Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Water SA
On-line version ISSN 1816-7950
Print version ISSN 0378-4738
Water SA vol.37 n.4 Pretoria Oct. 2011
Solid phase extraction of Beta(B)-N-methylamino-L-alanine (BMAA) from South African water supplies
M Esterhuizen-Londt; TG Downing*
Department of Biochemistry and Microbiology, Nelson Mandela Metropolitan University, PO Box 77000, Port Elizabeth 6031, South Africa
ABSTRACT
Beta(B)-N-methylamino-L-alanine (BMAA) has been implicated in amyotrophic lateral sclerosis/parkinsonism dementia complex (ALS/PDC) and is assumed to cause or contribute to this neurodegenerative disease after bioaccumulation and slow release of BMAA from a protein-associated form and conversion to the excitatory carbamate form. BMAA has been detected in varying quantities in freshwater cyanobacteria, causing some concern regarding the potential for direct dietary consumption of BMAA-containing water and subsequent intoxication. Considering the BMAA content reported in cyanobacteria and the concentrations cyanobacterial cells can reach in a bloom in freshwater impoundments, BMAA concentrations could potentially reach the mg·ℓ-1 range. BMAA has been shown to cause neuronal injury and even death at µM exposure ranges. Current analytical techniques are, however, insufficiently sensitive to detect the molecule at concentrations of less than 250 ng·ℓ-1 without prior concentration. Safe levels have yet to be determined for BMAA in potable water but these levels may be far below this analytical limit. It is therefore necessary to quantify potential exposure at these relatively low levels. A simple method is described here for high levels of BMAA recovery from a range of waters (78-103 ± 5%), as well as an amino acid matrix (57 ± 5%), saline solution (63 ± 5%), tap water (61 ± 5%) and a preliminary analysis of BMAA concentrations from bloom and non-bloom freshwater supply samples. No exogenous BMAA was detected in water supplies, despite high concentrations in the bloom material, suggesting that BMAA is not released or exported by the cyanobacteria or that rapid degradation, binding or uptake of BMAA occurs in these environments. This method is not suggested for marine samples as very low recovery percentages are seen in the presence of sodium.
Keywords: Beta(B)-N-methylamino-L-alanine, BMAA, cyanobacterial bloom, solid phase extraction
Introduction
The isolation of the neurotoxin BMAA from freshwater and marine cyanobacteria (Cox et al., 2005; Banack et al., 2007; Esterhuizen and Downing, 2008; Metcalf et al., 2008; Faassen et al., 2009) caused considerable debate. BMAA has been linked to ALS/PDC, and possibly to other neurodegenerative diseases (Murch et al., 2004; Rao et al., 2006; Lobner et al., 2007). BMAA promotes neurological damage in its carbamate form by acting as an excitotoxin on the N-methyl-D-aspartate (NMDA) receptor, by activating metabotropic glutamate receptor 5 (mGluR5) and by inducing oxidative stress (Lobner et al., 2007; Liu et al., 2009). It was suggested that BMAA bioaccumulated, both as free and protein-associated forms (Cox et al., 2003) through terrestrial and aquatic food chains (Murch et al., 2004; Jonasson et al., 2008; Jonasson et al., 2010; Brand et al., 2010). Protein-associated BMAA is possibly released gradually from proteins during cell metabolism (Murch et al., 2004), explaining the latency in onset of neurological degeneration and suggesting prolonged exposure to small concentrations with accumulated effects. The extent of animal and human exposure however remains unknown.
No published reports exist on the export or extracellular occurrence of BMAA in bloom-containing waters. It is therefore assumed that BMAA is contained intracellularly in cyanobacteria. Additionally, no mechanism for export is known to exist. However, upon bloom senescence it is likely that the intracellular BMAA is released into the surrounding environment. In the case of freshwater cyanobacteria, BMAA may therefore be released into raw water, which in turn may be used as a potable water source. The efficacy of current water treatment systems for BMAA removal is unknown and the potential risk to consumers is therefore also unknown, but can be determined by addressing the extent of raw water contamination and treatment efficacy.
Solid phase extraction has previously been described as a simple and reliable BMAA concentration protocol; however, these applications have been limited to BMAA concentration from small volumes (30 mℓ or less) of cyanobacterial extract (Kubo et al., 2008; Moura et al., 2010; Li et al., 2011). Since biotoxins are often present in low concentrations, toxins from large volumes of water need to be concentrated in order to quantify BMAA. A simple analyte concentration protocol is presented here, based on a strong cation exchanger with hydrophobic interaction, developed for extraction and concentration of BMAA from water samples,along with the method's application and results from selected water supplies and cyanobacterial culture media.
Experimental
Solid phase extraction recovery tests
The Strata-X-C (33 µm particle size, 85 A pore size, 800 m2·g -1 surface area) mixed mode polymeric strong cation sorbent GigaTM tubes (Phenomenex) were conditioned with 20 mℓ of pure methanol (LC grade, Merck) at a flow rate of 20 mℓ-min-1. This was followed by an equilibration step using 20 mℓ of 100 mM formate buffer (pH 4) at a flow rate of 20 mℓ-min-1.
BMAA (2.38 µg or 0.2382 µg) was added in triplicate to 1 ℓ of sterile reverse osmosis (RO) (Consolidated water conditioning CWC Series 7T) water and concentrated using the solid phase extraction (SPE) GigaTM tube columns (Phenomenex). All samples were acidified prior to application. A flow rate of 10 mℓ-min-1 was used for sample application. The columns were washed with 20 mℓ each of 2% formic acid in water and 100% methanol (20 mℓ-min-1) before elution in a final concentration of 5% ammonium hydroxide in a 1:1 ratio of methanol:acetonitrile (10 mℓ-min-1). The sample was subsequently dried under a low nitrogen gas stream to remove hydroxide ions that may interfere with the derivatisation. The samples were resuspended in 1 mℓ of sterile RO water and 500 µℓ of this was derivatised and analysed by liquid chromatography mass spectrometry (LC-MS) as described below. Flow through and wash fractions were collected and analysed for BMAA.
The testing was scaled-up to investigate BMAA recovery in a larger volume. BMAA (2.38 µg) was added to 20 ℓ of RO water in triplicate and concentrated as described. The collected eluent was dried under a stream of nitrogen gas and resuspended in 1 mℓ RO water and the entire volume was derivatised. BMAA (0.238 µg) recovery from 1 ℓ tap water was investigated with prior SPE concentration as described.
BMAA recovery from an amino acid matrix was investigated by adding 2.382 µg BMAA to 1 ℓ of acidified deionised water (16.8 MΩ.cm; Millipore Simplicity® System/Simplicity UV) containing an amino acid matrix of the 20 proteinogenic amino acids at a concentration of 20 nM each (n=3). These samples were processed as previously described.
BMAA recovery from a salt matrix was tested in triplicate by adding BMAA (2.38 µg) to 1 ℓ of deionised water containing 10 parts per thousand (g·ℓ-1) NaCl (analytical grade, Sigma) to simulate marine conditions. Samples were processed as previously described.
The GigaTM tubes SPE column specificity was tested (n=3) by adding BMAA (2.382 µg) to 1 ℓ non-sterile pond water containing unidentified green phytoplankton. After a short exposure period (approx. 10 min), the pond water was centrifuged (10, 000 g, 10 min, 4ºC, Beckman Avanti) to remove the biomass. The supernatant was filtered through a Whatman's glass microfiber filter (GFA, Merck), followed by concentration on a preconditioned GigaTM tube with washing and eluting as described. BMAA in the exposed pond biomass was evaluated by hydrolysis in 6 N hydrochloric acid and 2% thioglycolic acid at 110ºC for 24 h in an inert atmosphere. The hydrolysate was filtered through a 0.22 µm cellulose acetate filter (Lasec), pH adjusted (pH 2.5 ± 0.5) with 0.1 N NaOH, derivatised as before and analysed on LC-MS. All sample tests and analyses were conducted in triplicate.
Sample evaluation using SPE
The extracellular free BMAA content of cyanobacterial culture media was determined for a known BMAA-producing cyanobacterial culture at mid-log phase (n=3). The biomass was removed by centrifugation (Beckmann Avanti JA 10, 10, 000 g, 10 min, 4ºC). The resulting supernatant was concentrated as previously described. The samples were derivatised and analysed on LC-MS as described below.
Tap water (20 ℓ, in triplicate) was passed through a preconditioned GigaTM tube column. The column was washed and the analytes were eluted as previously described. Elutant was analysed on LC-MS with pre-derivatisation as described below.
Samples (approx. 20 ℓ) from 3 raw water supplies (Water Source 1, 2 and 3; South Africa) were pre-filtered with glass microfibre filters (Whatman GFA, Merck) and concentrated on SPE GigaTM tubes as previously discussed. Total BMAA in the collected bloom material was determined by hydrolysing the glass fibre filters in 6 N HCl with 2% thioglycolic acid at 110ºC for 24 h in an inert atmosphere. The hydrolysed extracts were filtered through a 0.22 µm cellulose acetate filter (Lasec) and the pH was corrected to pH 2.5 ± 0.5 before derivatisation.
BMAA analysis
All samples were derivatised as described by Esterhuizen and Downing (2008) using the EZ:faastTM amino acid analysis kit for LC-MS (Phenomenex KH0-7338). BMAA was separated from other amino acids by liquid chromatography on a commercial column (Phenomenex AAA-MS 250 x 2.0 mm amino acid analysis column) using a liquid chromatography system (Shimadzu LC 20AB) coupled to a mass spectrometer (Shimadzu 2010 EV). A solvent gradient was used with A: 10 mM ammonium formate in water and B: 10 mM ammonium formate in methanol (0.0 min = 68% B, 13.00 = 83% B, 13.01 = 68% B, 17.00 68% B) at a flow rate of 0.25 mℓ-min-1. Column temperature was kept constant at 35ºC. The mass spectrometer ESI source (positive ion mode) temperature was set at 250ºC. The ion scan range was between 20 and 600 m/z. The detector voltage was set at 1.5 kV. The interface voltage was set at 4.5 kV and the curved desolvation line voltage at -20 V with the heating block at 200ºC. Data was analysed using LC-MS solutions Ver. 3 software. The LC-MS system was validated using a range of dilutions of an authenticated BMAA standard (Sigma) and negative controls, as well as by spiking 20 standard amino acids with the BMAA authenticated standard (Esterhuizen-Londt et al., 2011). The BMAA was quantified against the homophenylalanine (HPHE) internal standard peak using the peak area ratio to compensate for any sample losses during the derivatisation process.
Results and discussion
Solid phase extraction optimisation
Use of the SPE method yielded high recovery rates for BMAA from both small and large-scale volumes (Table 1). Despite working well below the SPE resin capacity, the BMAA recovery percentage decreased in the presence of other compounds competing for cation binding on the column. Recoveries of BMAA from sterile distilled water samples, spiked with 0.238 µg and 2.38 µg BMAA, were 96 and 103%, respectively (Table 1) indicating complete recovery (SD = 5%). The reduced recovery of BMAA from 20 ℓ of RO water was attributed to losses due to degradation and the long filtering time required for SPE of 20 ℓ (> 24 h). BMAA left over a period of 24 h at room temperature exhibited a 5% loss. These losses fell within the range of degradation and binding in controls. No BMAA was detected from flow through or wash fractions.

The Strata X-C column is a mixed mode polymeric resin with reverse phase selectivity and strong cation exchange groups exhibiting various retention mechanisms including hydrophobic interactions, hydrogen bonding, π-π bonding and strong cation exchange. Under the extraction conditions used and considering the pKa values of BMAA, the acidic and amino groups of BMAA will be dissociated and protonated, respectively (Nunn et al., 1989), making the SPE column ideal for concentration of the analyte. BMAA recovery from an amino acid matrix (Fig. 1) yielded a lower recovery percentage (57%) (Table 1). Amino acids with the most protonation sites at the given pH will bind to the column more tightly. As BMAA has 2 protonation sites it is expected to bind to the resin. Lowering the pH should be considered to keep the acid functions protonated. Column specificity and overloading should also be considered. The Strata X-C column is not ideal for eliminating matrix components as all amino acids form strong ionic bonds with the sulphonic acid moieties on this sorbent. This however does allow for washing with strong organic solvents to remove organic impurities.
Similarly, the salt (NaCl) matrix resulted in reduced BMAA recovery (Table 1), presumably due to competition for solid phase during concentration and derivatisation. At high concentrations, NaCl counterions outcompete analytes in binding to the column which has a higher affinity for the charge on the salt (Ladiwala et al., 2005). Therefore the method is not recommended to be use with marine samples.
Losses from spiked pond water were also substantial (Table 1), despite a relatively low amino acid or salt load suggesting removal of BMAA by filtration of the pond water prior to SPE and derivatisation. The biomass from the filtered pond water was also tested and contained 159% of the spiked amount, yielding 199% of the total added and suggesting a biomass contribution of approximately 2.3 µg despite the lack of a bloom or scum. The sample did contain cyanobacteria, which may have contributed to the total BMAA content. No blank sample was quantified prior to BMAA addition. The phytoplankton BMAA contribution in this experiment prompted evaluation of 3 surface water sources intended for potable use.
Numerous water treatment processes cause cell lysis, which would in turn make BMAA available for uptake by other organisms (Esterhuizen et al., 2011). Safe exposure level guidelines for BMAA have not yet been determined; however, the amount of BMAA in cyanobacteria (Cox et al., 2005; Esterhuizen and Downing, 2008; Banack et al., 2007; Metcalf et al., 2008) is similar to the amounts of microcystin and anatoxin, and similar concentrations are thus expected in bloom-containing waters. The concentrations of microcystin and anatoxin may range from 0 to more than 30 µg·ℓ-1 and the amount of algal biomass per volume may range from 0 to over 20 mg·ℓ-1 (Park et al., 1997; Sedmak and Kosi, 1998; Pawlik-Skowronska et al., 2004). BMAA concentrations are therefore likely to reach up to 20 µg·ℓ-1 assuming total senescence of a dense bloom. Even at low BMAA concentrations, daily intake of water will therefore result in high BMAA exposure.
Sample evaluation using SPE
No free BMAA was detected in treated water or in raw water (Table 2) suggesting either complete removal by treatment processes, adsorption to particulate matter or rapid uptake of BMAA by aquatic organisms (Esterhuizen et al., 2011). As BMAA is a highly polar molecule it may adsorb onto particulate matter through electrostatic interactions. Normal water treatment processes such as flocculation, clarification, and filtration will result in the removal of the majority of, but not all, cyanobacterial cells (Falconer et al., 2005), thereby reducing the BMAA in the water but not eradicating it completely.
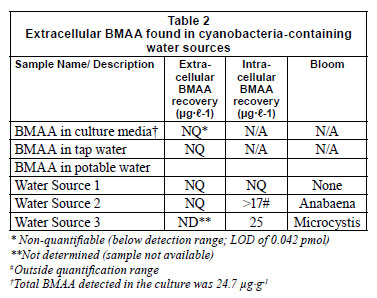
The absence of BMAA detected from the culture media of a healthy mid-log phase cyanobacterial bloom (Table 2), suggested that BMAA is not exported.
No extracellular BMAA was detected in any of the surface waters tested (Table 2). Water Source 1 had no visible cyanobacterial bloom and the filtrand was largely inorganic. No BMAA was detected in the hydrolysed filtrand sample. Water Source 2 contained an Anabaena sp. bloom. Filtrands from Water Source 2 were hydrolysed and BMAA was detected at concentrations in excess of the highest point on the calibration curve (Fig. 2). Samples were not diluted for accurate quantification. The biomass collected from the third dam (Table 2) contained 25 µg BMAA in the filtrand from 1 ℓ of sample.
Conclusion
The solid phase extraction procedure proved to be efficient in concentrating BMAA from large volumes of freshwater. The SPE recovery efficiency decreases with the increase of competing compounds despite adequate column capacity. This method is not suitable for marine samples. No BMAA was detected in raw bloom water samples, suggesting BMAA adsorption or uptake by other aquatic organisms, and raising concerns over human exposure.
In this study, BMAA was not found exogenously in natural water systems. When BMAA was added to raw water (pond water), extracellular BMAA was not detected after a short exposure period. However, BMAA was detected intracellularly, strongly suggesting that uptake by organisms may be occurring, as suggested by the previous uptake study (Esterhuizen et al., 2011). The presence of BMAA in the biomass from these samples, and the absence of free BMAA in the water and cyanobacterial culture media, suggests that BMAA is not transported out of the cells. However, cell lysis is a common event in blooms and the complete absence of BMAA at the detection sensitivities obtained with our LC-MS method, suggests rapid uptake of BMAA in the environment. This supports the possibility of bioaccumulation in natural aquatic systems, raising concerns about exposure via food webs.
Acknowledgements
This study was funded by the Water Research Commission (WRC) of South Africa: Project No. K5/1885.
References
BANACK SA, JOHNSON HE, CHENG R and COX PA (2007) Production of the neurotoxin BMAA by a marine cyanobacterium. Mar. Drugs 5 (4) 180-196. [ Links ]
BRAND LE, PABLO J, COMPTON A, HAMMERSCHLAG N and MASH DC (2010) Cyanobacterial blooms and the occurrence of the neurotoxin, Beta(B)-N-methylamino-L-alanine (BMAA), in South Florida aquatic food webs. Harmful Algae 9 (6) 620-635. [ Links ]
COX PA, BANACK SA and MURCH SJ (2003) Biomagnification of cyanobacterial neurotoxins and neurodegenerative disease among the Chamorro people of Guam. PNAS 100 (23) 13380-13383. [ Links ]
COX PA, BANACK SA, MURCH SJ, RASMUSSEN U, TIEN G, BIDIGARE RR, METCALF JS, MORRISON LF, CODD GA and BERGMAN B (2005) Diverse taxa of cyanobacteria produce Beta(B)-N-methylamino-L-alanine, a neurotoxic amino acid. Proc. Natl. Acad. Sci. 102 (14) 5074-5078. [ Links ]
ESTERHUIZEN M and DOWNING TG (2008) p-N-Methylamino-Lalanine (BMAA) in novel South African cyanobacterial isolates. Ecotoxicol. Environ. Saf. 71 (2) 309-313. [ Links ]
ESTERHUIZEN M, PFLUGMACHER S and DOWNING TG (2011) Beta(B)-N-methylamino-L-alanine (BMAA) uptake by the aquatic macrophyte Ceratophyllum demersum. Ecotoxicol. Environ. Saf. 74 (1) 74-77. [ Links ]
ESTERHUIZEN-LONDT M, DOWNING S and DOWNING TG (2011) Improved sensitivity using liquid chromatography mass spectrometry (LC-MS) for detection of propyl chloroformate derivatised Beta(B)-N-methylamino-L-alanine. Water SA 37 (2) 133-138. [ Links ]
FAASSEN EJ, GILLISSEN F, ZWEERS HAJ and LÜRLING M (2009) Detection of the neurotoxins BMAA (Beta(B)-N-methylaminoL-alanine) and DAB (α-γ-diaminobutyric acid) by LC-MS/MS in Dutch urban waters with cyanobacterial blooms. Amytroph. Lateral Scler. 10 (2) 79-84. [ Links ]
FALCONER IR (2005) Cyanobacterial toxins of drinking water supplies: cylindrospermopsins and microcystins. CRC, Boca Raton. [ Links ]
JONASSON S, ERIKSSON J, BERNTZON L, RASMUSSEN U and BERGMAN B (2008) A novel cyanobacterial toxin (BMAA) with potential neurodegenerative effects. Plant Biotechnol. 25 (3) 227-232. [ Links ]
JONASSON S, ERIKSSON J, BERNTZON L, SPÁCIL Z, ILAG LL, RONNEVI L-O, RASMUSSEN U and BERGMAN B (2010) Transfer of a cyanobacterial neurotoxin within a temperate aquatic ecosystem suggests pathways for human exposure. Proc. Natl. Acad. Sci. U.S.A. 107 (20) 9252-9257. [ Links ]
KUBO T, KATO N, HOSOYA K and KAYA K (2008) Effective determination method for a cyanobacterial neurotoxin, Beta(B)-N-methylamino-L-alanine. Toxicon 51 (7) 1264-1268. [ Links ]
LADIWALA A, REGE K, BRENEMANT CM and CRAMER SM (2005) A priori prediction of adsorption isotherm parameters and chromatograph behaviour in ion-exchange systems. Proc. Natl. Acad. Sci. U.S.A. 102 (33) 11710-11715. [ Links ]
LOBNER D, PIANA PMT, SALOUS AK and PEOPLES RW (2007) Beta-N-methylamino- L-alanine enhances neurotoxicity through multiple mechanisms. Neurobiol. Dis. 25 (2) 360-366. [ Links ]
LI A, TIAN Z, LI J, YU R, BANACK S and WANG Z (2008) Detection of the neurotoxin BMAA within cyanobacteria isolated from freshwater in China. Toxicon 55 (5) 947-953. [ Links ]
LIU X, RUSH T, ZAPATA J and LOBNER D (2009) Beta(B)-N-methylamino-L-alanine induces oxidative stress and glutamate release through action on system Xc-. Exp. Neurol. 217 (2) 429-433. [ Links ]
METCALF JS, BANACK SA, LINDSAY J, MORRISON LF, COX PA and CODD GA (2008) Co-occurrence of Beta(B)-N-methylamino-L-alanine, a neurotoxic amino acid with other cyanobacterial toxins in British waterbodies, 1990-2004. Environ. Microbiol. 10 (3) 702-708. [ Links ]
MOURA S, DE ALMEIDA ULTRAMARI M, MENDEZ LOUZADA DE PAULA D, YONAMINE M and PINTO E (2009) 1H NMR determination of Beta(B)-N-methylamino-L-alanine (L-BMAA) in environmental and biological samples. Toxicon 53 (5) 578-583. [ Links ]
MURCH SJ, COX PA and BANACK SA (2004) A mechanism for slow release of biomagnified cyanobacterial neurotoxins and neurodegenerative disease in Guam. Proc. Natl. Acad. Sci. 101 (33) 12228-12231. [ Links ]
NUNN PB, O'BRIEN P, PETTIT LD and PYBURN SI (1989) Complexes of zinc, copper and nickel with nonprotein amino acid L-alpha-amino beta methylamino propionic acid: a naturally occurring neurotoxin. J. Inorg. Biochem. 37 (2) 175-183. [ Links ]
PARK H, KIM B, KIM E and OKINO T (1997) Hepatotoxic microcystins and neurotoxic anatoxin -a in cyanobacterial blooms form Korean Lakes. Environ. Toxicol. Water Qual. 13 225-234. [ Links ]
PAWLIK-SKOWRONSKA B, SKOWRONSKA T, PIRSZEL J and ADAMCZYK A (2004) Relationship between cyanobacterial bloom composition and anatoxin -a and microcystin occurrence in the eutrophic dam reservoir (SE Poland). Pol. J. Ecol. 52 (4) 479-490. [ Links ]
RAO SD, BANACK SA, COX PA and WEISS JH (2006) BMAA selectively injures motor neurons via AMPA/kainate receptor activation. Exp. Neurol. 201 (1) 244-252. [ Links ]
SEDMAK B and KOSI G (1998) The role of microcystins in heavy cyanobacterial bloom formation. J. Plankton Res. 20 (4) 691-708. [ Links ]
Received 25 January 2011; accepted in revised form 5 September 2011.
* To whom all correspondence should be addressed. +27 41 504-2359; fax: +27 86 614-7129; e-mail: tim.downing@nmmu.ac.za














