Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
Water SA
versão On-line ISSN 1816-7950
versão impressa ISSN 0378-4738
Water SA vol.38 no.2 Pretoria Jan. 2012
ARTICLES
An ion-imprinted polymer for the selective extraction of mercury(II) ions in aqueous media
Bareki S BatlokwaI, *; Luke ChimukaII; Zenixole TshentuI; Ewa CukrowskaII; Nelson TortoI
IDepartment of Chemistry, Rhodes University, PO Box 94, Grahamstown, 6140 South Africa
IISchool of Chemistry, University of the Witwatersrand, P/Bag 3, WITS 2050, Johannesburg, South Africa
ABSTRACT
A double-imprinted polymer exhibiting high sensitivity for mercury(II) in aqueous solution is presented. Polymer particles imprinted with mercury(II) were synthesised by copolymerising the functional and cross-linking monomers, N'Â[3Â (Trimethoxysilyl)-propyl]diethylenetriamine (TPET) and tetraethylorthosilicate (TEOS). A double-imprinting procedure employing hexadecyltrimethylammonium bromide (CTAB), as a second template to improve the efficiency of the polymer, was adopted. The imprinted polymer was characterised by FTIR, scanning electron microscopy (SEM) and the average size determined by screen analysis using standard test sieves. Relative selective coefficients (k') of the imprinted polymer evaluated from selective binding studies between Hg2+ and Cu2+ or Hg2+ and Cd2+ were 10 588 and 3 147, respectively. These values indicated highly-favoured Hg2+ extractions over the 2 competing ions. The results of spiked and real water samples showed high extraction efficiencies of Hg2+ ions, (over 84%) as evaluated from the detected unextracted Hg2+ ions by ICP-OES. The method exhibited a dynamic response concentration range for Hg2+ between 0.01 and 20 μg/mℓ, with a detection limit (LOD, 3o) of 0.000036 μg/mℓ (36 ng/ℓ) that meets the monitoring requirements for the USA EPA of 2 000 ng/ℓ for Hg2+ in drinking water. Generally, the data (n=10) had percentage relative standard deviations (%RSD) of less than 4%. Satisfactory results were also obtained when the prepared sorbent was applied for the pre-concentration of Hg2+ from an aqueous certified reference material. These findings indicate that the double-imprinted polymer has potential to be used as an efficient extraction material for the selective pre-concentration of mercury(II) ions in aqueous environments.
Keywords: Ion-imprinted polymer, selective extraction, mercury(II) ion, pre-concentration
Introduction
In recent years the release of various harmful heavy metal ions into the environment has attracted great attention worldwide because of their toxicity and widespread use. Mercury(II) is among those which are of great concern (Byüyüktiryaki et al., 2007). It is a widely-distributed environmental pollutant in aqueous environments and its toxicity to humans and animals even at low concentrations is well known. Mercury(II) is included in all lists of priority pollutants as a result, and different regulations and guidelines have been developed for monitoring its levels in water and sediments (Hayes, 1997). Considering the extreme toxicity of mercury, the United States Environmental Protection Agency (EPA) has mandated an upper limit of 10 nM (2 000 ng/ℓ) for Hg2+ in drinking water (EPA 2001).
The toxicity of mercury depends considerably on its chemical form and, as such, analytical methods that are capable of distinguishing between the various forms of mercury and other competing inorganic ions are of special interest. Although there are currently some sensitive instruments to detect mercury(II), the most widely used methods for analysing these harmful ions employ inductively-coupled plasma optical emission spectros-copy (ICP-OES; Leopold et al., 2009) and atomic absorption spectroscopy (AAS; Detcheva and Grobecker, 2006), but their sensitivity and selectivity are usually insufficient for direct determination of these contaminants at very low concentration levels in complex matrices of environmental samples.
Therefore a sample preparation/pre-concentration step prior to analysis is usually necessary. The step must rely on inexpensive, intelligent and robust functional materials with high sensitivity, selectivity and specificity for the targeted analytes. Solid phase extraction (SPE) has been used for pre-concentration of mercury and other heavy metals due to its flexibility, environmental-friendliness, speed, simplicity, safety and ease of automation (Thurman et al., 1998). The choice of sorbent is a key point in SPE because it can control the analytical parameters such as selectivity, affinity and capacity (Dean, 1998). The main challenge of the available SPE sorbents has always been selectivity of the analyte of interest in the presence of closely-related analogues.
Several solid supports, such as chelating resins (Nastasovic et al., 2004), modified silica (Tzvetkova et al., 2010), modified clay (Guerra et al., 2009), alumina (Duan et al., 2003) and ion exchange resins have also been used for the pre-concentration of mercury or its other forms. For example, Duolite GT-73 resin has been used for the pre-concentration of mercury(II) and gold from hydrochloric acid media in the presence of co-existing metal ions. Due to the high affinity of the resin to the transition metals, the mercury was adsorbed alongside other metals. The competing metals were released by leaching with mineral acids, leaving behind the mercury which was then desorbed by digestion of the resin with peroxide and the acid (Pohl et al., 2005). The extraction selectivity of these materials was found to be inadequate, and as such the development of highly-selective materials for mercury species extraction continues to be of great interest (Wu et al., 2007). More recently, ion-imprinted polymers (IIPs) or molecular-imprinted polymers (MIPs) have been identified as suitable materials and are increasingly used in contaminant or trace analysis, as they are suitable for applications where analyte selectivity is essential.
IIPs are nano-porous polymeric materials, which upon leaching the imprint ion can thereafter selectively rebind the ion in the presence of closely-related ions. Ion-imprinting procedures are similar to those of molecular imprinting, except that metal ions rather than molecules are the ones used for imprinting. Unlike the MIPs, the IIP field is still in its infancy (Rao et al., 2006).The preparation of IIPs involves the complexation of the target ion (known as template or print ion) with the functional monomer (known as the ligand or the substrate) (Suede et al., 1999). This is followed by a polymerisation reaction with an excess cross-linking agent that fixes the preassembled binding groups around the print ion (Vlatakis et al., 1993). Eventually the print ion is leached out with a suitable solvent, leaving behind specific recognition sites with a memory for the original print ion (Sellergren et al., 2001; Bartsch et al., 1998). Consequently, the recognition vacancies left behind, when the print ion is leached out from the formed polymer, will be selective and complementary to it in shape, size and functionality (Rao et al., 2006). Therefore, IIPs show higher selectivities and affinities in rebinding the print ion than its analogues (Ferrer et al., 1999; Masque et al., 2001). A particularly promising application of ion-imprinting polymers is the selective (i) SPE pre-concentration of analytes present in trace amounts (Martin-Esteban et al., 2001; Sellergren, 1999) or (ii) separation from other coexisting species (Tsukaghoshi et al., 2001) or complex matrix, which may lead to selective environmental clean-up of analytes, not achievable by the conventional methods (Li et al., 2007).
In this paper, a mercury(II) IIP that was synthesised and applied to water samples collected in the vicinity of Grahamstown, Eastern Cape Province, South Africa, will be discussed. The synthesis procedure employed was the hierarchical double-imprinting approach proposed by Wu et al. (2007). Our group used a monomer with more nitrogen (N) donor atoms (3 in the triamine as opposed to 2 in the diamine which Wu et al. used) to improve coordination during the pre-assembly step in the imprinting process. In order to improve selectivity, a more rigorous method was used to leach out the template ion thus resulting in more available cavities for rebinding. This was carried out to improve on the relative selectivity coefficients (k'), {300 - 500}, that were reported by Wu et al. (Wu et al., 2007). A large k' value means selectivity of the prepared polymer material relative to the competing ions is high.
Experimental
Chemicals
N'-[3-(trimethoxysilyl)-propyl] diethylenetriamine (TPET) and tetraethylorthosilicate (TEOS), hexadecyltrimethylammo-nium bromide (CTAB), sodium hydroxide, nitric acid, sodium acetate and acetic acid were supplied by Sigma-Aldrich (Saint Louis, MO, USA), and mercury(II) nitrate monohydrate and Hg(NO3)2.H2O by BDH AnalR (London, England). Reagents used were at least of analytical grade. All water used was obtained from Direct Q 3UV millipore system (Billerica, MA, USA). NIST traceable mercury(II) certified reference material of water, lot number D2-MEB338111MCA was obtained from Inorganic Ventures (Christiansburg, VA, USA).
Instrumentation and apparatus
ICP-OES, ICAP 6000 series, Thermo Electron Corporation, (Waltham, MA, USA) was used to measure the concentration of the unextracted Hg2+ as well as that of Cd2+ and Cu2+ in aqueous media (at 194.4, 214.438, 324.754 nm respectively). To ensure that Hg2+ as the imprint ion was thoroughly washed off the imprinted polymer, an XRF EDX 900 spectrometer, Pan Analytical, Shimadzu, (Kyoto, Japan), was used to detect the concentration of mercury from the washings of the polymer as well as in the dried polymer itself.
For morphology and characterisation, scanning electron microscope (SEM) micrographs for the imprinted (washed and unwashed) and the non-imprinted polymer powders were obtained at 20 kV on a JSM 840 field emission scanning electron microscope JEOL, (Tokyo, Japan). FTIR spectra (4 000 - 400 cm-1) were recorded by a Bruker Tensor 27 FTIR spectrophotometer (Ettlingen, Germany).
The polymer particles were obtained by centrifuging with MSE Mistral 1000 centrifuge, Sanyo Gallenkamp, (Loughborough, England), at 45 000 r/min for 10 min. A Jenway 3510 pH meter, (Dunmow, England) was used to measure the pH values. Standard Test sieves Retsch GmbH & Co., (Haan, Germany), were used to obtain the average size of the polymer particles by screen analysis.
Preparation of the mercury(II) ion-imprinted polymer and removal of the print species (templates)
The mercury(II) ion-imprinted polymer was prepared by following a literature procedure (Wu et al., 2007) with some modifications. Hg(NO3)2-H2O (print ion), CTAB (surfactant micelle as second print species), TPET (monomer), TEOX (cross linking agent), 1 M NaOH (pH modulator) and ultrapure water (porogen) were mixed according to the following optimal molar ratios; 1:2:2.5:10:4:1500, respectively. The mixture was magnetically stirred at 900 r/min for 4 h. Off-white gels were yielded. The gels were mixed with more water, refluxed at 90°C for 1 h and recovered by centrifugation.
The gels that resulted were washed with 3 M NaOH until the pH of the washings was at 7.5. The gels were further washed several times with water, before drying in the oven at 60°C for about 4 h. The resultant granules were ground and wet-sieved to a homogenous off-white powder to yield the mercury(II) ion-imprinted polymer of 25-30 µm particle size as measured by the standard test sieves. The particles still contained the mercury(II) ions and CTAB templates and were referred to as the unwashed ion-imprinted polymer (IIP) particles.
The mercury(II) ions and CTAB templates were exhaustively removed from the unwashed IIP particles by refluxing with 3 M HNO3 and 99.99% ethanol in the ratio 1:1 v/v, respec-tively, for a total of 7 h of 1 h cycles. At the end of every 1 h cycle the solid IIP particles were recovered by centrifugation. The procedure was repeated 7 times, which resulted in a total of 7 h, for optimal template removal. A fresh solvent of the nitric acid and ethanol was added at the beginning of every hour. The concentration of mercury(II) ions in both the supernatant and the IIP, on the other hand, were determined at the end of every 1 h removal cycle. The concentration of mercury in the supernatant liquids and corresponding IIP particles for each of the 1 h removal cycles were analysed by XRF spectrophotometer.
Optimal template removal at the 7th cycle was marked by no further change in the quantity of mercury(II) ions detected in the supernatant liquid. The IIP particles that resulted after the template removal provided the washed IIP particles. A non-imprinted polymer (NIP), referred to as the control polymer, was prepared in the same manner as the mercury(II) IIP, with the exception that the mercury(II) was not included in the synthetic procedure for the control polymer and hence was not templated.
Binding studies
Optimisation of IIP quantity needed for maximum extraction of Hg2+
20 mℓ aliquots of 1 µg/mℓ Hg2+ spiked water, each containing increasing concentrations of the Hg2+ imprinted polymer (5, 10, 15, 20, 30, 35, 25 and 40 mg) as well as the sodium acetate/acetic acid buffer, were mechanically shaken and kept for 24 h. The mixture was then filtered and the concentration of the unextracted Hg2+ measured by ICP-OES. The experiment was performed in triplicate. Mean values and standard deviations were determined. From the values, the extraction efficiencies (EEs) were evaluated using Eq. (1):
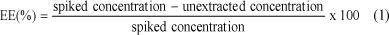
Optimisation of time needed for maximum extraction of Hg2+
Following the procedure for optimisation of quantity, unextracted Hg2+ ions (at 5 min intervals) were determined until a constant value was reached. This marked the optimum time needed for the polymer to bind.
Effect of pH on extraction of Hg2+
The optimal time and quantity of the IIP were used in the evaluation of the effect of pH by performing binding experiments at different pH. The pH of the solutions was adjusted using sodium acetate/nitric acid for pH 1-3, sodium acetate/ acetic acid for pH 4-7.5, ammonium hydroxide/ammonia for pH 8-10 and ammonium hydroxide/sodium hydroxide for pH 10-12.
Selectivity experiments
Using the optimised conditions, competitive and selectivity experiments were performed. Both the imprinted and non-imprinted polymers were used to obtain 2 sets of experimental data. Polymer material (25 mg) was added to 20 mℓ aqueous solutions containing 1 μg/mℓ Hg2+/Cd2+ and 1 μg/mℓ Hg2+/Cu 2+. The pH was then adjusted accordingly to pH 7.2. These were placed in sealed containers and stirred magnetically at 900 r/min for 15 min. After the adsorption-equilibrium, the mixtures were filtered and the concentration of each ion in the remaining solution was measured by ICP-OES. The measured values gave the concentrations of the unextracted ions, from which EEs were evaluated. The experiments were performed in triplicate and the results subjected to statistical analysis at the 95% confidence limit.
The effect of imprinting on selectivity was defined by:

where:
Kd is the distribution coefficient
Ci and Cf the initial and final concentrations, respectively
V the volume of the solution used for the extraction
m the mass of the polymer used for extraction.
The selectivity coefficient (k), for the binding of a particular metal ion in the presence of a competing ion can be obtained by:

The relative selectivity coefficient k':

The results allow an estimation of the effect of imprinting on selectivity.
Sample preparation and analysis
Real water samples (tap, sea, river, pulverised coal solution, treated and untreated sewage, from the vicinity of Grahamstown, South Africa) were filtered through the Millipore Millex-HV hydrophilic PVDF 0.45 μm filter and refluxed for 1 h with 1% H2O2 to oxidise the organic matter. The pH of the resulting water samples was adjusted accordingly to pH 7.2. For each of the samples the concentration of Hg2+ was determined by ICP-OES in 100 mℓ aliquots, for back-ground, spiked (1μg/mℓ) and spiked with IIP (1μg/mℓ + 75 mg IIP). The samples with the IIP were continually shaken for 1 h to allow for equilibration after which the unextracted concentration of the ions was determined. EEs were then evaluated. The imprinted powder with rebound Hg2+ was then eluted with millipore water, 3M HNO3, millipore water in sequence, and the desorbed Hg2+ was subsequently determined with ICP-OES. This procedure was followed for the determination of Hg2+ in the certified reference material (CRM).
Results and discussion
Characterisation of the polymers
Spectroscopic and physical characteristics of the polymer material were in agreement with those reported in literature (Wu et al., 2007). The SEM micrograph of the imprinted polymer displayed a regular, spherical morphology with numerous pores on the spherical surface (figure not shown), which is a suitable geometrical and textural property for a potential adsorbent. This indicates that there are many well-defined binding sites on the imprinted polymer. The irregular, amorphous morphology exhibited in the non-imprinted SEM micrographs showed no well-defined binding sites, hence indicating its lack of suitability to act as an adsorbent.
Characterisation of the imprinted (unwashed and washed) and non-imprinted polymers by FTIR showed similar locations and appearances of major bands. Of particular interest was the peak at 1 472 cm-1 due to the existence of the N-Hg-N stretching vibrations. It was strong in the unwashed polymer, relatively weak in the washed polymer and absent in the non-imprinted polymer. The strong peak in the unwashed polymer spectrum indicated the abundant existence of a coordination complex, [Hg(TPET)]2+ in its polymer structure. The relatively weak peak in the washed polymer was due to the removal of most of the Hg2+ ions from the polymer, thus resulting in very little coordination remaining between Hg2+ and TPET after washing. The Hg2+ ions were not included during the synthesis of NIP, hence the expected absence of the characteristic peak in its spectrum.
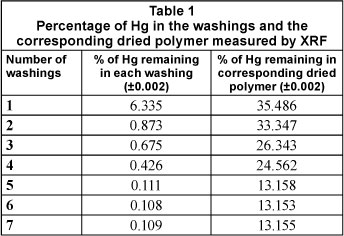
Washing off the mercury(II) ions and CTAB
The very weak N-Hg-N stretch at 1 472cm-1 in the FTIR spectrum of the washed polymer, as well as the low concentration of mercury (0.111%) determined by XRF in the final washing of the IIP, suggested that Hg2+ ions were thoroughly washed out. By contrast, the direct determination of mercury concentration of the washed IIP by XRF was 13.158% (see Table 1). This was noted to be very high for a polymer which was thoroughly washed, and for a material to be used in trace analysis. A logical explanation to these discrepancies is that some of the mercury may have gotten bound to the structure of the polymer during synthesis, to the extent that it could not be removed by the methods that were used for washing in this study. Another assumption is that, since XRF is a very sensitive technique and measures total mercury, it could be that the mercury that was detected in the washed IIP, even after thorough washing, was not the one involved in the selective nano-pore formation of the IIP, and is therefore of little concern to our study as it would not affect the performance of the polymer if it was not involved in the initial binding of the template.
Extraction behaviours of the polymer
The percentage of Hg2+ extracted increased with the quantity of polymer from 5 mg up to 25 mg, after which further increase in the quantity of the polymer did not yield any increase, as shown in Fig. 1. This marked the optimum quantity of the polymer powder (25 mg) needed to bind maximally. The highest extraction efficiency (EE) of Hg2+ achieved was calculated as 88.8 ± 0.1%.
Figure 2 shows the time dependence of the adsorption capacities of Hg2+ ions on the polymer powder (25 mg) as a function of time. Hg2+ ion adsorption increases with time during the first 15 min, after which it levels off (Fig. 2), exhibiting fast kinetics for binding the Hg2+ ions. A good EE (%) of the bound ions was recorded (89.9 ± 0.1%), even at these short equilibration-adsorption times.
The effect of pH on Hg2+ ion adsorption by the polymer powder (25 mg) is shown in Fig. 3. The polymer exhibited low affinities for Hg2+ ion extraction in very acidic and alkaline conditions, as indicated by the low EEs, with the highest calculated being 87.5 ± 0.1% at pH 7.2 ± 0.2. Low pH (acidic) solutions have a greater affinity for metal ions such as the Hg2+ ion; hence the ion was distributed more in the acidic solution than on the IIP particles. Thus the low EEs were recorded at low pH. Under alkaline conditions (high pH), it is likely that the Hg2+ ion complexed with the hydroxide ions forming soluble amphoteric hydroxides instead of being adsorbed on the IIP particles. As a result, low EEs were recorded.

Selectivity studies of the imprinted polymer powder (25 mg) for Hg2+ versus closely related ions, Cd2+ and Cu2+
Competitive adsorption of Hg2+/Cd2+ and Hg2+/Cu2+ couples were investigated in an equilibration-adsorption batch system (see Table 2).
Cd2+ ion was chosen as a competing ion because, like Hg2+, it binds well with amine ligands, while Cu2+ has a higher affinity for the same type of ligand (Wu et al., 2007). Additionally, both of the competing ions have the same charge, have comparative ionic radii, and often coexist with Hg2+ ions, exhibiting certain interference properties in aqueous environments. Table 3 summarises the distribution coefficient (Kd), the selectivity coefficient (k) and the relative selectivity coefficient (k') values of the competing ions with respect to the target ions, i.e. Hg2+ ions.
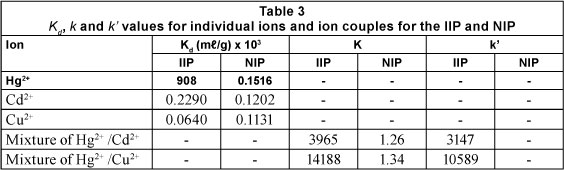
As indicated in Eq. (1) the Kd values are the ratios of the concentration of a particular ion between the imprinted polymer and the aqueous environment. It signifies the extraction ability of a unit quantity of the polymer for a particular ion in a unit volume of solution. Kd values for the imprinted polymer for all ions were higher than those for the non-imprinted polymer, indicating that even the non-imprinted polymer has non-selective sites which any ion may be bound to. The Kd value for the imprinted polymer used to extract Hg2+ ions in the spiked samples was significantly higher (908.0909 x 103 mℓ/g), more than threefold, than that of Cd2+ and Cu2+ (152 and 120 ml/g respectively). This demonstrated the ability of the imprinted polymer to bind Hg2+ ions as they perfectly fitted the fabricated recognition sites, and to a far greater extent than the competing ions. The selectivity coefficient (k) for Hg2+ binding in the presence of Cd+2 ions was found to be 3 965; that is, the polymer will extract Hg2+ 3 965 times more than it can extract Cd2+ ions. For Cu2+ this is 14 189 times more, making Cu2+ the less interfering ion, as the Hg2+ ion out-competes it by a large k value. The k values for the non-imprinted polymer were of the same order of magnitude: 1.26 for the Hg2+/Cd2+ and 1.34 for the Hg2+/Cu2+ competition systems. The closeness of these values shows that the non-imprinted polymer had similar if not the same affinity for all of the ions, as there were no recognition sites that were originally created for any particular ion in its structure. From the values the effect of imprinting on selectivity can be estimated. The high values of k' exhibited by the prepared imprinted polymer powder between Hg2+/Cd2+ (3 147) and Hg2+/Cu2+ (10 588) indicate that the prepared polymer is highly selective to Hg2+ even in the presence of its closely-related analogues.
Validation and application to water samples
Calibration was performed using Hg2+ standards at different concentrations in the range of 0-20 μg/mℓ .The obtained linear range regression equation and correlation coefficient (r) for Hg2+ were Cins = 0.995 C cal + 0.004 and 0.9997, respectively, where Cms and Ccalwere instrumental (ICP-OES) signal and calculated (prepared) concentrations of Hg2+ for each standard, respectively. Results showed that the linear range was several orders of magnitude for the determination of Hg2+ in aqueous environments. The limit of detection (LOD, 3σ) was calculated as 0.036 ng/mℓ (36 ng/ℓ) and meets the monitoring requirements for the USA EPA of 2 000 ng/ℓ for Hg2+ in drinking water.
The accuracy of the method was validated by determining the Hg2+ concentration of an aqueous NIST traceable CRM, with certified Hg2+ concentration of 9.99 ± 0.03 μg/mℓ. After concentrating the CRM with the imprinted sorbent, mean Hg2+ concentrations, of 10.01 ± 0.01 μg/mℓ for the 9.99 μg/mℓ CRM and 0.0997 ± 0.04 μg/mℓ for a hundred-times diluted original CRM, were determined with ICP-OES for n = 10. The accuracy and precision of the method were found to be acceptable at 95% confidence limit for the concentration and analysis of Hg2+ in aqueous solutions.
When the method was applied to real water samples, high extraction efficiencies, over 84% in all cases (see Table 4), were obtained. This demonstrated the suitability of the sorbent to selectively extract mercury(II) ions from complex aqueous matrices.
Conclusions
In this study, a mercury(II) ion-imprinted functionalised polymer with exceedingly high performance, as marked by the fast equilibration-adsorption kinetics, the very large relative selectivity coefficients, high extraction efficiency percentages of the targeted ion (Hg+2), even in the presence of other closely-related ions, was successfully prepared. The polymer was simple and relatively easy to prepare. To the best of our knowledge, this is the first time such relative coefficients (k'), in the ranges of several thousands, have been reported. Experimental results obtained show that the polymer has high analytical potential for selective extraction and pre-concentration of mercury(II) ions in the presence of closely-related ions. Its use as a solid-phase extraction sorbent can be further evaluated in future.
Acknowledgements
This work was supported by funding from the National Research Foundation (Republic of South Africa).
References
BARTSCH RA and MAEDA M (1998) Molecular and Ionic Recognition with Imprinted polymers. ACS Symposium Series, Vol.703. Oxford University Press, Washington, DC. [ Links ]
BÚYÚKTIRYAKI S, SAY R, DENIZLI A and ERSOZ A (2007) Mimicking receptor for methylmercury preconcentration based on ion-imprinting. Talanta 71 699-705. [ Links ]
DEAN JR (1998) Extraction Methods for Environmental Analysis. Wiley, New York. [ Links ]
DETCHEVAA and GROBECKER KH (2006) Determination of Hg, Cd, Mn, Pb and Sn in seafood by solid sampling Zeeman atomic absorption spectrometry. Spectrochim. Acta PartB 61 454-459. [ Links ]
DUAN T, KANG J, CHEN H and ZENG X (2003) Determination of ultra-trace concentrations of elements in high purity tellurium by inductively coupled plasma mass spectrometry after Fe(OH)3 co-precipitation. Spectrochim. Acta Part B 58 1679-1685. [ Links ]
EPA (ENVIRONMENTAL PROTECTION AGENCY, UNITED STATES) (2001) Mercury update: Impact of fish advisories. In: EPA Fact Sheet EPA823-F-01-011. Office of Water, USEPA, Washington, DC. [ Links ]
FERRER I and BARCELO D (1999) Validation of new solid-phase extraction materials for the selective enrichment of organic contaminants from environmental samples. Trends Anal. Chem. 18 180-192. [ Links ]
GUERRA DL, VIANA RR and AIROLDI C (2009) Adsorption of mercury cation on chemically modified clay. Mater. Res. Bull. 44 485-491. [ Links ]
HAYES RB (1997) The carcinogenicity of metals in humans. Cancer Causes Control 8 371-385. [ Links ]
LEOPOLD K, FOULKES M and WORSFOLD PJ (2009) Preconcen- tration techniques for the determination of mercury species in natural waters. Trends Anal.Chem. 28 426-435. [ Links ]
LI F, JIANG H and ZHANG S (2007) An ion-imprinted silica- supported organic-inorganic hybrid sorbent prepared by a surface imprinting technique combined with a polysaccharide incorporated sol-gel process for selective separation of cadmium(II) from aqueous solution. Talanta 7 1487-1493. [ Links ]
MARTIN-ESTEBAN A (2001) Molecularly imprinted polymers: New molecular recognition materials for selective solid-phase extraction of organic compounds. Fresenius J. Anal. Chem. 370 795-802. [ Links ]
MASQUE N, MARCE RM and BORRULL F (2001) Molecularly imprinted polymers: new tailor-made materials for selective solid-phase extraction. Trends Anal. Chem. 20 477-486. [ Links ]
NASTASOVIC A, JOVANOVIC S, DORDEVIC D, ONJIA D, JAKOVLJEVIC D and NOVAKOVIC T (2004) Metal sorption on macroporous poly(GMA-co-EGDMA) modified with ethylene diamine. React. Funct. Polym. 58 139-147. [ Links ]
POHL P and PRUSISZ B (2005) On the applicability of Duolite GT-73 to column preconcentration of gold and palladium prior to determination by inductively coupled plasma atomic emission spectro-metry. Microchim. Acta 150 159-165. [ Links ]
RAO TP, KALA R and DANIEL S (2006) Metal ion-imprinted polymers - Novel materials for selective recognition of inorganics. Anal. Chim. Acta 578 105-116. [ Links ]
SELLERGREN B and LANZA F (2001) Molecularly Imprinted Polymers. Elsevier, New York. [ Links ]
SELLERGREN B (1999) Polymer- and template-related factors influencing the efficiency in molecularly imprinted solid-phase extractions. Trends Anal. Chem. 18 164-174. [ Links ]
SUEDE R, SRICHANA T, SAELIM J and THAVORNPIBULBUT T (1999) Chiral determination of various adrenergic drugs by thin-layer chromatography using molecularly imprinted chiral stationary phase prepared with a-agonists. Analyst 124 1003-1009. [ Links ]
THURMAN MS and MILLS MS (1998) Solid-Phase Extraction: Principles and Practice. Wiley, New York. [ Links ]
TSUKAGHOSHI K, YU KY, MAEDA M and TAKAGI M (2001) In: Sellergren B (ed.). Molecularly Imprinted Polymers. Elsevier, New York. [ Links ]
TZVETKOVA P, VASSILEVA P and NICKOLOV R (2010) Modified silica gel with 5-amino-1,3,4-thiadiazole-2-thiol for heavy metal ions removal. J. Porous Mater. 17 459-463. [ Links ]
VLATAKIS G, ANDERSSON LI, MÜLLER R and MOSBACH K (1993) Drug assay using antibody mimics made by molecular imprinting. Nature 361 645-647. [ Links ]
WU G, WANG Z, WANG J and HE C (2007) Hierarchically imprinted organic-inorganic hybrid sorbent for selective separation of mercury ion from aqueous solution. Anal. Chim. Acta 582 304-310. [ Links ]
Received 16 February 2011;
Accepted in revised form 2 April 2012.
* To whom all correspondence should be addressed. +27 82 349-7832; fax: +27 46 622-5109; e-mail: shimabatlokwa@yahoo.com














