experiments. This observation was attributed to the relative kinetics of production of aqueous As and Fe species under the experimental conditions and their impact on the formation of a protective film on Fe0. Accordingly, no protective film could be built at the surface of the least reactive materials. The results corroborated the urgent need for unified experimental procedures to characterise Fe0 materials.
Keywords: Column study, intrinsic reactivity, ore mineral, water treatment, zerovalent iron
Introduction
Elemental metals are efficient reactive agents for the remediation of several classes of environmental contaminants including arsenic, azo dyes, bacteria, halogenated organic compounds, heavy metals, nitrates, nitroaromatics, radionuclides, and viruses (O'Hannesin and Gillham, 1998; Bojic et al., 2004; Bartzas et al. 2006; Bojic et al., 2007; Henderson and Demond, 2007; Komnitsas et al.2007; Bojic et al., 2009; Antia, 2010; Bartzas and Komnitsas, 2010; Bundschuh et al., 2010; Luna-Velasco et al., 2010; Noubactep, 2010a; Phillips et al., 2010; Sarathy et al., 2010, Comba et al., 2011; Giles et al., 2011; ITRC, 2011; Lin et al., 2011; Noubactep, 2011a; Salter-Blanc et al., 2011). Metallic iron (Fe0) is currently the most used material for field applications (Gillham, 2010; Comba et al., 2011; Gheju, 2011; Henderson and Demond, 2011; ITRC, 2011; Salter-Blanc et al., 2011).
Despite the wide variety of environmental contaminants and their possible specific interactions with Fe0, tested materials were characterised mainly by their surface area, size and interface chemistry (e.g. surface state). However, it has been traceably demonstrated that none of these structural and physical characteristics is really determinant for the chemical reactivity of Fe0 (Reardon, 1995; Landis et al., 2001: Noubactep et al., 2005; Reardon, 2005; Noubactep et al., 2009). For instance, Landis et al. (2001) reported that Fe0 materials of comparable particle size (comparable surface area) exhibited reactivity differences greater than 3-fold for cDCE and VC degradation rates in column studies. This example substantiates that a broad understanding of the chemical reactivity is urgently needed.
Several sources of Fe0 materials have been reported in literature to be efficient for aqueous contaminant removal (Landis et al., 2001; Miehr et al., 2004; Leupin and Hug, 2005; Noubactep et al., 2005; Gheju and Iovi, 2006; Satapanajaru et al., 2006; Yang et al., 2006; Ngai et al., 2007; Gheju et al., 2008; Gheju and Balcu, 2010; Gheju and Balcu, 2011, Wanner et al. 2011). These include commercial Fe0 for contaminant removal (e.g. Connelly iron, Peerless iron, iron from G. Maier GmbH), commercial iron for other purposes (e.g. construction steel, iron nails, steel wool), scrap iron, production by-products, and Fe0 prepared in situ by reduction of iron salts. Although many tested materials have been reported to be highly reactive and recommendable for field application, efficacy of Fe0 in terms of high removal capacity for a specific contaminant in short-term experiments is not a guarantee for high removal capacity in field applications. Moreover, researchers working with nano-scale Fe0 usually compare their results to that of conventional micro-scale Fe0 (Noubactep et al., 2012 and references cited therein). The question is what is the reference material to which innovative materials should be compared?
The present study is a continuation of a series of works aiming at introducing reliable tools for the evaluation of the intrinsic reactivity of various Fe0 materials. A method based on the characterisation of Fe dissolution in a 2 mM EDTA solution was first proposed (Noubactep et al., 2004; 2005; 2009). The method was proven less efficient for powdered materials and for materials with high proportion of fines (Noubactep, 2010b). On the other hand, Fe0 dissolution is not necessarily coupled to contaminant removal. These limitations have led to the development of a second experimental tool in which Fe0 is characterised by the extent of the discoloration of methylene blue (MB) in the presence of manganese dioxide (MnO2) (MB-test) (Noubactep, 2009). The MB-test was shown to be more efficient and affordable than the EDTA-test but was limited by the lack of reference MnO2 materials. Both tests could enable an advanced material screening. However, of the 18 tested materials, 7 were still exhibiting very similar reactivity. Therefore, new approaches are needed.
A further possibility to characterise the reactivity of Fe0 materials is to stress them in systems where building of a protective film at their surface is likely to occur. Such a system was identified recently while characterising the solubilisation of toxic species from natural rocks (Noubactep et al., 2008a; 2008b). It was shown that elevated amounts of As could be leached from an ore material for a long time (up to 99 days). Accordingly, introducing the same amount of various Fe0 materials in a system capable of producing As concentrations as high as 1 000 mg/£ could be a powerful tool to investigate the impact of As on the formation of the oxide film (mixed oxides) in the process of contaminant removal by Fe0. As a rule, the more reactive a material, the more rapid the passivation process (protective film formation). In other words, the system with the most reactive material will exhibit the lowest contaminant removal efficiency.
The objective of this study is to present a new contribution to the effort for the development of reliable protocols for the comparison of the intrinsic reactivity of different Fe0 materials. For this purpose, 4 selected materials from former works were tested. One of the materials was essentially less reactive than the others. The three other materials were very closely matched in their reactivity by both tests described above (Noubactep, 2010b). The results confirmed the suitability of the used method and opened new routes for coupling the investigation of contaminant release and contaminant removal under relevant conditions.
Materials and methods
Solid materials
As-bearing rock
The As ore material used originates from the Otto-Stollen in Breitenbrunn/Erzgebirge (Saxony, Germany). The material was selected on the basis of its high arsenic content (80%). A qualitative SEM analysis showed the presence of As, Ca, F, Fe, O, S and Si (Noubactep et al., 2008). The ore material is primarily a hydrothermal vein material and arsenic occurred as native arsenic (As0) and Loellingite (FeS2 - As-I) (Jones and Nesbitt, 2002) in paragenesis with hydrothermal vein carbonates (for example Fe-bearing calcite or dolomite). The mineral was ground and sieved. The particle size fraction 0.063 < d (mm) < 0.10 was used without any pre-treatment.
Fe0 materials
One scrap iron (ZVI1), and three commercially-available Fe0 materials were tested. The main characteristics of these materials are summarised elsewhere (Noubactep, 2010b). ZVI2 is a spherical material (d = 1.2 mm) from Würth (Germany) termed 'HartguBgranulat'. ZVI3 are iron chips from G. Maier GmbH
Rheinfelden (Germany) termed 'GrauguBgranulat'. ZVI4 is a direct reduced iron from ISPAT GmbH (Germany), termed 'Schwammeisen'. Before use ZVI4 was crushed and sieved; the size fraction 1.0-2.0 mm was used. The specific surface area of the materials varies between 0.043 and 0.63 m2/g. These data were compiled from the literature (Table 1). The materials were compared solely on the basis of the extent of As removal by the same initial mass of Fe0 (e.g. 5.0 g in columns) under similar experimental conditions. The materials differ regarding their characteristics, such as iron content, nature and proportion of alloying elements, and shape.
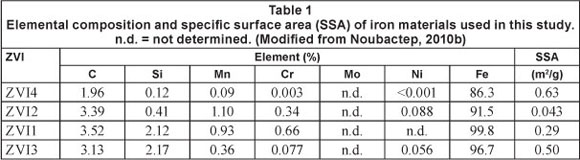
The 4 materials used were selected from 9 materials which were recently characterised by leaching with 2 mM EDTA in column study (Noubactep, 2010b). In turn, the 9 materials tested were selected from 18 materials after screening in batch experiments using the EDTA-test (Noubactep et al., 2005; Noubactep et al., 2009). Both tests could not really differentiate the reactivity of ZVI1, ZVI3 and ZVI4. The reactivity of these three materials toward As removal from a natural rock was investigated in this study. For comparison the least reactive commercial Fe0 (ZVI2) was incorporated in this study.
Sand
The sand used was a commercial material for aviculture ("Papagaiensand" from RUT - Lehrte/Germany). Papagaiensand was used as received without any further pretreatment or characterisation. This sand was the operational reference non-adsorbing material.
Leaching solution
The leaching solution was tap water of the city of Göttingen (Lower Saxonia, Germany). Tap water is considered to be a better proxy for natural groundwater than synthetic solutions (Noubactep, 2003; Noubactep et al., 2008). The rationale behind this assumption is that, in many cases, natural water is only treated for iron and manganese removal. The average composition (in mg/£) of the tap water used was: Cl-: 7.7; NO3-: 10.0; SO42-: 37.5; HCO3-: 88.5; Na+: 7.0; K+: 1.2; Mg2+: 7.5; Ca2+: 36.1; and an initial pH of 8.3.
As leaching and immobilisation
Air-homogenised batch experiments
These experiments were conducted in special reaction vessels allowing the system to be homogenised by a humid current of air supplied by a small aquarist pump. The goal was to homogenise the experimental systems at atmospheric pressure (PCO2 = 0.035 %) without breaking down the materials. 10.0 g/£ of the ore material and 0.0 or 5.0 g/£ of Fe0 were allowed to react in sealed vessels containing 100 m£ of tap water at laboratory temperature (22 ± 3°C) for up to 30 days. At given dates, 1.5 m£ of the solution was retrieved and diluted for As analysis and the same volume of tap water was added to the system.
Column experiments
The tap water was pumped upwards from PE bottles using a peristaltic pump (Ismatec, ICP 24). Tygon tubes were used to connect inlet reservoir, pump, column and outlet. Ten glass columns (40 cm long, 2.6 cm inner diameter) were used in two series of experiments. The columns were mostly packed with sand. The effective length, bulk density and porosity of the packed columns were not characterised as this was not necessary for the discussion of the results. The extent of As dissolution by water and the extent of its removal by selected Fe0 materials were the sole targets. The experiments were performed at room temperature (22 ± 3°C). A stable flow rate was maintained throughout the experiment.
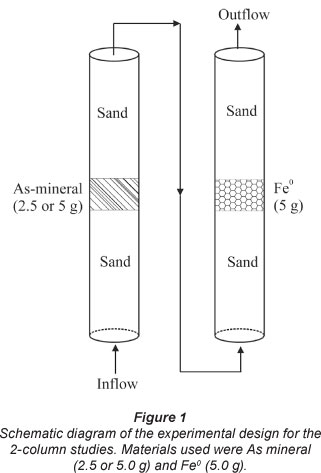
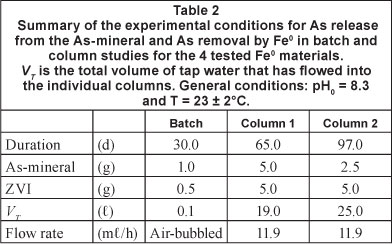
Five parallel experiments were performed in each series. The same mass of the rock (2.5 or 5.0 g) was placed in a first column and 5.0 g of each tested Fe0 was placed in the second column (Fig. 1). In the reference system, the second column contained only sand (no Fe0). The experiments were stopped after 65 or 97 days when each column was leached by 19 or 25.0 £ of tap water (Table 2). The water flow rate was constant at 12.0 ml/h.
Analytical methods
Analysis for As was performed by inductively coupled plasma mass spectrometry (ICP-MS) at the Department of Geochemistry (Centre of Geosciences, University of Göttingen). All chemicals used for the experiments and analyses were of analytical grade. The pH value was measured by combining glass electrodes (WTW Co., Germany). The electrodes were calibrated with 5 standards following a multi-point calibration protocol and in agreement with the new IUPAC recommendation (Meinrath and Spitzer, 2000; Buck et al., 2002).
Expression of experimental results
The mass (m) of leached As (mg) at any time (t) is calculated from the concentration of the effluent using Eq. (1):

where
P is the As concentration (in mg/l)
and V the volume (l).
At the end of the experiment the total amount of leached As can be calculated by addition and the extent of As leaching by tap water deduced. Knowing the As percentage in the natural rock (80%), the maximal leachable mass (m0) of As can be calculated. The percentage (P) As leaching at each time is given by Eq. (2):

At each time the amount of As leached in the reference system (Pref) can be set to 100 and the relative leaching percent (Prel) for all other systems deduced by Eq. (3):

Finally, the relative percentage of As removal (Pfix) by each material is given by Eq. (4):

Results and discussion
The particularity of the As-rock/Fe0 systems investigated here is that aqueous As and the solid Fe hydroxides and oxides for their removal are generated in situ. It has been traceably demonstrated, that AsIII and AsV are removed in Fe0/H2O systems by adsorption and co-precipitation (Lackovic et al., 2000; Farrell et al., 2001; Noubactep, 2010a; Noubactep, 2011b; Noubactep, 2011c; Noubactep, 2012). As released from the ore material used was recently characterised (Noubactep et al., 2008a) and the process of As dissolution will not be discussed here. The basis for the characterisation of Fe0 materials is that the smallest As concentration (relative to the reference system) is encountered in the system with the most reactive material under testing conditions.
Batch experiments
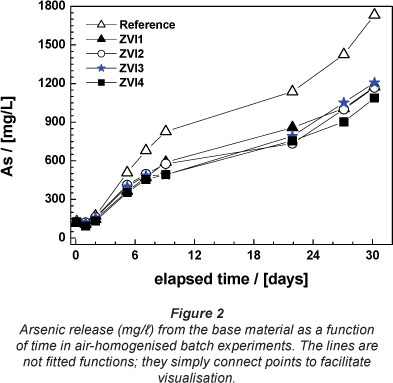
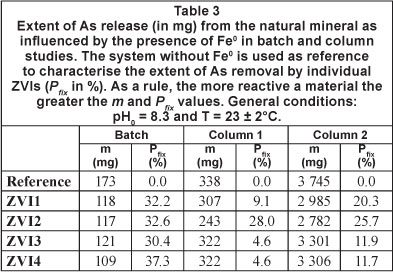
Figure 2 illustrates As dissolution in the absence (reference) or presence of tested Fe0 materials (ZVI1 through ZVI4), as a function of time. Both leaching kinetics and the extent of As release substantially decreased with the addition of Fe0. From Fig. 2 no difference in reactivity can be visually detected. It appears that the reactivity of all 4 materials is very similar. Examination of the Pfix values (Table 3) shows that the relative fixation efficiency varies from 30.4 to 37.3 %. A tentative order of increasing reactivity based on these values is: ZVI3 < ZVI1 < ZVI2 < ZVI4. Note that the order of reactivity after the EDTA-test (Noubactep et al., 2005; 2009) and the MB-test (Noubactep, 2009) were univocally: ZVI2 < ZVI1 = ZVI3 = ZVI4. Accordingly, air-homogenised batch experiments are not appropriate for the differentiation of the reactivity of ZVI1, ZVI3 and ZVI4. It is well known that batch systems cannot accurately reflect processes occurring in nature (Wang et al., 2009). The first reason for this, in regard to the experimental conditions of this work, is the possibility of super-saturation of the As solution given the excessive contact time (30 days) and the relatively strong homogenisation with air-bubbles. To account for this, further characterisations were performed under dynamic conditions.
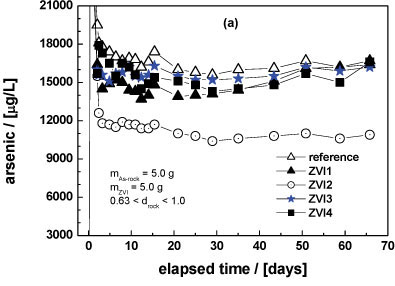
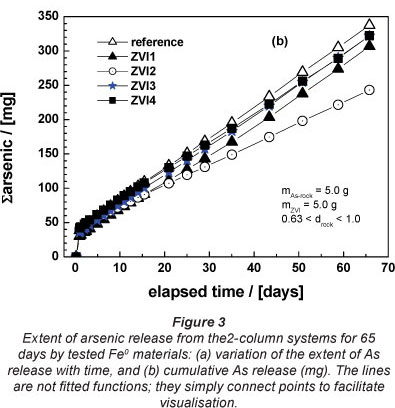
Column experiments for 65 days
Figure 3a shows the effect of tested ZVIs on As leaching from the natural ore as a function of time (< 65 days). 5.0 g of the ore material was placed in the first column and 5.0 g of ZVI in the second column (Fig. 1). From Fig. 3a a visual differentiation of ZVI2 is evident. It is also evident that the reference system exhibited the highest As concentration. The cumulative sum of released As (Fig. 3b) confirmed this trend. The m- and Pfix-values (Table 3) confirmed these observations. The deduced order of increasing reactivity is: ZVI3 = ZVI4 < ZVI1 < ZVI2. This classification, showing that ZVI2 was the most reactive material, is acceptable, but the experimental conditions should be further modified to obtain a clear trend. The following modifications were made:
- the mass of ore material was halved;
- the first 2 l of leaching solution in Fe0/As-rock systems was discarded; and
- the duration of the experiment was lengthened to 97 days.
All other parameters (flow rate, temperature) were kept constant. The rationale behind discarding the first 2 l of leaching solution was the elevated As concentration which occurred in this initial phase (Fig. 3a).
The next important feature to note from Fig. 3a is that 5.0 g of the used ore material is capable of producing about 17 mg/l (reference system) As for more than 2 months. High As concentration was intentionally tested here. By varying the mass of the ore material and the particle size, As concentrations relevant for each specific size could be achieved.
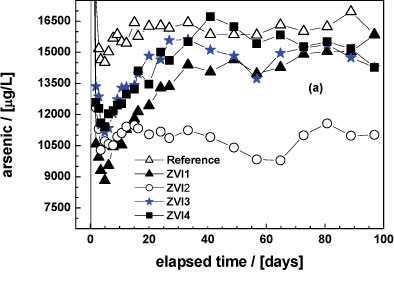
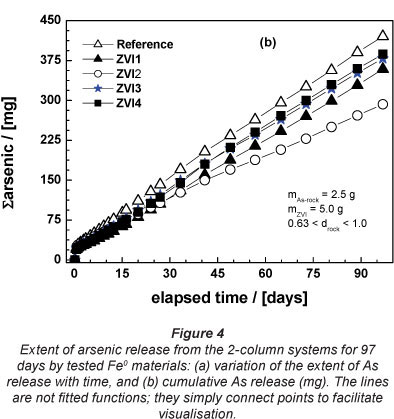
Column experiments for 97 days
Figure 4a shows the effect of tested ZVIs on As leaching from the natural ore as a function of time (< 97 days). A visual reactivity difference can be better performed than in Fig. 3a. The visual increasing order of reactivity is: ZVI4 < ZVI3 < ZVI1 < ZVI2. The m- and Ρ -values shown in Table 3 confirmed this trend, with the additional information that ZVI3 and ZVI4 are very closely matched in their reactivity, as the per cent As removal (Pfix -values) for each was 11.9 and 11.7, respectively. Figure 4b clearly confirmed the results shown in Table 3.
From Fig. 4a is clear that 2.5 g of the As-rock is able to produce about 16 mg/£ As for more than 3 months. These results clearly show that long-term experiments regarding As removal can be coupled with As leaching from natural ores. By reducing the ore mass, changing the particle size and using different ores it is possible to perform long-term leaching experiments in the laboratory. Such experiments could help to bridge the huge gap between the laboratory and the field (Wang et al., 2009). On the other hand, parameters from such systems could help to develop more reliable models to predict contaminant leaching in the environment.
Discussion
The use of Fe0 materials for environmental remediation is severely handicapped by the lack of methods for characterisation of the chemical reactivity. The current procedure of testing the reactivity of Fe0 for individual contaminants (Landis et al., 2001; Miehr et al., 2004; Leupin and Hug, 2005; Gheju and Iovi, 2006, Wanner et al. 2011) is not very useful as no comparison between two independent works is possible, even for the same contaminant. Ideally, there should be a universally-acceptable/accepted method to evaluate various Fe0 for their chemical reactivity. Accordingly, it is contemplated to propose protocols, which could be used to compare the efficiency of ifferent Fe0.
From available works, only the characterisation of Fe0 by the extent of H2 production (Reardon, 1995; 2005) could be regarded as a universally applicable method to characterise Fe0 intrinsic reactivity. However, this protocol is not necessarily affordable and uses relatively high Fe0 masses (15.0 to 400 g). Accordingly, more simple and affordable tests should be developed. The EDTA-test (Noubactep et al., 2005; 2009) and the MB-test (Noubactep, 2009) are simple and affordable but they could not address the passivation of tested material.
By using elevated As concentrations, the present work has corroborated warnings to perform contaminant removal experiments with over-saturated solutions (e.g. Kalin et al., 2005). However, in addition to the instability of used solutions introducing biases in the extent of contaminant removal by the tested process, this study has delineated the impact of elevated concentrations on the passivation process. In fact, in ature contaminants are rarely available at high concentration (Henderson and Demond, 2011; Kümmerer, 2011) and contaminated water enters the zone containing Fe0 when an oxide scale is already formed at its surface. In other words, while using elevated contaminant concentrations, an artificial system is created that could not be reproduced in nature. On the other hand, elevated contaminant concentrations necessarily impact the process of film formation on Fe0 (Noubactep, 2010c).
Concluding remarks
In an attempt to access their intrinsic chemical reactivity, the performance of 3 commercial Fe0 (ZVI2, ZVI3 and ZVI4) and 1 scrap iron (ZVI1) for the removal of As was evaluated in long-term column studies. As was leached from a natural rock using the tap water of the city of Göttingen as leaching solution. The results confirmed findings from previous works that ZVI2 is the least reactive material (Noubactep et al., 2005; 2009; Noubactep, 2010a, Noubactep 2011d). It could further be shown that ZVI1 is less reactive than ZVI3 and ZVI4.
The test methodology consisting of leaching As with tap water can be further improved or adapted to investigate several aspects of contaminant release and contaminant removal. For example, by reducing the mass of the ore material As concentrations relevant to field situations could be obtained and used to characterise the performance of Fe0 materials for As removal. On the other hand, using several leaching solutions could enable the characterisation of the impact of relevant ions on the process of As leaching (and/or removal). Such experiments could be designed on the basis of site-specific situations. It is hoped that this new experimental tool will accelerate efforts to characterise the intrinsic reactivity of Fe0 materials.
Acknowledgments
The arsenic-bearing ore used was purchased by the Department of Geology of the Technical University Bergakademie Freiberg. Dr. Klaus Simon (Centre of Geosciences, University of Göttingen) is acknowledged for the As analysis. Dr. Ralf. Köber (Institute Earth Science of the University of Kiel) kindly purchased the commercial ZVI samples. The scrap iron (Sorte 69) was kindly purchased by the branch of the MAZ (Metallaufbereitung Zwickau, Co) in Freiberg. Mohammad Azizur Rahman (Angewandte Geologie, Universitat Göttingen) is acknowledged for technical support. The work was supported by the Deutsche Forschungsgemeinschaft (DFG-No 626/2-2).
References
ANTIA DDJ (2010) Sustainable zero-valent metal (ZVM) water treatment associated with diffusion, infiltration, abstraction and recirculation. Sustainability 2 2988-3073. [ Links ]
BARTZAS G and KOMNITSAS K (2010) Solid phase studies and geochemical modelling of low-cost permeable reactive barriers. J. Hazard. Mater. 183 301-308. [ Links ]
BARTZAS G, KOMNITSAS K and PASPALIARIS I (2006) Laboratory evaluation of Fe0 barriers to treat acidic leachates. Miner. Eng. 19 505-514. [ Links ]
BOJIC A, PURENOVIC M and BOJIC D (2004) Removal of chro-mium(VI) from water by micro-alloyed aluminium based composite in flow conditions. Water SA 30 353-359. [ Links ]
BOJIC A, PURENOVIC M, BOJIC D and NDJELKOVIC T (2007) Dehalogenation of trihalomethanes by a micro-alloyed aluminium composite under flow conditions. Water SA 33 297-304. [ Links ]
BOJIC A, BOJIC D and ANDJELKOVIC T (2009) Removal of Cu2+ and Zn2+ from model wastewaters by spontaneous reduction-coagulation process in flow conditions. J. Hazard. Mater. 168 813-819. [ Links ]
BUCK RP, RONDININI S, COVINGTON AK, BAUCKE FGK, BRETT CMA, CAMOES MF, MILTON MJT, MUSSINI T, NAU-MANN R, PRATT KW, SPITZER P and WILSON GS (2002) Measurement of pH. Definition, standards, and procedures (IUPAC Recommendations 2002). Pure Appl. Chem. 74 2169-2200. [ Links ]
BUNDSCHUH J, LITTER M, CIMINELLI VST, MORGADA ME, CORNEJO L, HOYOS SG, HOINKIS J, ALARCÓN-HERRERA MT, ARMIENTA MA and BHATTACHARYA P (2010) Emerging mitigation needs and sustainable options for solving the arsenic problems of rural and isolated urban areas in Latin America - A critical analysis. Water Res. 44 5828-5845. [ Links ]
COMBA S, DI MOLFETTA A and SETHI R (2011) A Comparison between field applications of nano-, micro-, and millimetric zero-valent iron for the remediation of contaminated aquifers. Water Air Soil Pollut. 215 595-607. [ Links ]
FARRELL J, WANG J, O'DAY P and CONKLIN M (2001) Electrochemical and spectroscopic study of arsenate removal from water using zero-valent iron media. Environ. Sci. Technol. 35 2026-2032. [ Links ]
GHEJU M (2011) Hexavalent chromium reduction with zero-valent iron (ZVI) in aquatic systems. Water Air Soil Pollut. 222 103-148. [ Links ]
GHEJU M and IOVI A (2006) Kinetics of hexavalent chromium reduction by scrap iron. J. Hazard. Mater. 135 66-73. [ Links ]
GHEJU M, IOVI A and BALCU I (2008) Hexavalent chromium reduction with scrap iron in continuous-flow system: Part 1: Effect of feed solution pH. J. Hazard. Mater. 153 655-662. [ Links ]
GHEJU M and BALCU I (2010) Hexavalent chromium reduction with scrap iron in continuous-flow system. Part 2: Effect of scrap iron shape and size. J. Hazard. Mater. 182 484-493. [ Links ]
GHEJU M and BALCU I (2011) Removal of chromium from Cr(VI) polluted wastewaters by reduction with scrap iron and subsequent precipitation of resulted cations. J. Hazard. Mater. 196 131-138. [ Links ]
GILES DE, MOHAPATRA M, ISSA TB, ANAND S and SINGH P (2011): Iron and aluminium based adsorption strategies for removing arsenic from water. J. Environ. Manage. 92 3011-3022. [ Links ]
GILLHAM RW (2010) Development of the granular iron permeable reactive barrier technology (good science or good fortune). In: Chen Y, Tang X and Zhan L (eds.). Advances in Environmental Geotechnics: Proceedings of the International Symposium on Geoenvironmental Engineering, Hangzhou, China, September 8-10, 2009. Springer, Berlin/London. 5-15. [ Links ]
HENDERSON AD and DEMOND AH (2007) Long-term performance of zero-valent iron permeable reactive barriers: a critical review. Environ. Eng. Sci. 24 401-423. [ Links ]
HENDERSON AD and DEMOND AH (2011) Impact of solids formation and gas production on the permeability of ZVI PRBs. J. Environ. Eng. 137 689-696. [ Links ]
ITRC (INTERSTATE TECHNOLOGY & REGULATORY COUNCIL) (2011) Permeable reactive barrier: Technology update. PRB-5. Washington, D.C.: Interstate Technology & Regulatory Council, PRB: Technology Update Team. URL: www.itrcweb.org (Accessed 9 March 2012). [ Links ]
JONES RA and NESBITT HW (2002) XPS evidence for Fe and As oxidation states and electronic states in loellingite (FeAs2). Am. Miner. 87 1692-1698. [ Links ]
KALIN M, WHEELER WN and MEINRATH G (2005) The removal of uranium from mining waste water using algal/microbial bio-mass. J. Environ. Radioact. 78 151-177. [ Links ]
KOMNITSAS K, BARTZAS G, FYTAS K and PASPALIARIS I (2007) Long-term efficiency and kinetic evaluation of ZVI barriers during clean-up of copper containing solutions. Miner. Eng. 20 1200-1209. [ Links ]
KÜMMERER K (2011) Emerging contaminants versus micro-pollutants. Clean - Soil, Air, Water 39 889-890. [ Links ]
LACKOVIC JA, NIKOLAIDIS NP and DOBBS GM (2000) Inorganic arsenic removal by zero-valent iron. Environ. Eng. Sci. 17 29-39. [ Links ]
LANDIS RL, GILLHAM RW, REARDON EJ, FAGAN R, FOCHT RM and VOGAN JL (2001) An examination of zero-valent iron sources used in permeable reactive barriers. Proc. 3rd International Containment Technology Conference, 10-13 June 2001, Florida State University, Tallahassee. Orlando, FL. 5 pages. [ Links ]
LEUPIN OX and HUG SJ (2005) Oxidation and removal of arsenic (III) from aerated groundwater by filtration through sand and zero-valent iron. Water Res. 39 1729-1740. [ Links ]
LIN H, ZHU L, XU X, ZANG L and KONG Y (2011) Reductive transformation and dechlorination of chloronitrobenzenes in UASB reactor enhanced with zero-valent iron addition. J. Chem. Technol. Biotechnol. 86 290-298. [ Links ]
LUNA-VELASCO A, SIERRA-ALVAREZ R, CASTRO B and FIELD JA (2010) Removal of nitrate and hexavalent uranium from ground-water by sequential treatment in bioreactors packed with elemental sulfur and zero-valent iron. Biotechnol. Bioeng. 107 933-942. [ Links ]
MEINRATH G and SPITZER P (2000) Uncertainties in determination of pH. Mikrochem. Acta 135 155-168. [ Links ]
MIEHR R, TRATNYEK GP, BANDSTRA ZJ, SCHERER MM, ALOWITZ JM and BYLASKA JE (2004) Diversity of contaminant reduction reactions by zerovalent iron: Role of the reductate. Environ. Sci. Technol. 38 139-147. [ Links ]
NGAI TKK, SHRESTHA RR, DANGOL B, MAHARJAN M and MURCOTT SE (2007) Design for sustainable development -Household drinking water filter for arsenic and pathogen treatment in Nepal. J. Environ. Sci. Health A 42 1879-1888. [ Links ]
NOUBACTEP C (2003) Investigations for the passive in-situ immobilization of uranium (VI) from water (in German). Dissertation, TU Bergakademie Freiberg, Wiss. Mitt. Institut für Geologie der TU Bergakademie Freiberg, Band 21.ISSN1433-1284. 140 pp. [ Links ]
NOUBACTEP C (2009) Characterizing the reactivity of metallic iron upon methylene blue discoloration in Fe0/MnO2/H2O systems. J. Hazard. Mater. 168 1613-1616. [ Links ]
NOUBACTEP C (2010a) The fundamental mechanism of aqueous contaminant removal by metallic iron. Water SA 36 663-670. [ Links ]
NOUBACTEP C (2010b) Characterizing the reactivity of metallic iron in Fe0/EDTA/H2O systems with column experiments. Chem. Eng. J. 162 656-661. [ Links ]
NOUBACTEP C (2010c) Elemental metals for environmental remediation: Learning from cementation process. J. Hazard. Mater. 181 1170-1174. [ Links ]
NOUBACTEP C (2011a) Metallic Iron for Safe Drinking Water Production. Freiberg Online Geology 27. ISSN 1434-7512 (www. geo.tu-freiberg.de/fog). 38 pp. [ Links ]
NOUBACTEP C (2011b) Aqueous contaminant removal by metallic iron: Is the paradigm shifting? Water SA 37 419-426. [ Links ]
NOUBACTEP C (2011c) Metallic iron for water treatment: A knowledge system challenges mainstream science. Fresenius Environ. Bull. 20 2632-2637. [ Links ]
NOUBACTEP C (2011d) Characterizing the reactivity of metallic iron in Fe0/UVI/H2O systems by long-term column experiments. Chem. Eng. J. 171 393-399. [ Links ]
NOUBACTEP C (2012) Investigating the processes of contaminant removal in Fe0/H2O systems. Korean J. Chem. Eng. DOI: 10.1007/ s11814-011-0298-8. [ Links ]
NOUBACTEP C, CHEN-BRAUCHER D and SCHLOTHAUER T (2008) Arsenic release from a natural rock under near-natural oxidizing conditions. Eng. Life Sci. 8 622-630. [ Links ]
NOUBACTEP C, FALL M, MEINRATH G, and MERKEL B (2004) A simple method to select zero valent iron material for ground-water remediation. Paper presented at Quebec 2004, 57th Canadian Geotechnical Conference, 5th Joint CGS/IAH-CNC Conference, Session 1A. 6-13. 24-27 October, Quebec. [ Links ]
NOUBACTEP C, LICHA T, SCOTT TB, FALL M and SAUTER M (2009) Exploring the influence of operational parameters on the reactivity of elemental iron materials. J. Hazard. Mater. 172 943-951. [ Links ]
NOUBACTEP C, MEINRATH G, DIETRICH P, SAUTER M and MERKEL B (2005) Testing the suitability of zerovalent iron materials for reactive walls. Environ. Chem. 2 71-76. [ Links ]
NOUBACTEP C, SCHÖNER A and SCHUBERT M (2008) Characterizing As, Cu, Fe and U solubilization by natural waters. In: Merkel BJ andn Hasche-Berger A (eds.) Uranium in the Environment. Springer, Berlin, Heidelberg. 549-558. [ Links ]
NOUBACTEP C, CARÉ S and CRANE RA (2012) Nanoscale metallic iron for environmental remediation: prospects and limitations. Water Air Soil Pollut. 223 1363-1382. [ Links ]
O'HANNESIN SF and GILLHAM RW (1998) Long-term performance of an in situ "iron wall" for remediation of VOCs. Ground Water 36 164-170. [ Links ]
PHILLIPS DH, VAN NOOTEN T, BASTIAENS L, RUSSELL MI, DICKSON K, PLANT S, AHAD JME, NEWTON T, ELLIOT T and KALIN RM (2010) Ten year performance evaluation of a field-scale zero-valent iron permeable reactive barrier installed to remediate trichloroethene contaminated groundwater. Environ. Sci. Technol. 44 3861-3869. [ Links ]
REARDON EJ (2005) Zerovalent irons: Styles of corrosion and inorganic control on hydrogen pressure buildup. Environ. Sci. Tchnol. 39 7311-7317. [ Links ]
REARDON EJ (1995) Anaerobic corrosion of granular iron: Measurement and interpretation of hydrogen evolution rates. Environ. Sci. Technol. 29 2936-2945. [ Links ]
SALTER-BLANC AJ and TRATNYEK PG (2011) Effects of solution chemistry on the dechlorination of 1,2,3-trichloropropane by zerovalent zinc. Environ. Sci. Technol. 45 4073-4079. [ Links ]
SARATHY V, SALTER AJ, NURMI JT, JOHNSON GO, JOHNSON RL and TRATNYEK PG (2010) Degradation of 1,2,3-trichloro-propane (TCP): Hydrolysis, elimination, and reduction by iron and zinc. Environ. Sci. Technol. 44 787-793. [ Links ]
SATAPANAJARU T, ANURAKPONGSATORN P, SONGSASEN A, BOPARAI H and PARK J (2006) Using low-cost iron byproducts from automotive manufacturing to remediate DDT. Water Air Soil Pollut. 175 361-374. [ Links ]
WANG T-H, LI M-H and TENG S-P (2009) Bridging the gap between batch and column experiments: A case study of Cs adsorption on granite. J. Hazard. Mater. 161 409-415. [ Links ]
WANNER C, EGGENBERGER U and MÀDER U (2011) Reactive transport modelling of Cr(VI) treatment by cast iron under fast flow conditions. Appl. Geochem. 26 1513-1523. [ Links ]
YANG JE, KIM JS, OK YS, KIM S-J and YOO K-Y (2006) Capacity of Cr(VI) reduction in an aqueous solution using different sources of zerovalent irons. Korean J. Chem. Eng. 23 935-939. [ Links ]
Received 3 June 2011; accepted in revised form 28 June 2012.
* To whom all correspondence should be addressed. ffi +49 551 39 3191; fax: +49 551 399379; e-mail: cnoubac@gwdg.de