Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
Water SA
versión On-line ISSN 1816-7950
versión impresa ISSN 0378-4738
Water SA vol.37 no.3 Pretoria jul. 2011
REVIEW
Water quality in the Okavango Delta
Lesego C Mmualefe; Nelson Torto*
Department of Chemistry, Rhodes University, PO Box 94, Grahamstown 6140, South Africa
ABSTRACT
The Okavango Delta ecosystem sustains a large number of plant and animal species as well as providing resources for the livelihood of the riparian human population. Despite changes in flow patterns, rainfall and other climatic conditions over the past decades, the system has responded well to maintain low salt-water balances through evapotranspiration and chemical precipitation processes. The electrical conductivity and total dissolved solids are generally low, with values less than 200 µS·cm-1 and averaging 40 mg·ℓ-1, respectively. The dissolved oxygen and dissolved organic carbon range from 1.8 to 8.8 and 5 to 15 mg·ℓ-1, respectively, while pH ranges from 6.7 to 10.3. Total nitrogen and phosphorus are generally low with maximum concentrations of 1.7 and 1.6 mg·ℓ-1, respectively, recorded downstream of the Delta. Even though most of these quality parameters are within limits for potable water, the Delta's ecosystem needs to be protected from anthropogenic activities. Past use of persistent organic pollutants requires monitoring of impacts of their residues on the plants and animal species within the ecosystem, in order to maintain its rich biodiversity. This review focuses on chemical quality data for water and sediments in the Okavango Delta published between 2000 and 2010. Despite the shortage of published data, it is hoped that this review will provide an overall picture of the status quo of the Delta's water and will set the direction for future monitoring efforts.
Keywords: Okavango Delta, water quality, pollution, metals, pesticides
Introduction
Freshwater ecosystems consist of producers (plants and phytoplankton), consumers (zooplankton, fish and crocodiles) and decomposers (bacteria and fungi). Their interactions with light, water, dissolved nutrients and suspended solids determine the water quality of the ecosystem at any particular point in time (Humbert et al., 2010). Due to anthropogenic activities, freshwater systems worldwide are confronted with numerous xenobiotics and overloaded with nutrients (Ternes, 1998). A major contribution to chemical contamination originates from wastewater discharges that negatively impact on water quality, due to both the organic and inorganic constituents of wastewater (Kolpin et al., 2004; Snyder et al., 2001; Gao et al., 2010). Additional contamination comes from agricultural activities in which millions of tons of fertilisers and pesticides are employed annually (Schriks et al., 2010). High levels of nitrates (NO3-) and phosphates (PO43-) are common pollutants associated with fertilisers. Nitrates are highly toxic to aquatic life as they easily convert to the even more toxic nitrites (NO2-) (Bowie et al., 1985). The ammonium ion (NH4+) tends to leach slowly but may be converted by soil bacteria to NO3- or NO2- which spreads more rapidly through the soil if not taken up by plants. Phosphate usually precipitates from solution with calcium, iron and aluminium or is incorporated into organic matter. Nitrogen and phosphate are often the limiting factor in eutrophication of water bodies (Mubyana et al., 2003). The resultant increase in growth of algae may cut off the supply of oxygen and sunlight to other aquatic organisms and thus result in subsequent loss of biodiversity within the ecosystem.
Figure 1 shows the location of the Okavango Delta - an alluvial fan situated in the northern part of Botswana. Its economic importance and hydrological functioning have been described extensively elsewhere (McCarthy and Metcalfe, 1990; McCarthy et al., 2005; Bauer-Gottwein et al., 2007; Magole and Magole, 2009).

This review will discuss levels of water quality parameters, such as conductivity, pH, dissolved oxygen, dissolved organic carbon, total nitrogen and phosphorus as well as metals and pesticides, as a reflection of the status of water quality in the Okavango Delta.
Water quality parameters
Electrical conductivity
Electrical conductivity (EC) of an aqueous body is a measure of dissolved ions that is expressed in milliSeimens or microSeimens per centimeter (mS or µS·cm-1) - the higher the concentration of dissolved salts in an aquatic system the higher the EC (Daniel et al., 2002). Large quantities of salts enter the Okavango Delta ecosystem annually, however, the EC of water in the Delta remains low (less than 200 µS·cm-1) despite the high loss of water to evapotranspiration (Zimmermann et al., 2006). The salt-water balance is kept in check by several processes, such as the accumulation of salt under islands (McCarthy et al., 2005), and by a combination of the removal of salts through seasonal flooding, uptake of solids in peat (McCarthy et al., 1989) as well as leakage of salts into underground aquifers (McCarthy and Metcalfe, 1990). In their evaluation of the salt mass balance of the entire Okavango Delta, Bauer et al. (2006) showed that density-driven flow (precipitation of salts onto islands due to evaporation and transpiration) was a significant salt removal process in the Okavango Delta. This is illustrated by the conceptual model in Fig. 2.

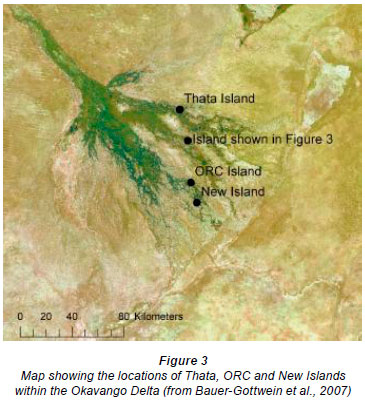
Bauer-Gottwein et al. (2007) analysed the geochemical evolvement of salt islands in the Okavango Delta in 2004 (Fig. 3). They focused on Thata Island (located in the seasonal swamps of the Delta on the Nqoga reach), and the Okavango Research Centre (ORC) and New Islands, which are located on the Boro and lower Boro reaches, respectively. Thata, ORC and New Islands recorded maximum ECs of 20 000, 30 000 and 10 000 µS·cm-1, respectively.
However ECs of surface water in the main channels can be as low as 30 µS·cm-1, with slightly higher values downstream as well as away from the channel centres, indicating evaporative concentration of dissolved salts (Ellery et al., 2003). Mladenov et al. (2005) reported a 21 µS·cm-1 increase of conductivity of water in floodplains downstream compared to water at the entry point (Mohembo).
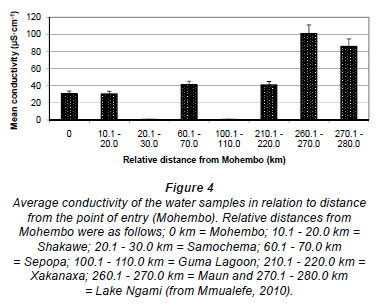
ECs of water along the Thamalakane-Boteti River (a river that floods mostly from June to September but sometimes flows throughout the year, especially in the upper reaches) are generally less than 200 µS·cm-1 at flooded points and higher at the flood fronts (Masamba and Mazvimavi, 2008). The high ECs are attributed to the dissolution of dried salts from the previous flooding season by the approaching flood. In their study, Masamba and Mazvimavi (2008) reported that some sampling points that had not flooded for 5 previous consecutive years recorded high ECs, around 600 µS·cm-1. However, the ECs reduced as the flood passed these points and the water was further diluted by local rainfall. Mmualefe (2010) reported an increase in ECs from 30.9 µS·cm-1 at Mohembo to 101.0 µS·cm-1 at Maun, during flooded periods between 2005 and 2009 (Fig. 4). Measurements had been taken at several points from Mohembo to Maun and Lake Ngami in the downstream end of the Delta.
pH
The pH of an aquatic system determines the concentrations of phosphates, nitrates and organic materials dissolved in the water, which are essential for various metabolic processes in primary producers such as plants and algae. Slight changes in pH (0.1 or 0.2) will not change the diversity of these species but may affect their abundance (McDonald et al., 1989). However, large changes (0.5 or 1.0) of pH may kill the primary producers and have a cascading negative impact on species at higher trophic levels, such as fish, and eventually change the species composition of the aquatic ecosystem (Smith et al., 1986). The pH of most freshwater systems is dependent on the mineral content of the surrounding rocks, soils and other landforms, and often ranges from 6 to 8, which is ideal for most fish and plant species supported by such ecosystems (Sampaio et al., 2010).
Water in the main channels of the Okavango Delta is slightly acidic to neutral (pH 6.5 - 7.0) and becomes alkaline downstream in the floodplains, especially around cattle farming villages, but generally remains within the recommended range (pH 6.5 - 8.5) for potability (WHO, 1984). Mmualefe (2010) reported that the pH of water at sampling points from Mohembo to Lake Ngami ranged from 6.7 to 9.0 during flooded periods from 2005 to 2009. Samples were collected from points as close to the middle of the channel, lagoon or pool as possible, except for Lake Ngami where accessibility to the centre of the lake was difficult. According to Fig. 5 the lowest pH (6.7) was recorded at Xakanaxa - a permanent pool frequently occupied by hippopotami (Hippopotamus amphibus). Lake Ngami - the furthest downstream sampling point from Mohembo, recorded the highest pH 9.0. The local riparian community utilises this sampling point as a drinking spot for livestock and the high pH may be due to the dissolution of ammonia (present in cattle dung) in the water. pH measurements ranging from 6.91 to 10.33 have been reported at points along the Thamalakane-Boteti River (Masamba and Mazvimavi, 2008). Sampling points downstream of Maun, where major land uses involved flood recession cultivation and livestock grazing/watering, recorded the highest pH values, as in the case of Lake Ngami. Bauer-Gottwein et al. (2007) recorded pH values between 7.15 and 8.90 in underground water beneath islands. The highest pH values and ECs were recorded at the centre of these Islands.

Dissolved oxygen
Dissolved oxygen (DO) (expressed in mg·ℓ-1) is the concentration of oxygen that is dissolved in water. Oxygen is introduced into water through photosynthesis by plants and phytoplankton or via diffusion from atmospheric air and through aeration during turbulent mixing (Breitburg, 1990; Sanford et al., 1990). The presence of pollutants in the water may either suppress oxygen production or kill plants and phytoplankton, hence the relevance of DO as an indirect measure of pollution (Engle et al., 1999). Byproducts from sewage effluents, runoff from livestock waste, food waste, decomposing plants and animals may also reduce dissolved oxygen in the water (Rabalais and Turner, 2001; Rabalais et al., 2001).
In the Okavango Delta concentrations of DO are generally higher than the minimum (2.4 mg·ℓ-1) required by aquatic life (Koukal et al., 2004). Measurements made between 2005 and 2009 during flooded seasons showed that a majority of sampling points had mean dissolved oxygen values between 2.7 and 5.5 mg·ℓ-1 (Mmualefe, 2010). Masamba and Mazvimavi (2008) reported DO values between 1.85 and 8.81 mg·ℓ-1 from July to November 2005 along the Thamalakane-Boteti River. Low DO in the water was attributed to the decomposition of organic matter or anthropogenic introduction of oxygen-consuming material (such as livestock waste).
Dissolved organic carbon
Dissolved organic carbon (DOC) refers to micro-particulate (<0.45 µm) organic material found in water and is derived from decomposed plants and animals (Boyer et al., 1996). DOC is an essential component of the carbon cycle and serves as a primary food source for aquatic life (Raymond and Bauer, 2000; Findlay et al., 1993). It contributes to the acidification of weakly-buffered freshwater systems and forms strong complexes with trace metals thus enhancing metal solubilities while reducing their toxic ionic states (McKnight et al., 1985). However DOC and other particulate matter can reduce the penetration of light in aquatic ecosystems and thus negatively impact on phototrophs on the river beds that need light to subsist (Morris and Hargreaves, 1997).
Concentrations of DOC in undisturbed wetlands may range from 1 to 20 mg·ℓ-1 and may vary in flood-pulsed systems such as the Okavango Delta, as annual floods deposit sediments and dissolved constituents from upstream (Mladenov et al., 2007). DOC concentrations range from 5 to 15 mg·ℓ-1 in both channels and floodplains (Mladenov et al., 2005). It has been observed that during flooding DOC concentrations increase at the flood wave front in seasonal swamps due to the inundation of vegetative sources on the floodplains. Higher DOC concentrations on the floodplains compared to channels suggests that floodplains may be sources of DOC. Furthermore, the presence of a downstream increase in vegetation-derived DOC during flooding may be attributed to increasing inundation of DOC sources along the flood path and the evaporative concentration as the water flows downstream.
Total nitrogen and phosphorus
Low concentrations of nitrogen and phosphorus are essential for the growth of aquatic plants as high concentrations can cause rapid growth of algae and plants that may clog waterways and result in blooms of toxic blue-green algae. Okavango Delta's water has been classified as hyperoligotrophic (Wetzel, 1983), with extremely low concentrations of total nitrogen (TN) and phosphorus (TP) (Ellery et al., 1991). In their investigation of TN and TP between February and July 1999, Mubyana et al. (2003) reported non-detectable levels of nitrogen at different soil profile depths (0.1, 0.5, 2.0, 3.0, 4.0 and 5.0 m) from the ORC Island in the Delta. This was attributed to possible leaching in the sandy floodplain and island soils or denitrification in the Delta's woodland areas (Staring, 1978; Davidson and Leonardson, 1998). In the same study by Mubyana et al. (2003), TP was reported to range from 0.02 to 0.52%. It was observed that flooding significantly increased levels of TP on the islands, most probably due to desorption of phosphorus from flooded soils and peat areas in the presence of excess water (Brady, 1990).
Masamba and Mazvimavi (2008) reported maximum TN (1.72 mg·ℓ-1) at the onset of the flood downstream in August 2005, and a subsequent decrease in October and November of the same year. High TN was attributed to fertiliser use in the horticultural gardens close to the sampling points or effluent from hotels upstream of the sampling points. While the TP was below detection at most sampling points, values up to 1.6 mg·ℓ-1 were recorded at the two furthest sampling points in the month of August 2005. These levels reduced in September due to dilution, consumption by aquatic organisms, or deposition into sediment. An increase of TP in October may have been due to concentration effects as a result of evaporation of the water from the system.
Total dissolved solids
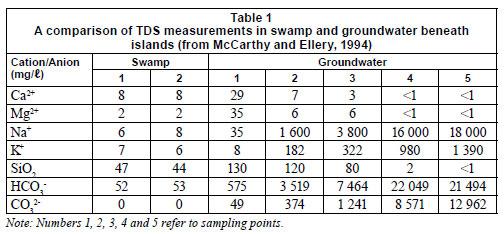
Water in the Okavango Delta has very low total dissolved solids (TDS) content, averaging 40 mg·ℓ-1 (McCarthy, 2006). In their study of the Delta water chemistry and stable isotope composition, Hutton and Dincer (1976) reported TDS measurements downstream of the Delta to be twice those in the upstream flow due to a concentration effect of evapotranspiration processes. Sawula and Martins (1991) characterised water in the main channel of the Delta and Boro River as generally soft, with calcium and magnesium as the major cations with bicarbonate as the main anion. However, TDS measurements of groundwater under islands have been reported to be high - up to 3 orders of magnitude greater than swamp water (McCarthy et al., 1991). According to Table 1, most cations were present at much higher concentrations in water under islands, with the exception of Ca2+ and Mg2+, the concentrations of which were higher in surface water than under islands. This could be because the cations are precipitated out of solution as concentrations of Na+ and K+ increase towards the centre of islands.
A large proportion of the dissolved solids are fixed in the soil as insoluble carbonates and silicates. McCarthy and Ellery (1994) reported that in the seasonal swamps aquatic vegetation causes precipitation of silica below the soil surface, while trees growing on islands cause localised calcium and magnesium carbonate precipitation. Potassium is fixed in insoluble forms as silicate minerals (McCarthy et al., 1991) while sodium exists mainly as a bicarbonate and occurs in the soil and at the soil surface, sometimes at biologically toxic concentrations (Ellery et al., 1993; McCarthy et al., 1986). Nevertheless, the confinement of sodium to the interiors of the islands by transpirational processes shields its toxic impact from the entire system (Ellery and McCarthy, 1994). A change of flooding patterns causes an accumulation of salts in dried-up channels, as was noted by Masamba and Mazvimavi (2008), who reported high sodium and potassium concentrations at points along the Boteti River that had not been flooded for 5 years prior to 2005. In another study, Masamba and Muzila (2005) reported a general increase of sodium and potassium from Mohembo to the Okavango-Maunachira-Khwai channel of the Delta. No anthropogenic source of these metals was identified. Similarly, magnesium levels were high at the onset of the flood wave but reduced as the flood front passed and subsequently increased due to evaporative concentration.
Metals
Metals are introduced into aquatic systems through natural processes such as volcanic eruptions and the weathering of rocks and soils, as well as through human activities such as manufacturing, mining and waste disposal of substances containing metals. Metal pollution in aquatic systems is related to pH, i.e., when the pH of water decreases, the solubility and speciation of metals are enhanced thus increasing their toxicity (Campbell and Stokes, 1985; Starodub et al., 1987; Rai et al., 1993). Arsenic, cadmium, copper, lead, and mercury are the most common heavy metal contaminants.
Low levels for iron (below 0.01 mg·ℓ-1), lead (0.01 to 0.25 mg·ℓ-1) and manganese (0.01 mg·ℓ-1) have been reported at all sampling points along the Thamalakane-Boteti River during the months of June to November 2005, with the exception of the most downstream point during the dry season in November, when the concentrations increased to 6.89 mg·ℓ-1, 0.37 mg·ℓ-1 and 0.53 mg·ℓ-1 for iron, lead and manganese, respectively (Masamba and Mazvimavi, 2008). Lower levels of manganese have been reported along the Boro River (a secondary channel and upstream of the Thamalakane-Boteti River), ranging from 0.007 to 0.019 mg·ℓ-1 (Sawula, 2004). This may be due to dilution effects since the Boro is a bigger river and upstream of the Thamalakane River.
Cadmium, copper, cobalt, nickel and zinc have been reported at trace level concentrations of 0.0006, 0.0021, 0.0004, 0.0003 and 0.0005 mg·ℓ-1, respectively, along the Boro River (Sawula, 2004). These metal concentrations are higher than those reported in an equally pristine wetland, the Niger (Nriagu, 1986), but lower than in relatively polluted environments e.g. the Rhine (Van der Weijden and Middleburg, 1989). The lowest metal concentrations were recorded in the main channel of the Boro River where there was more efficient water mixing compared to zones of low water flow such as flat swamps and isolated pools. Dilution effect of metal ions was also observed during flooding and the rainy season.
Huntsman-Mapila et al. (2006) carried out an extensive investigation of water quality in surface water from the Boro River and in underground water beneath islands adjacent to the river. Table 2 shows an overall concentration of cations and anions as well as higher pH, DO, EC, etc., with increasing depth in underground water. Underground water from a 44 m depth recorded As concentrations higher than the current 0.010 mg·ℓ-1 maximum set by the World Health Organization, with As(III) more predominant than As(V).
The elevated As concentrations prompted a further survey on its distribution in groundwater of the Delta and its interactions with other metals as well as with the physicochemical properties of the water (Huntsman-Mapila et al., 2009). Their findings indicated a positive correlation with sodium and chloride ions, EC, alkalinity, DOC, pH, potassium and sulphate ions, as well as a negative correlation to the easily reducible nitrate ion (Table 3). An enrichment of As together with sodium, chloride, potassium and sulphate ions, as well as EC, in groundwater beneath islands was attributed mainly to a concentration effect of evapotranspiration processes. The increase in pH and alkalinity towards the centre of islands favours the desorption of As from sediments while higher levels of DOC prevent As from percolation, thus promoting higher As concentrations in water beneath islands. In addition the reductive dissolution of iron and manganese oxides or hydroxides could result in the release of As from sediment to groundwater, thereby further increasing its concentration.

Pesticides
Organochlorine pesticides, such as dichlorodiphenyltrichloroethane (DDT) and endosulfan, have been employed in the area surrounding the Okavango Delta in attempts to control the malarial and trypanosome vectors, from as early as the 1940s, until the late 1990s when deltamethrin (a less persistent pyrethroid) was introduced (Allsopp, 2002; Mabaso et al., 2004). These pesticides persist in the environment and hence regular monitoring of their levels is essential for the preservation of the Delta ecosystem.
Mbongwe et al. (2003) investigated DDT in water, plants, invertebrates and fish, between July and December 1999, in lagoons within the Delta. The sum total DDT (DDT and its metabolites) ranged from 0.009 ng·ℓ-1 in water to 18.76 ng·g-1 wet weight in fish. The predominance of dichlorodiphenyldichloroethane (DDE) indicated that there has been no recent application of DDT in the Delta.
Daka et al. (2006) analysed Okavango Delta sediments for deltamethrin along the channel, lagoon and pool sites of Xakanaxa, following aerial spraying between July and August 2002. Concentrations of deltamethrin ranged from 0.013 to 0.291 µg·g-1, with the highest concentrations observed in the pool sites. Analysis of total organic carbon (TOC) revealed a positive correlation between deltamethrin and TOC. Figure 6 shows that sediments with high TOC (pool samples) had the highest deltamethrin levels indicating that high carbon content favoured the adsorption of deltamethrin onto sediment.

In their study to establish baseline levels of persistent organic pollutant (POPs) in the sediments of the Okavango Delta, Mmualefe et al. (2008) reported HCB, aldrin and 4, 4'-DDT in sediments at concentration ranges of 1.1 - 30.3, 0.5 - 15.2 and 1.4 - 55.4 µg·g-1, respectively. Figure 7 shows that there was an increase in pesticide concentrations in the direction of water flow, from the Panhandle (point of entry) to the lower delta.

This trend may be due to the low topographic gradient of the Delta causing low flow rates downstream (Andersson et al., 2003). The low flow rates allow partitioning of water-insoluble components such as pesticides, which are adsorbed onto suspended matter that subsequently settles to the bottom of the river becoming part of sediment. Thus pesticides are more likely to be adsorbed onto organic-rich sediment as compared to the sandy sediment in the Panhandle, as reported by Daka and co-workers (Daka et al., 2006).
In the same study, Mmualefe et al. (2009) reported the presence of hexachlorobenzene (HCB), trans-chlordane, 4,4'-DDD and 4,4'-DDE in water samples, at concentrations ranging from 2.4 to 61.4 µg·ℓ-1. The presence of DDT metabolites in the water and sediments from the Okavango Delta confirm historical exposure to the pesticide. Phthalates were also detected in the water samples but could not be quantified. Long-chain hydrocarbons were detected in samples collected downstream next to lodges and human settlements and could be indicative of petroleum contamination.
Conclusions
Several studies carried out at different times by different researchers on water quality parameters are in agreement. The ecosystem has a unique way of removing from the surface water excessive amounts of dissolved salts that are introduced annually by floods. Concentrations of major anions and cations were within acceptable levels in surface water but were much higher in borehole water and sometimes at toxic levels in islands. Metabolites of DDT have been reported in the water and sediments and their cumulative increase in concentration downstream requires further investigation of the causes and point sources.
There is, however, a need for extensive and organised water quality monitoring in the Delta all year round to provide accurate continuous information through the different seasons and flood patterns. This data would contribute to the establishment of trends and spatial variations of water characteristics. This review has highlighted the need to understand the reasons behind fluctuation of several water quality parameters in response to flood pulses. There is a need for Government departments such as Water Affairs to expand their monitoring exercises beyond simple measurements, such as pH and conductivity, to metals, pesticides and other emerging contaminants.
References
ALLSOP R (2001) Aerial spraying and environmental monitoring in Botswana. Report of the seventh PAAT advisory group of co-ordinators meeting held in Ouagadougou, Burkina Faso, 26-28 September 2001. http://www.fao.org/Ag/againfo/programmes/en/paat/documents/reports/Report-7th%20PAG%20Meeting.pdf. [ Links ]
ANDERSSON L, GUMBRICHT T, HUGHES, D, KNIVETON D, RINGROSE S, SAVENIJE H, TODD M, WILK J and WOLSKI P (2003) Water flow dynamics in the Okavango River Basin and Delta - a prerequisite for the ecosystems of the Delta. Phys. Chem. Earth 28 1165-1172. [ Links ]
BAUER P, SUPPER R, ZIMMERMANN S and KINZELBACH W (2006) Geoelectrical imaging of groundwater salinization in the Okavango Delta, Botswana. J. Appl. Geophys. 60 126-141. [ Links ]
BAUER-GOTTWEIN P, LANGER T, PROMMER H, WOLSKI P and KINZELBACH W (2007) Okavango Delta Islands: Interaction between density-driven flow and geochemical reactions under evapo-concentration. J. Hydrol. 335 389-405. [ Links ]
BOWIE GL, MILLS WB, PORCELLA DB, CAMPBELL CL, PAGENKOPF JR, RUPP GL, JOHNSON KM, CHAN PWH, GHERINI SA and CHAMBERLIN CE (1985) Rates, Constants and Kinetics Formulations in Surface Water Quality Modelling. US Environmental Protection Agency, Athens. [ Links ]
BOYER EW, HORNBERGER GM, BENCALA KE and MCKNIGHT DM (1996) Overview of a simple model describing variation of dissolved organic carbon in an upland catchment. Ecol. Model. 86 183-188. [ Links ]
BRADY NY (1990) The Nature and Properties of Soils (10th edn.). Macmillan Publishing Company, New York. [ Links ]
BREITBURG DL (1990) Near-shore hypoxia in the Chesapeake Bay: Patterns and relationships among physical factors. Estuarine Coastal Shelf Sci. 30 593-609. [ Links ]
CAMPBELL PGC and STOKES PM (1985) Acidification and toxicity of metals to aquatic biota. Can. J. Fish Aquat. Sci. 42 2034-2049. [ Links ]
DAKA PS, OBUSENG VC, TORTO N and HUNTSMAN-MAPILA P (2006) Deltamethrin in sediment samples of the Okavango Delta, Botswana. Water SA 32 483-488. [ Links ]
DANIEL MHB, MONTEBELO AA, BERNARDES MC, OMETTO JPHB, DE CAMARGO PB, KRUSCHE AV, BALLESTER MV, VICTORIA RL and MARTINELLI LA (2002) Effects of urban sewage on dissolved oxygen, dissolved inorganic and organic carbon and electrical conductivity of small streams along a gradient of urbanization in the Piracicaba River Basin. Water Air Soil Pollut. 136 189-206. [ Links ]
DAVIDSON TE, LEONARDSON L (1998) Seasonal dynamics of denitrification activity in two water-logged meadows. Hydrobiologia 364 189-198. [ Links ]
ELLERY K, ELLERY WN, ROGERS KH and WALKER BH (1991) Water depth and biotic insulation: major determinants in back-swamp plant community composition. Wetl. Ecol. Manage. 1 149-162. [ Links ]
ELLERY WN, ELLERY K and MCCARTHY TS (1993) Plant-distribution in islands of the Okavango Delta, Botswana - determinants and feedback interactions. Afr. J. Ecol. 31 118-134. [ Links ]
ELLERY WN and McCARTHY TS (1994) Principles for the sustainable utilization of the Okavango Delta ecosystem, Botswana. Biol. Conserv. 70 159-168. [ Links ]
ELLERY WN, McCARTHY TS and SMITH ND (2003) Vegetation, hydrology, and sedimentation patterns on the major distributary system of the Okavango Fan, Botswana. Wetlands 23 357-375. [ Links ]
ENGLE VD, SUMMERS JK and MACAULEY JM (1999) Dissolved oxygen conditions in northern Gulf of Mexico estuaries. Environ. Monit. Assess. 57 1-20. [ Links ]
FINDLAY S, STRAYER D, GOUMBALA C and GOULD K (1993) Metabolism of streamwater dissolved organic carbon in the shallow hyporheic zone. Limnol. Oceanogr. 38 1493-1499. [ Links ]
GAO HW, LIN J, LI WH, HU ZJ and ZHANG YL (2010) Formation of shaped barium sulfate-dye hybrids: waste dye utilization for eco-friendly treatment of wastewater. Environ. Sci. Pollut. Res. 17 78-83. [ Links ]
HUMBERT JF, QUIBLIER C and GUGGER M (2010) Molecular approaches for monitoring potentially toxic marine and freshwater phytoplankton species. Anal. Bioanal. Chem. 397 1723-1732. [ Links ]
HUNTSMAN-MAPILA P, MAPILA T, LETSHWENYO M, WOLSKI P and HEMOND C (2006) Characterization of arsenic occurrence in the water and sediments of the Okavango Delta, NW Botswana. Appl. Geochem. 21 1376-1391. [ Links ]
HUNTSMAN-MAPILA P, NSENGIMANA H, TORTO N and DISKIN S (2011, in press) Arsenic distribution and geochemistry in groundwater of a recharge wetland in NW Botswana. In: Van Steenbergen (Ed.) Sustaining Groundwater Resources - A Critical Element in the Global Water Crisis. Springer, The Netherlands. [ Links ]
HUTTON LG and DINCER T (1976) Chemical and stable isotope composition of Okavango Delta waters. Technical Note 23. United Nations Development Project, Gaborone. [ Links ]
KOLPIN DW, SKOPEC M, MEYER MT, FURLONG ET and ZAUGG SD (2004) Urban contribution of pharmaceuticals and other organic wastewater contaminants to streams during differing flow conditions. Sci. Total Environ. 328 119-130. [ Links ]
KOUKAL B, DOMINIK J, VIGNATI D, ARPAGAUS P, SANTIAGO S, OUDDANE B and BENAABIDATE L (2004) Assessment of water quality and toxicity of polluted Rivers Fez and Sebou in the region of Fez (Morocco). Environ. Pollut. 131 163-172. [ Links ]
MABASO MLH, SHARP B and LENGELER C (2004) Historical review of malarial control in southern African with emphasis on the use of indoor residual house-spraying. Trop. Med. Int. Health 9 846-856. [ Links ]
MAGOLE L and MAGOLE LI (2009) The Okavango: Whose Delta is it? Phys. Chem. Earth 34 874-880. [ Links ]
MASAMBA WRL and MAZVIMAVI D (2008) Impact on water quality of land uses along Thamalakane-Boteti River: An outlet of the Okavango Delta. Phys. Chem. Earth 33 687-694. [ Links ]
MBONGWE B, LEGRAND M, BLAIS JM, KIMPE LE, RIDAL JJ and LEAN DRS (2003) Dichlorodiphenyltrichloroethane in the aquatic ecosystem of the Okavango Delta, Botswana, South Africa. Environ. Toxicol. Chem. 22 7-19. [ Links ]
McCARTHY J, GUMBRICHT T and MCCARTHY TS (2005) Ecoregion classification in the Okavango Delta, Botswana from multitemporal remote sensing. Int. J. Remote Sens. 26 4339-4357. [ Links ]
McCARTHY TS (2006) Groundwater in the wetlands of the Okavango Delta, Botswana, and its contribution to the structure and function of the ecosystem. J. Hydrol. 320 264-282. [ Links ]
McCARTHY TS and ELLERY WN (1994) The effect of vegetation on soil and ground-water chemistry and hydrology of islands in the seasonal swamps of the Okavango-fan, Botswana. J. Hydrol. 154 169-193. [ Links ]
McCARTHY TS, ELLERY WN and ELLERY K (1993) Vegetation-induced, subsurface precipitation of carbonate as an aggradational process in the permanent swamps of the Okavango (delta) fan, Botswana. Chem. Geol. 107 111-131. [ Links ]
McCARTHY TS, MCIVER JR and CAIRNCROSS B (1986) Carbonate accumulation on islands in the Okavango Delta, Botswana. S. Afr. J. Sci. 82 588-591. [ Links ]
McCARTHY TS, MCIVER JR, CAIRNCROSS B, ELLERY WN and ELLERY K (1989) The inorganic-chemistry of peat from the maunachira channel-swamp system, Okavango Delta, Botswana. Geochim. Cosmochim. Acta 53 1077-1089. [ Links ]
McCARTHY TS, MCIVER JR and VERHAGEN BT (1991) Groundwater evolution, chemical sedimentation and carbonate brine formation on an island in the Okavango Delta swamp, Botswana. Appl. Geochem. 6 577-595. [ Links ]
McCARTHY TS and METCALFE J (1990) Chemical sedimentation in the semiarid environment of the Okavango Delta, Botswana. Chem. Geol. 89 157-178. [ Links ]
McDONALD DG, READER JP and DALZIEL TRK (1989) The combined effects of pH and trace metals on fish ionoregulation. In: Morris R., Taylos EW, BrownDJA, Brown JA (eds.) Acid Toxicity and Aquatic Animals. Cambridge University Press, New York. 221 pp. [ Links ]
McKNIGHT DM, THURMAN EM, WERSHAW RL and HEMOND H (1985) Biogeochemistry of aquatic humic substances in Thoreau's Bog, Concord, Masssachusetts. Ecology 66 1339-1352. [ Links ]
MLADENOV N, MCKNIGHT DM, WOLSKI P and RAMBERG L (2005) Effects of annual flooding on dissolved organic carbon dynamics within a pristine wetland, the Okavango Delta, Botswana. Wetlands 25 622-638. [ Links ]
MMUALEFE L (2010) Sample preparation in environmental monitoring: A case study of the Okavango Delta, Botswana. PhD Thesis, Department of Chemistry, Rhodes University. 140 pp. [ Links ]
MMUALEFE LC, TORTO N, HUNTSMAN-MAPILA P and MBONGWE B (2008) Supercritical fluid extraction of pesticides in sediment from the Okavango Delta, Botswana, and determination by gas chromatography with electron capture detection (GC-ECD) and mass spectrometry (GC-MS). Water SA 34 405-410. [ Links ]
MMUALEFE LC, TORTO N, HUNTSMAN-MAPILA P and MBONGWE B (2009) Headspace solid phase microextraction in the determination of pesticides in water samples from the Okavango Delta with gas chromatography-electron capture detection and time-of-flight mass spectrometry. Microchem. J. 91 239-244. [ Links ]
MORRIS DP and HARGREAVES BR (1997) The role of photodegradation of dissolved organic carbon in regulating the UV transparency of 3 lakes on the Pocono plateau. Limnol. Oceanogr. 42 239-249. [ Links ]
MUBYANA T, KRAH M, TOTOLO O and BONYONGO M (2003) Influence of seasonal flooding on soil total nitrogen, organic phosphorus and microbial populations in the Okavango Delta, Botswana. J. Arid Environ. 54 359-369. [ Links ]
NRIAGU JO (1986) Chemistry of the River Niger II. Trace metals. Sci. Total Environ. 58 89-92. [ Links ]
RABALAIS NN and TURNER RE (2001) Coastal Hypoxia: Consequences for Living Resources and Ecosystems. Coastal and Estuarine Studies. Technical Report 58. American Geophysical Union, Washington, D.C. [ Links ]
RABALAIS NN, TURNER RE and WISEMAN JR WJ (2001) Hypoxia in the Gulf of Mexico. J. Environ. Qual. 30 320-329. [ Links ]
RAI PK, MALLICK N and RAI LC (1993) Physiological and biochemical studies on an acid tolerant Chlorella vulgaris under copper stress. J. Gen. Appl. Microbiol. 39 529-540. [ Links ]
RAYMOND PA and BAUER JE (2000) Bacterial consumption of DOC during transport through a temperate estuary. Aquat. Microb. Ecol. 22 1-12. [ Links ]
SAMPAIO FG, BOIJINK CL, DOS SANTOS LRB, OBA ET, KALININ AL and RANTIN FT (2010) The combined effect of copper and low pH on antioxidant defenses and biochemical parameters in neotropical fish pacu, Piaractus mesopotamicus (Holmberg, 1887). Ecotox. 19 963-976. [ Links ]
SANFORD LP, SELLNER KG and BREITBURG DL (1990) Covariability of dissolved oxygen with physical processes in the summertime Chesapeake Bay. J. Mar. Res. 48 567-590. [ Links ]
SAWULA G and MARTINS E (1991) Major ion chemistry of the lower Boro River, Okavango Delta, Botswana. Freshwater Biol. 26 481-493. [ Links ]
SAWULA GM (2004) On-site preconcentration and trace metal ions determination in the Okavango Delta water system, Botswana. Talanta 64 80-86. [ Links ]
SCHRIKS M, HERINGA MB, VAN DER KOOI MME, DE VOOGT P and VAN WEZEL AP (2010) Toxicological relevance of emerging contaminants for drinking water quality. Water Res. 44 461-476. [ Links ]
SMITH DL, UNDERWOOD JK, OGDEN JG and SABEAN BC (1986) Fish species distribution and water chemistry in Nova Scotia lakes. Water Air Soil Pollut. 30 489-496. [ Links ]
STARODUB ME, WONG PTS, MAYFIELD CI and CHAU YK (1987) Influence of complexation and pH on individual and combined heavy metal toxicity to a freshwater green alga. Can. J. Fish Aquat. Sci. 44 1173-1180. [ Links ]
SNYDER SA, VILLENEUVE DL, SNYDER EM and GIESSY JP (2001) Identification and quantification of estrogen receptor agonists in wastewater effluents. Environ. Sci. Technol. 35 3620-3625. [ Links ]
STARING GJ (1978) Soils of the Okavango Delta. UNESCO, Gaborone, 68 pp. [ Links ]
TERNES T (1998) Occurrence of drugs in German sewage treatment plants and rivers. Water Res. 32 3245-3260. [ Links ]
VAN DER WEIJDEN CH and MIDDLEBURG JJ (1989) Hydrogeochemistry of the River Rhine: Long term and seasonal variability, elemental budgets, base levels and pollution. Water Res. 23 1247-1266. [ Links ]
WETZEL RG (1983) Limnology. WB Saunders Co., Philadelphia. [ Links ]
WHO (1984) Guidelines for Drinking Water Quality. World Health Organization, Switzerland. [ Links ]
ZIMMERMANN S, BAUER P, HELD R, KINZELBACH W and WALTHER JH (2006) Salt transport on islands in the Okavango Delta: Numerical investigations. Adv. Water Resour. 29 11-29. [ Links ]
Received 12 March 2010; accepted in revised form 31 May 2011.
* To whom all correspondence should be addressed. +27 46 6038924; fax: +27 46 6225109; e-mail: N.Torto@ru.ac.za














