Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Water SA
On-line version ISSN 1816-7950
Print version ISSN 0378-4738
Water SA vol.38 n.4 Pretoria Jan. 2012
Cr(VI) formation during ozonation of Cr-containing materials in aqueous suspension - implications for water treatment
W van der Merwe; JP Beukes*; PG van Zyl
Chemical Resource Beneficiation, North-West University, Potchefstroom Campus, Private Bag X6001, Potchefstroom 2520, South Africa
ABSTRACT
Ozonation, or advanced oxidation processes (utilising ozone decomposition products as oxidants) are widely used in industrial wastewater and drinking water treatment plants. In these applications the use of ozone is based on ozone and its decomposition by-products being strong oxidants. In this paper, the possible oxidation of non-Cr(VI) Cr-containing materials suspended in water during ozonation, is presented. This study is of particular interest within the South African context, considering that South Africa holds the majority of global chromium ore resources and has the largest Cr-related industry in the world. Chromium also occurs commonly in other industrial waste materials and is a naturally-occurring element in the crust of the earth. Results indicated that in situ formation of Cr(VI) is possible during aqueous ozonation. pH had a significant influence, since the decomposition products of aqueous O3, i.e. hydroxyl radicals formed at higher pH levels, were found to be predominantly responsible for Cr(VI) formation. Increased ozonation contact time, water temperature and solid loading also resulted in higher Cr(VI) concentrations being formed.
Keywords: hexavalent chromium, Cr(VI), ozone, ozonation, advanced oxidation processes (AOP)
Introduction
The use of ozone or use of ozone in conjunction with other compounds and catalysts (e.g. advanced oxidation processes) to treat industrial waste waters and effluents is well documented (Nawrocki and Kasprzyk-Hordern, 2010; Coca et al., 2007; Selcuk, 2005; Gogate and Pandit, 2004; Beltrán, 2003). Ozonation is also used extensively in drinking water treatment plants (Audenaert et al., 2010; Beltrán, 2003; Camel and Bermond, 1998). While O3 is not yet widely used in the South African water treatment sector, its popularity for this use is gaining momentum. A case study revealed that there are several waterworks in South Africa where O3 is used successfully as a pre-oxidant for the treatment of raw waters (Rajagopaul et al., 2008).
Although ozonation has many advantages, there are also some disadvantages associated with its use, which include it being an energy-intensive process option and the potential formation of harmful disinfection by-products (Rajagopaul et al., 2008; Legube et al., 2004; Beltrán, 2003). The use of O3 in water treatment is based on ozone and its decomposition by-products, i.e. hydroxyl radicals, being strong oxidants (Audenaert et al., 2010; Lovato et al., 2009; Beltrán, 2003). The potential for the formation of Cr(VI), a known carcinogen (Stern, 2010; Proctor, 2002; IARC, 1997), by aqueous O3 has received limited research attention. Rodman et al. (2006) investigated the conversion of Cr(III) propionate to Cr(VI) by the advanced oxidation process, as a means of pre-treatment for an analytical technique. However, as far as the authors could assess, an investigation into the formation of Cr(VI) via aqueous O3 oxidation of non-Cr(VI) containing materials, with relevance to water treatment, has not yet been conducted. Such a study is of particular interest within the local context, considering South Africa's considerable chromium ore reserves and the associated industries.
South Africa holds more than three quarters of the world's viable chromium ore (chromite) reserves (Murthy et al., 2011; Cramer et al., 2004) and produced approximately 40% of the world's ferrochrome in 2009 (ICDA, 2010; Beukes et al., 2010). Upper Group 2 chromite (UG2) is also processed in South Africa to produce platinum group metals (PGMs) (Cramer et al., 2004; Beukes et al., 2010), with SA producing an estimated 80% of annual global PGMs (Xiao and Laplante, 2004; Cawthorn, 1999). Cr(VI) chemicals are also produced in South Africa (Lanxess, 2011). All these industries produce Cr-containing wastes, albeit wastes containing mostly Cr(III). Due to chromium being part of trace minerals occurring in coal, all of the coal combustion industries in South Africa (e.g. coal-fired power stations, coal-to-liquid fuel production, boilers) also produce fly ash and clinker containing chromium (Nel et al., 2011; Wagner and Hlatshwayo, 2005).
Considering the abundance of Cr-containing wastes in South Africa and the possibility that some of these wastes might be very fine and airborne (e.g. combustion off-gas particles), it is not unlikely that some Cr-containing materials might be suspended in raw water entering water treatment facilities. Chromium also occurs in natural sediments, since chromium is the 21st most abundant element in the earth's crust with an average concentration of approximately 100 mg-kg-1 (Emsley, 2003).
In this paper, the possible oxidation of non-Cr(VI) Cr-containing materials to Cr(VI) by aqueous ozonation is presented. UG2 chromite ore, typically utilised by the PGM industry in South Africa (Xiao and Laplante, 2004; Cramer et al., 2004; Beukes et al., 2010), as well as slag (waste material) from a local ferrochrome producer (Beukes et al., 2010), were used as case study materials. Various experimental parameters including pH, contact time, solid material loading, ozone concentration and water temperature were investigated.
Experimental
Materials
All commercial chemicals used were analytical grade (AR) reagents obtained from the different suppliers and used without any further purification. Standard Cr(VI) solutions were prepared from a 1 000 mg-l-1 aqueous chromate analytical solution (Spectrascan, distributed by Teknolab AB, Sweden), which was used for calibration and verification of the analytical technique employed. s-Diphenyl carbazide (FLUKA) was used during Cr(VI) analysis (Thomas et al., 2002)). Solutions of sodium hydroxide (Merck) and perchloric acid (Merck) were used to adjust the pH of aqueous solutions/mixtures. Ultra-pure water (resistivity, 18.2 ΜΩ-cm-1), produced by a Milli-Q water purification system, was used for all dilutions and aqueous extractions. Medical grade oxygen (99.5% minimum O2, with remainder consisting of N2, Ar, CO2, CO and H2O), utilised for O3 generation, was supplied by Afrox. Ferrochrome slag and UG2 ore (< 1 mm) samples were received from local ferrochrome and PGM producers, respectively. SARM 8 and SARM 18, obtained from Industrial Analytical (Pty) Ltd, were used as reference materials during the analysis of the slag and UG2 ore.
Methods
Pulverising
Since the above-mentioned Cr-containing case study materials, i.e. UG2 ore and ferrochrome slag, were relatively coarse, the materials were pulverised to 90% less than 191 and 499 μm respectively. This facilitated the suspension of these solids in the aqueous medium via agitation, as described later. The slag and UG2 ore were dried at 40°C for 1 day and then cooled in airtight containers to avoid possible water absorption prior to pulverising. A Siebtechnik pulveriser, commonly used to pulverise solid samples prior to chemical analysis, was used. All parts of the pulveriser, which made contact with the sample materials, were made of tungsten carbide. This prevented possible iron contamination, since it is well-known that metallic iron particles can reduce Cr(VI) to Cr(III) in aqueous medium. Pulverising time was kept as short as possible (20 s for the slag and 25 s for the UG2 ore) to reduce the possible formation of Cr(VI) during pulverisation (Glastonbury et al., 2010; Beukes and Guest, 2001).
Particle size analysis
A Malvern Mastersizer 2000, was used to determine the particle size distribution of the pulverised samples (Etxebarria et al., 2005). A diluted suspension of pulverised Cr-containing material was ultra-sonicated for 1 min prior to the particle size measurement, in order to disperse the individual particles and to prevent the use of a chemical dispersant.
Chemical and surface analyses
A Spectro Ciros Vision inductively coupled plasma optical emission spectrometer (ICP-OES) was used to characterise the chemical composition of the Cr-containing materials used in this case study.
Scanning electron microscopy with energy dispersive x-ray detectors (SEM-EDS) was used to detect potential surface chemical compositional changes that occurred as a result of ozonation of the Cr-containing materials. A Zeiss MA 15 SEM incorporating a Bruker AXS XFlash® 5010 Detector x-ray EDS system operating with a 20 kV electron beam and a working distance of 17.4 mm was used.
Ozone generation
A Sanders Certizon Series (corona discharge) ozone generator (100 mg-h-1 specification) was used to generate the O3 from medical grade oxygen. A constant flow of 210 ml-min-1 O2 through the generator was maintained during all experiments, including blank experiments. An Agilent Flow Tracker Series 1000 was used to verify the O2 flow. The maximum gas concentration of O3 that could be achieved with this instrument was 0.0058 mg-ml-1 (approximately 73 mg-h-1) at the above-mentioned flow rate. The gaseous O3 concentration was determined with UV-visible spectrophotometry as described by McElroy et al. (1997), on a Cary 50 Conc UV-visible spec-trophotometer at 254 nm. This gaseous O3 concentration was maintained during all experiments, except when explicitly indicated otherwise.
Ozonation experimental set-up
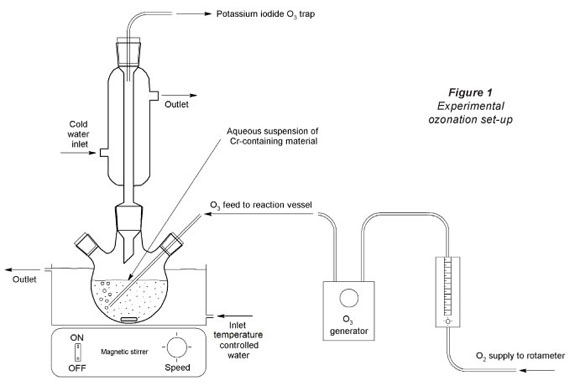
A predetermined mass of the pulverised Cr-containing material was suspended for a selected ozonation contact time in 100 ml pH adjusted water in a 250 ml sealed glass beaker, with a bubble diffuser through which the O3-rich O2 gas was fed. A magnetic stirrer (18 mm x 2.5 mm dimensions, rotating at 750 r-min-1) was used to keep the particles in suspension. A circulation pump fed water from a temperature-controlled water bath to a water jacket in which the reaction vessel (glass beaker) was housed. This simple experimental setup (Fig. 1) allowed monovariance investigation of parameters (e.g. pH, temperature, contact time, etc.). After each specific experiment was completed the gas supply was stopped, the beaker removed from the experimental setup, and the water filtered off by milli-pore filtering (0.45 μπι). The aliquot was then analysed for Cr(VI) as soon as possible after filtering. All monovariance reaction conditions investigated were repeated at least 3 times, to ensure repeatability. Blank experiments in which all the reaction conditions were kept identical (including solid loading and O2 flow), except that the O3 generator was switched off, were also conducted. These blank experimental results were indicated with the word 'blank' in all relevant figures.
Cr(VI) analysis
The Cr(VI) concentration of aliquots was determined with a Dionex ICS 3000 according to the Dionex application update 144 (Dionex, 2003) and the procedure published by Thomas et al. (2002). Analysis was conducted with a 2 x 750 μΐ knitted reaction coil (for better peak response) and at a wavelength of 530 nm. 250 mM ammonium sulphate was used as an eluent and 2 mM s-diphenyl carbazide (DPC) solution for post column coloration. The flow rates of the eluent and colorant were 1.0 ml-min-1 and 2.0 ml-min-1 respectively. With this technique, a quantification limit of 0.06 μg-l-1 could be achieved (Thomas et al., 2002). Results were expressed by indicating the mean Cr(VI) concentration obtained under specific conditions, as well as the standard deviation resulting from the repetitions for a particular monovariance case.
Case study material characterisation
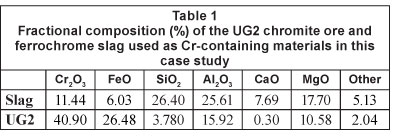
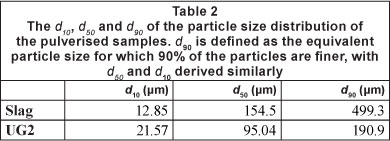
The chemical compositions of the Cr-containing case study materials utilised in the investigation are indicated in Table 1. The d10, d50 and d90 of the particle size distribution of these materials after pulverising are presented in Table 2. The d90 is defined as the equivalent particle size for which 90% of the particles are finer, with definitions of d50 and d10 derived similarly.
pH dependence
In order to confirm or reject the notion of possible Cr(VI) formation during ozonation, 100 mg of Cr-containing material was suspended in 100 ml water, of which the pH was pre-adjusted. The pre-adjusted pH was varied between pH 2 and pH 12, while all other parameters were kept constant. Ozonation contact time was selected to be 6 min, which is similar to contact times applied in operational ozonation plants (Rajagopaul et al., 2008). The results of the pH-dependence study are shown in Fig. 2. From these results it is clear that Cr(VI) can be formed during aqueous ozonation of Cr-containing materials. Blank experiments confirmed that Cr(VI) was indeed formed and not merely liberated by the aqueous contact of the case study materials. Zero or very low concentrations of Cr(VI) were detected at pH 2-4. However, the Cr(VI) concentration increased as the pH was increased further, reaching a maximum at approximately pH 10. Although it was not the aim of this paper to establish the chemical reaction mechanism of the Cr-containing material oxidation to Cr(VI), possible reasons for the observations can be postulated. At low pH values aqueous O3 decomposition is relatively slow (Lovato et al., 2009; Beltrán, 2003; Sotelo et al., 1987); hence chromium oxidation is mostly dependent on the ability of dissolved O3 to serve as oxidant. Gurol and Singer (1982) specifically reported that the rate of ozone decomposition was slow and relatively insensitive to pH below pH 4. However, at higher pH levels, aqueous O3 decomposition accelerates, which result in the formation of higher concentrations of the hydroxyl radicals (Lovato et al., 2009; Beltrán, 2003). These radicals are stronger oxidants than dissolved O3 (Beltrán, 2003), hence increased Cr(VI) formation. Since aqueous O3 is replenished all of the time during ozonation, the formation of hydroxyl radicals at higher pH levels is also not diminished by O3 decomposition.
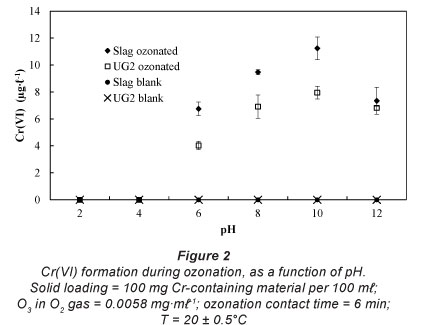
Each type of Cr-containing material will obviously have different characteristics, which will influence its ability to be oxidised during aqueous ozonation. In this particular case study, higher Cr(VI) levels were in general formed for the ferrochrome slag than for the UG2 ore (Fig. 2), notwithstanding the fact that the ore had a higher total chromium content (Table 1). It is well documented that chromite ore is chemically relatively inert due to its spinel crystal structure (Gu and Wills, 1988), therefore it was more resistant to oxidation than the slag. Additionally the Cr:Fe ratio of the slag is much higher than that of the ore.
The actual concentrations of Cr(VI) formed during the pH investigation were well below the 50 μg·m£-1 drinking water standard. However, it would be misleading to extrapolate these bench-top experimental results directly to practical ozonation plant operations, since many parameters are likely to differ (e.g. solid loading, contact time, pH, O3 generator efficiency, agitation). It is, however, apparent from the results presented here that Cr(VI) could be formed through ozonation if Cr-containing materials are suspended in the water.
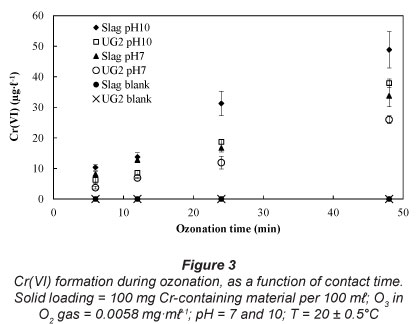
Influence of ozonation contact time
Rajagopaul et al. (2008) reported that the contact times during ozonation in operational plants are in the order of 3-6 minutes. However, contact time could even be longer, depending on the objective of the treatment and the compounds present (Beltrán, 2003). In order to establish the effect of ozonation contact time on Cr(VI) formation, contact times of 6, 12, 24 and 48 min were investigated at 2 pH values, i.e., pH 7, representing a neutral water system, and pH 10, since it was the optimum observed pH for Cr(VI) formation (Fig. 2). These results (Fig. 3) indicate an almost linear increase in Cr(VI) concentrations as a function of contact time, for the experimental conditions investigated.
Temperature effect
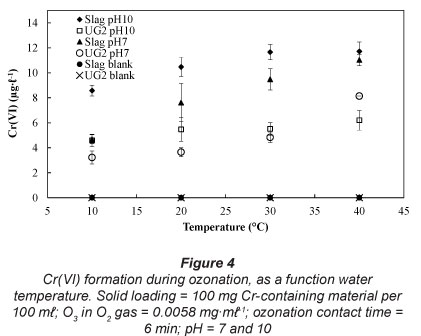
It is unlikely that ozonation will be applied at extremely high or extremely low water temperatures. Therefore, the effect of water temperature was investigated at 10, 20, 30 and 40°C. This temperature range is representative of normal and maybe slightly heated water, which might be applicable to some industrial waste waters (e.g. combution off-gas venturi scrubber water). As indicated in Fig. 4, increased water temperatures led to increased Cr(VI) formation. Aqueous O3 decomposition studies have indicated that higher temperatures lead to increased rates of ozone decomposition (Beltrán, 2003; Sotelo et al., 1987), hence higher concentrations of the more aggressive oxidation radicals, as discussed earlier.
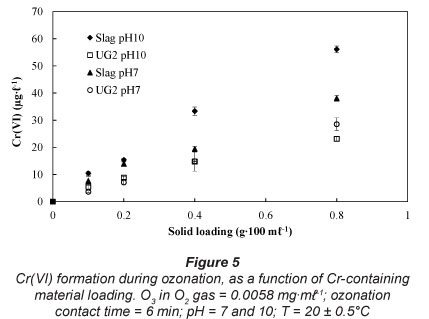
Effect of solid loading
As expected, higher solid loading of the Cr-containing materials led to higher Cr(VI) concentrations (Fig. 5). These results represent relatively high solid loadings, which might never be achieved in water treatment applications. However, these high solid loadings assisted in identifying a trend.
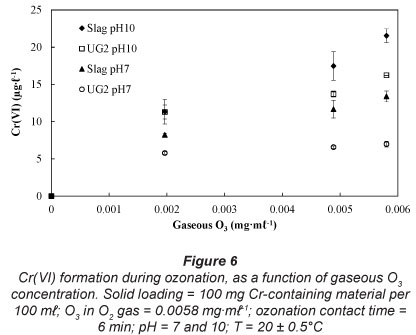
Effect of gaseous O3 concentrations
The maximum O3 concentration in the O2 gas stream was limited by the ability of the O3 generator utilised in this study. All results presented in the previous sections of this paper were conducted at the maximum setting of the O3 generator utilised, i.e, achieving 0.0058 mg-m£-1 O3 in O2 gas. In order to assess the effect of O3 gaseous concentration on the formation of Cr(VI) during aqueous ozonation, two lower settings of the O3 generator were tested. These results are shown in Fig. 6. It is evident that lower gas O3 concentrations resulted in lower Cr(VI) formation.
Mechanism of Cr-liberation
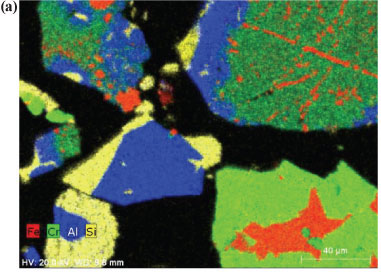
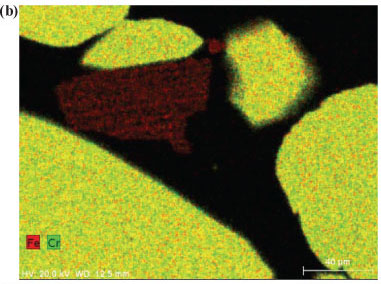
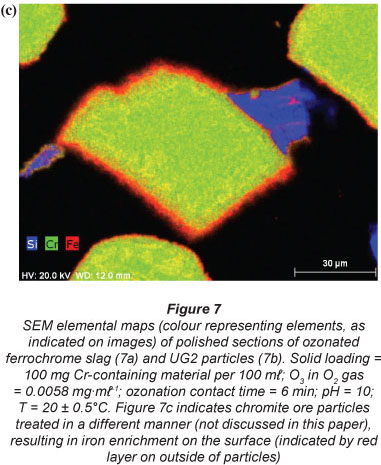
In order to obtain insight into the actual liberation mechanism of Cr(VI) from the solid Cr-containing materials during ozonation, SEM elemental maps were generated for polished sections of the Cr-containing materials exposed to ozonation. Figures 7a and 7b indicate ozonated slag and ÜG2 ore particles, respectively. The colour distributions (colours representing different elements, as indicated on the images) of the two materials utilised clearly show that the slag is much more heterogeneous than the ÜG2 with regard to elemental distribution. This was expected, since the slag is a re-crystallised waste product, while the ÜG2 ore is more homogeneous. However, in neither of these two ozonated materials can any enrichment of a specific element be observed on the surface (outside) of the particles. For comparison, Fig. 7c is included, which indicates chromite ore particles which were treated in a different manner (not discussed in this paper), resulting in the enrichment of iron on the surface of the particles. The absence of enrichment of any elements on the surface of the ozonated slag and ÜG2 ore particles therefore indicates that ozonation did not selectively extract chromium or any other element. It is therefore most probable that entire surfaces of particles were eroded due to the strong oxidising conditions, resulting in the release and subsequent oxidation of chromium. Other elements present in the case study materials were therefore also likely to be liberated; however, these were not quantified since this was beyond the scope of the investigation.
The experimental differences observed between the two case study materials, i.e. UG2 ore and slag, could be due to several reasons. Chromite ore is likely to be more resistant to O3 erosion than the slag, due to the well-defined spinel crystal structure of the ore (Gu and Wills, 1988) and the less well-defined crystalline structure of the slag. Additionally Fe reduces at a lower temperature than Cr during the ferrochrome pyrometallurgical production process (Beukes et al., 2010). This results in the higher Cr:Fe ratio observed for the slag, if compared with the ore (Table 1). In the spinel crystalline structure of the ore most of the Fe occurs as Fe(II). Ore particle erosion by O3 oxidation, as indicated by the SEM elemental maps (Fig. 7), could therefore lead to the release of higher concentrations of Fe(II) than what would be expected for the slag O3 erosion. Fe(II) could consume O3 during its conversion to Fe(III) and Fe(II) is also a well-known reducing agent for Cr(VI). However, the possible release of Fe(II) from the ore spinel structure was not verified in this study and could be considered a future perspective in the clarification of the exact mechanism of this reaction system.
Conclusion
The experimental conditions employed in this study cannot be related directly to ozonation in drinking water or industrial wastewater treatment plants, since parameters (e.g. solid loading, ozone concentrations, mixing efficiencies) are likely to be different. Cr(VI) concentrations formed during the experimental ozonation conditions investigated can therefore not be used as a guide to predict possible Cr(VI) formation. However, the results clearly indicate that Cr(VI) can be formed in situ during ozonation of water with non-Cr(VI) Cr-containing materials in suspension. pH seems to be the most important parameter influencing the formation of Cr(VI), with higher pH levels favouring Cr(VI) formation. This can be attributed to the increased rate of aqueous O3 decomposition occurring at pH > 4, resulting in higher concentrations of hydroxyl radicals that are stronger oxidants than aqueous O3. Other parameters, such as contact time, water temperature, solid loading, ozone concentration and the characteristics of the Cr-containing material also have an influence on Cr(VI) formation.
The results indicate the importance of removing suspended particulates from water prior to ozonation. Although dissolved Cr(III) oxidation was not specifically investigated in this study, it can be assumed that dissolved Cr(III) would be more easily oxidised than the relatively inert chromite ore utilised as one of the case study Cr-containing materials. Although most Cr(III) compounds are precipitated out of solution at pH levels relevant to drinking water and wastewater treatment plants, some Cr(III) species are soluble (Bartlett, 1991).
Acknowledgements
The authors thank Prof Quentin Campbell and Prof Marthie Coetzee for the use of the particle size analyser and the pulveriser, respectively.
References
AUDENAERT WTM, CALLEWAERT M, NOPENS I, CROMPHOUT J, VANHOUCKE R, DUMOULIN A, DEJANS P and VAN HULLE SWH (2010) Full-scale modelling of an ozone reactor for drinking water treatment. Chem. Eng. J. 157 (2-3) 551-557. [ Links ]
BARTLETT RJ (1991) Chromium Cycling in Soils and Water: Links, Gaps, and Methods. Environ. Health Perspect. 92 17-24. [ Links ]
BELTRÁN FJ (2003) Ozone Reaction Kinetics for Water and Wastewater Systems. Lewis Publishers, London. 358 pp. [ Links ]
BEUKES JP, DAWSON NF and VAN ZYL PG (2010) Theoretical and practical aspects of Cr(VI) in the South African ferrochrome industry. J. S. Afr. Inst. Min. Metall. 110 (12) 743-750. [ Links ]
BEUKES JP and GUEST RN (2001) Cr(VI) generation during milling. Miner. Eng. 14 (4) 423-426. [ Links ]
CAMEL V and BERMOND A (1998) The use of ozone and associated oxidation processes in drinking water treatment. Water Res. 32 (11) 3208-3222. [ Links ]
CAWTHORN RG (1999) The platinum and palladium resources of the Bushveld Complex. S. Afr. J. Sci. 95 481-489. [ Links ]
COCA M, PENA M and GONZALEZ G (2007) Kinetic study of ozonation of molasses fermentation wastewater. J. Hazard. Mater. 149 (2) 364-370. [ Links ]
CRAMER LA, BASSON J and NELSON LR (2004) The impact of platinum production from UG2 ore on ferrochrome production in South Africa. J. S. Afr. Inst. Min. Metall. 104 (9) 517-527. [ Links ]
DIONEX (2003) Application Update 144. Dionex. URL: http://www.dionex.com/en-us/webdocs/4242-AU144_LPN1495.pdf (Accessed 9 October 2011). [ Links ]
EMSLEY J (2003) Nature's Building Blocks: An A-Z Guide to the Elements. Oxford University Press, Oxford. 538 pp. [ Links ]
ETXEBARRIA N, ARANA G, ANTOLÍN R, DIEZ E, BORGE G, POSADA T and RAPOSO JC (2005) Chromium powder as a reference material for the quality control of particle-size measurement by laser diffraction. Powder Technol. 155 (1) 85-91. [ Links ]
GLASTONBURY RI, VAN DER MERWE W, BEUKES JP, VAN ZYL PG, LACHMANN G, STEENKAMP CJH, DAWSON NF and STERART HM (2010) Cr(VI) generation during sample preparation of solid samples - A chromite ore case study. Water SA 36 (1) 105 -110. [ Links ]
GOGATE PR and PANDIT AB (2004) A review of imperative technologies for wastewater treatment I: oxidation technologies at ambient conditions. Adv. Environ. Res. 8 (3-4) 501-551. [ Links ]
GU F and WILLS BA (1988) Chromite- mineralogy and processing. Miner. Eng. 1 (3) 235-240. [ Links ]
GUROL MD and SINGER PC (1982) Kinetics of ozone decomposition: A dynamic approach. Environ. Sci. Technol. 16 (7) 377-441. [ Links ]
IARC (1997) International Agency for Research on Cancer. Monographs on the Evaluation of Carcinogenic Risks to Humans. Volume 49: Chromium, Nickel and Welding. URL: http://monographs.iarc.fr/ENG/Monographs/vol49/volume49.pdf (Accessed 1 September 2010). [ Links ]
ICDA (2010) International Chromium Development Association. Statistical Bulletin (2010 edn.). 1-65. [ Links ]
LANXESS (2011) URL: http://lanxess.co.za/en/about-lanxess-south-africa/locations/newcastle/ (Accessed 9 October 2011). [ Links ]
LEGUBE B, PARINET B, GELINET K, BERNE F and CROUE J (2004) Modeling of bromate formation by ozonation of surface waters in drinking water treatment. Water Res. 38 (8) 2185-2195. [ Links ]
LOVATO ME, MARTIN CA and CASSANO AE (2009) A reaction kinetic model for ozone decomposition in aqueous media valid for neutral and acidic pH. Chem. Eng. J. 146 (3) 486-497. [ Links ]
McELROY F, MIKEL D and NEES M (1997) Determination of ozone by ultraviolet analysis, A New Method for Volume II, Ambient Air Specific Methods, Quality Assurance Handbook for Air Pollution Measurement Systems. URL: http://mattson.creighton.edu/Ozone/OzoneEPAMethod.pdf (Accessed 9 October 2011). [ Links ]
MURTHY YR, TRIPATHY SK and KUMAR CR (2011) Chrome ore beneficiation challenges & opportunities - A review. Miner. Eng. 24 (5) 375-380. [ Links ]
NAWROCKI J and KASPRZYK-HORDERN B (2010) The efficiency and mechanisms of catalytic ozonation. Appl. Catal., B 99 (1-2) 27-42. [ Links ]
NEL MV, STRYDOM CA, SCHOBERT HH, BEUKES JP and BUNT JR (2011) Comparison of sintering and compressive strength tendencies of a model coal mineral mixture heat-treated in inert and oxidizing atmospheres. Fuel Process. Technol. 92 (5) 1042-1051. [ Links ]
PROCTOR DM, OTANI JM, FINLEY BL, PAUSTENBACH DJ, BLAND JA, SPEIZER N and SARGENT EV (2002) Is hexavalent chromium carcinogenic via ingestion? A weight of evidence review. J. Toxicol. Environ. Health, Part A 65 (10) 701-746. [ Links ]
RAJAGOPAUL R, MBONGWA NW and NADAN C (2008) Guidelines for the Selection and Effective use of Ozone in Water Treatment. WRC Report No. 1596/1/08. Water Research Commision, Pretoria. [ Links ]
RODMAN DL, CARRINGTON NA and XUE Z (2006) Conversion of chromium(III) propionate to chromium(VI) by the Advanced Oxidation Process Pretreatment of a biomimetic complex for metal analysis. Talanta. 70 (3) 668-675. [ Links ]
SELCUK H (2005) Decolorization and detoxification of textile wastewater by ozonation and coagulation processes. Dyes Pigm. 64 (3) 217-222. [ Links ]
SOTELO JL, BELTRÁN FJ, BENÍTEZ FJ and BELTRÁN-HEREDIA J (1987) Ozone Decompostion in Water: Kinetic Study. Ind. Eng. Chem. Res. 26 (1) 39-43. [ Links ]
STERN AH (2010) A quantitative assessment of the carcinogenicity of hexavalent chromium by the oral route and its relevance to human exposure. Environ. Res. 110 (8) 798-807. [ Links ]
THOMAS DH, ROHRER JS, JACKSON PE, PAK T and SCOTT JN (2002) Determination of hexavalent chromium at the level of the California Public Health Goal by ion chromatography. J. Chromatogr. 956 (1-2) 255-259. [ Links ]
WAGNER NJ and HLATSHWAYO B (2005) The occurrence of potentially hazardous trace elements in five Highveld coals. Int. J. Coal Geol. 63 (3-4) 228-246. [ Links ]
XIAO Z and LAPLANTE AR (2004) Characterizing and recovering the platinum group minerals - a review. Miner. Eng. 17 (9-10) 961-979. [ Links ]
Received 14 October 2011; accepted in revised form 11 July 2012.
* To whom all correspondence should be addressed. ffi +27 18 299 2337; fax: +27 18 299 2350; e-mail: paul.beukes@nwu.ac.za