Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Water SA
On-line version ISSN 1816-7950
Print version ISSN 0378-4738
Water SA vol.37 n.3 Pretoria Jul. 2011
Development of analytical procedures for the simultaneous determination of tri- to heptabrominated diphenyl ethers and hexabrominated biphenyl (BB 153) in sediment samples
Adegbenro P DasoI,*; Olalekan S FatokiII; James P OdendaalI
IDepartment of Environmental and Occupational Studies, Faculty of Applied Sciences, Cape Peninsula University of Technology, Cape Town, South Africa
IIDepartment of Chemistry, Faculty of Applied Sciences, Cape Peninsula University of Technology, Cape Town, South Africa
ABSTRACT
A simple analytical procedure was developed for simultaneous determination of trace levels of tri- to hepta-BDEs as well as 2,2′,4,4′,5,5′-hexabromobiphenyl (BB 153). The mechanical shaking extraction technique for the isolation of target analytes was optimised. Different extraction solvent combinations were tested under different extraction time periods of 3, 6, 12 and 16 h. The n-hexane:acetone (2:1, v/v) combination gave the best recovery at the optimum extraction of 12 h. Target analytes were quantified using a high capillary gas chromatograph (GC) equipped with an electron capture detector. Under the optimum GC conditions, the resolution of the co-eluting BDE 154 and BB 153 was achieved. The overall recoveries of target analytes in the matrix spike experiment conducted ranged between 84.4 and 110% for BDE 183 and BDE 28, respectively. The method was applied to 19 field sediment samples collected from a control site and from 3 sampling locations (upstream, point of discharge and downstream) of the Black River, which receives effluent from a wastewater treatment plant (WWTP). The sum of the mean concentrations of all of the 7 PBDE congeners was 4.63, 0.35, 'not detectable' and 4.43 ng/g, for the control site, upstream, point of discharge and downstream samples, respectively. The concentrations of BB 153 were generally low in these samples and ranged between ND and 0.89 ng/g. The developed method allows for the simultaneous determination of PBDE congeners and BB 153. It is efficient, moderately rapid and cost-effective.
Keywords: PBDEs, sediment, wastewater treatment plant (WWTP), Black River, Cape Town
Introduction
One of the greatest challenges confronting global water resources is water pollution caused by numerous human activities. Freshwater systems, particularly the rivers which are receptacles for most urban sewage, industrial and agricultural discharges, as well as highly contaminated wastes from informal settlements, are the most affected. The South African situation is similar to that experienced globally. However, in 1994 the South African Government, in a bid to address these concerns, inaugurated the River Health Programme (RHP), a component of the National Aquatic Ecosystem Health Monitoring Programme (NAEHMP), with the objective of assessing the health status of rivers in the entire country (DWA, 2007). To date, most studies conducted on rivers have focussed mainly on heavy metal determination (Binning and Baird, 2001; Fatoki and Awofolu, 2003a; Okonkwo and Mothiba, 2004; Awofolu et al., 2005) and on certain organic contaminants (Dalvie et al., 2003; Fatoki and Awofolu, 2003b; Sereda and Meinhardt, 2005; Fatoki et al., 2010). Generally, there is still insufficient information on the environmental levels of certain emerging contaminants, particularly brominated flame retardants (BFRs), in Sub-Saharan Africa.
BFRs are groups of industrial chemicals incorporated into various consumer products. These chemicals are generally added to products such as electronics, textiles, furniture, etc., to reduce the likelihood of ignition during their usage. Polybrominated diphenyl ethers (PBDEs) and polybrominated biphenyls (PBBs) are typical examples of these chemicals. Although the use of the latter as flame retardants has been completely phased-out, some commercial formulations of the former are still being manufactured and used, particularly in Africa and other regions where their usage has not been subjected to any regulatory scrutiny. Most of these BFRs, especially PBDEs and PBBs, are not covalently bonded to the polymeric materials to which they are added. Hence, the tendency for their eventual release from these materials is likely to occur. Their mode of release from these polymeric materials therefore constitutes an important exposure pathway to humans, particularly in indoor environments. Consumption of contaminated food, ingestion and inhalation of dust, dermal absorption and breastfeeding are some of the known exposure pathways of these contaminants to humans (Lorber, 2008; Raab et al., 2008; Shaw and Kannan, 2009; Daso et al., 2010).
There is growing scientific evidence on the health risks associated with exposure to PBDEs. Toxicological studies have shown that these contaminants are capable of inducing neurobehavioral defects in laboratory animals (Branchi et al., 2002; Dufault et al., 2005; Johansson et al., 2008). Similar studies have also demonstrated that PBDEs are capable of altering sex hormone and thyroid hormone homeostasis, which is essential for the regulation of important developmental and reproductive processes (Hallgren and Darnerud, 2002; Stoker et al., 2004). Recent findings have equally shown that women with considerable levels of PBDEs may experience a significant decrease in fecundability (Harley et al., 2010). Besides their deleterious effects, PBDEs, like other persistent organic pollutants (POPs), are highly toxic, persistent, and capable of bioaccumulation and may undergo long-range atmospheric transport (Hardy, 2002; McDonald, 2002; Wania and Dungani, 2003; Kuriyama et al., 2005). Hence, the need for proper assessment of their levels in the environment, particularly in freshwater systems, cannot be over-emphasised.
Several studies have established the presence of these contaminants in various environmental matrices, thus confirming their widespread distribution in the environment. PBDEs have been detected in soil (Wang et al., 2005a; Li et al., 2008; Fontana et al., 2009), sediment (Samara et al., 2006; Guzzella et al., 2008), sewage sludge (Knoth et al., 2007; Gevao et al., 2008), air particulate matter (Wang et al., 2005b) and indoor dust (Wilford et al., 2005; Gevao et al., 2006; Harrad et al., 2008), using different analytical techniques. Common analytical methods employed include: accelerated solvent extraction (ASE), ultrasonication, microwave-assisted extraction (MAE), supercritical fluid extraction (SFE), and soxhlet extraction. These extraction techniques are usually combined with sophisticated instrumental techniques such as gas chromatography (GC) - mass spectrometry (GC-MS), gas chromatography with electron capture detector (GC-ECD), liquid chromatography - mass spectrometry (LC-MS) and other hyphenated techniques (e.g. gas chromatography - atomic emission detection) for the analysis of BFRs (Johnson and Olson, 2001; Saito et al., 2007; Lagalante and Oswald, 2008; Zhou et al., 2010).
Generally, there has been tremendous improvement in the analysis of PBDEs over the years. Unfortunately, some of these analytical techniques are either too sophisticated or expensive to allow for the investigation of these contaminants in some regions of the world. This may provide a partial explanation for the lack of information on the environmental levels of these contaminants in most developing countries around the world. This study therefore aimed to develop a simple analytical procedure for the simultaneous determination of trace levels of selected PBDE congeners (BDE 28, 47, 99, 100, 153, 154 and 183), as well as 2,2′,4,4′,5,5′-hexabromobiphenyl (BB 153), in sediment samples. The technique employs mechanical shaking for the extraction of target analytes and GC-µECD for the final instrumental analysis. The technique offers numerous advantages, including: good sensitivity, simplicity and relatively low cost. However, a major setback with regard to this technique is its limited selectivity when compared with other techniques.
This study also investigated the imminent co-elution often encountered between BDE 154 and BB 153, especially when shorter capillary columns are employed. Previous studies have shown that the complete resolution of these species could be achieved either by employing longer capillary columns or through the use of a column with a more compatible stationary phase (De Boer et al., 2001; Covaci et al., 2003; Korytar et al., 2005). These measures have often resulted in longer analysis time, usually more than 60 min in most cases. Optimisation of some GC parameters was carried out to improve the performance of the instrument with a view to shortening the analysis time.
Although there are 209 possible PBDE congeners, the commercial formulations of PBDE (penta-, octa- and deca-BDE) only contain a limited number (approximately 20) of these congeners (Webster et al., 2009). The selection of the congeners employed for the development of this analytical procedure was done by taking into account their occurrence in the environment as well as their toxicity. The inclusion of BB 153 in this study was motivated by the need to provide a better understanding of the environmental significance of this congener. Like other PBDE congeners, BB 153 is a congener of a widely-used technical formulation of PBB, used as a flame retardant in the early 1970's. With the recent ban on its production and usage worldwide, many studies have continued to report appreciable levels of this PBB congener, the presence of which is indicative of its degree of persistence in the environment. The analytical procedure was then applied to the determination of both PBDEs and BB 153 in bottom sediment of a selected river in the City of Cape Town.
Experimental
Chemicals and materials
All organic solvents used for the analysis (n-hexane, dichloromethane, acetone and isooctane) were purchased from Merck (South Africa). These were doubly-distilled prior to use. Anhydrous sodium sulphate was purchased from Radchem (Pty) Ltd. (South Africa). Silica gel (60-200 mm) and copper powder were supplied by Sigma-Aldrich (South Africa). High purity gases (Helium - 99.999%; nitrogen 99.999%) were purchased from Afrox (Pty.) Ltd. (South Africa). Unlabelled individual reference PBDEs as well as BB 153 standards were used for identification and quantification. These were produced by Cambridge Isotope Laboratories (CIL) (Andover, MA, USA) and were supplied by Industrial Analytical (Pty.) Ltd. (South Africa).
Sample collection
For method development, sediment samples were collected from a wastewater-receiving pond (S33º56'06''; E18º38'22'') within the university premises. The sample was collected using a stainless steel scoop into a clean 1 ℓ wide-mouth amber glass bottle. The sediment sample was dried for 5 days in a dark room. The dried sample was then sieved with a 1 mm mesh size stainless steel sieve.
Dried sediment samples were then sequentially pre-extracted with combinations of organic solvents (n-hexane, acetone and dichloromethane) using a mechanical shaking technique (Schüttelmaschine RO 20, Bonn, Germany) until no traces of target compounds were detected. The pre-extracted sediment was employed for the recovery experiment. Sediment samples used for method validation were collected in May 2010 from 3 sampling points in the Black River, namely, upstream, downstream and at the point of discharge. The point of discharge receives final effluents from a nearby wastewater treatment plant (WWTP). Historically, the present day Black River was a seasonal river whose flow was heavily dependent on the prevailing climatic conditions. This river derives its name from the dark organic materials leaching out from the fynbos vegetation through which it flows.
Recently, the dark colour of the river water was equally attributed to the discharge of sewage sludge, treated and partially treated effluent from the nearby WWTP (Brown and Magoba, 2009). A number of canals constructed to convey urban stormwater also empty their contents into the river. The combination of these processes had changed the status of this river from a seasonal to perennial water body in recent years. The control site was a stream within the Kirstenbosch Botanical Gardens. The choice of this site was due to the general belief that fewer human activities take place within and around the garden. However, the water of the stream comes from the mountain top adjacent to the most visited tourist centre in the City of Cape Town. In addition, most of the ornamental plants grown within the garden were cultivated with compost materials probably derived from biosolid.
Sample extraction
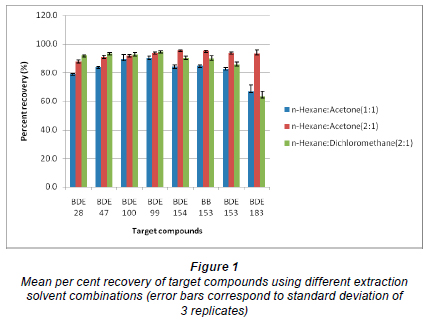
To assess the efficiency of the mechanical shaking technique employed, 3 extraction solvent combinations (n-hexane: acetone (1:1), n-hexane:acetone (2:1) and n-hexane:dichloromethane (2:1)) were evaluated. For this purpose, 10 g of the pre-extracted sediment samples (n = 3) were spiked with a known mixture of all target analytes. Prior to extraction, about 2 g of copper powder was added to each sample to remove any traces of sulphur which could interfere with analyte determination. The spiked samples were thereafter extracted with 120 mℓ of the extraction solvent combinations for 12 h. The n-hexane: acetone (2:1) solvent system gave the best recovery of target compounds as shown in Fig. 1. Hence, subsequent analyses of field-contaminated samples were extracted using this extraction solvent combination. The per cent total organic carbon (TOC %) was estimated for sediment samples analysed, using the weight loss on ignition method, with a muffle furnace operated at 440ºC for 4 h.
Extract clean up
Several approaches have previously been adopted for the purification of sample extracts containing PBDEs. These include the use of gel permeation chromatography (GPC) and multi-layer silica gel column chromatography (Knoth et al., 2007; Kupper et al., 2008; Yun et al., 2008). Owing to the enormous number of samples (n>300), we intended to analyse in other concurrent studies; he conventional multi-layer silica gel glass column chromatography was modified. This was done by packing the sorbents (0.1 g activated silica gel; 0.2 g (30% w/w, 1 N NaOH) basic silica gel; 0.1 g activated silica gel; 0.4 g (44% w/w, conc. H2SO4) acidic silica gel; 0.1 g activated silica gel; 1.0 g anhydrous sodium sulphate) in this sequence (from the bottom) into a Pasteur pipette, thus allowing the use of smaller quantities of the sorbents as well as the eluting solvent. Column pre-conditioning was done using about 10 mℓ n-hexane to remove background contaminants and air trapped within the column.
Prior to extract clean up, sample extracts were concentrated to about 2 mℓ using a rotary evaporator. The concentrated extracts in n-hexane were then cleaned on the pre-conditioned multi-layer silica gel column using n-hexane as eluting solvent. A good recovery of target compounds was achieved with the use of 10 mℓ of the eluting solvent. Although this approach gave excellent recoveries of the target compounds, the elution of analytes was totally dependent on the gravitational force, which made this technique time-consuming as compared to the use of solid-phase extraction cartridges. The cleaned extracts were further concentrated using a rotary evaporator to about 2 mℓ. This was followed by the addition of 1 mℓ of isooctane, serving as a keeper, before final concentration to about 500 µℓ in an amber sample vial under a gentle stream of nitrogen. The prepared samples were then kept in the refrigerator until the final instrumental analysis.
Instrumental analysis
Analyses of target compounds were performed using an Agilent 6890 gas chromatograph equipped with electron capture detector (GC-ECD). The GC-ECD was equipped with an Agilent 7890B autosampler. Chromatographic separation of analytes was performed using a DB-5 MS column (60 m length, 0.25 mm internal diameter and 0.1 µm film thickness). Helium gas was employed as the carrier gas with a flow rate of 1.5 mℓ/min using a constant flow mode. High purity nitrogen gas was used as a make-up gas for the detector at a flow rate of 30 mℓ/min. The injector and detector temperatures were set at 280ºC and 300ºC, respectively. The oven temperature was programmed as follows: 100ºC held for 2 min, ramped at 20ºC/min to 220ºC, and further ramped at 4ºC/min to 300ºC and held for 7 min. 1 µℓ of standard and experimental samples was injected using a splitless injection mode. Quantification was based on peak areas of target compounds using an external calibration technique. 5 to 6 calibration levels containing 1, 5, 10, 25, 50 and 100 ng/mℓ for all target compounds, except BDE-183 which had a calibration level of 5, 10, 25, 50 and 100 ng/mℓ. Identification of analyte was done by comparing the retention times with those of reference standards.
For quality control and assurance purposes, regular injection of solvent blanks and standard solutions was carried out. The retention times of target analytes were only considered if they fell within the retention time window relative to those of the reference standards. A matrix spike (pre-extracted sediment) and a procedural blank (anhydrous sodium sulphate) were run with the batch of 9 field samples. No detectable levels of analytes were found in the blank samples. In addition, a calibration standard of 10 ng/mℓ was run as a check after every 5 samples to provide assurance that less than 20% variation was found from the initial calibration standards. All data were processed with Microsoft Excel software (2007 version).
Results and discussion
Optimisation of extraction technique
The following experimental factors were considered in the proposed mechanical shaking method: sample size, extraction solvent combination and extraction time. The choice of 10 g of sediment sample employed in this method was based on the recommendation contained in the draft USEPA's Method 1614 for PBDEs in environmental samples (USEPA, 2007). Commonly used solvent combinations for the extraction of PBDEs include: n-hexane:acetone (1:1), n-hexane:acetone (3:1) and n-hexane:dichloromethane (2:1). In this study, we evaluated the extraction efficiencies of these solvent combinations. However, an alteration in one of the solvent combinations was made to allow for comparative evaluation of both n-hexane:dichloromethane (2:1) and n-hexane:acetone (2:1).
As shown in Fig. 1, the former gave better recoveries of the lower brominated congeners while the latter had a relatively better recovery of all target compounds, particularly for the highly brominated congeners. The influence of extraction time on the overall efficiency of the proposed method was also investigated. This test was performed during the preliminary investigation, in which 4 extraction times were evaluated. Spiked sediment samples were extracted for 3, 6, 12 and 16 h. No significant difference (P > 0.05) was observed when the extraction time was increased beyond 12 h.
However, a reduction in the overall recovery of BDE 28 was observed when spiked samples were extracted for 16 h. This was probably due to its relatively lower molecular weight, which could possibly enhance its volatilisation. In general, possible losses of analytes could result from volatilisation during concentration steps, adhesion of analytes onto the inner surface of glass containers, thermal degradation in GC injector and/or incomplete recovery of analytes during extract clean-up steps.
Chromatographic separation of target compounds
Many studies have demonstrated that GC-ECD has excellent sensitivity for halogen-containing compounds such as PBDEs and PBBs (Stapleton, 2006; Odusanya et al., 2009). This technique is particularly versatile due to its simplicity and relatively low cost of maintenance. Being a non-selective detector, however, many unwanted compounds containing these chemical species could readily interfere with the investigation of compounds of interest. In this study, the advantages this technique offers were explored by developing an analytical procedure for the analysis of 7 commonly investigated PBDE congeners (BDE 28, 47, 99, 100, 153, 154 and BDE 183), as well as BB 153, in sediment samples. The optimum chromatographic conditions allowed for a good separation of all of the target compounds investigated (Fig. 2). However, a substantial co-elution between BDE 154 and BB 153 was observed during the preliminary investigations when a shorter capillary GC column (HP-5, 30 m x 0.25 mm x 0.25 µm) was employed. Although, several studies had attempted to separate these co-eluting compounds by employing a high resolution gas chromatograph coupled to a high resolution mass spectrometer (Clarke et al., 2008), this measure is very expensive and requires good technical skills. Alternatively, the use of 2-dimensional GC x GC-µECD can be explored to overcome the inherent co-elution of lower brominated diphenyl ethers with other halogenated compounds.
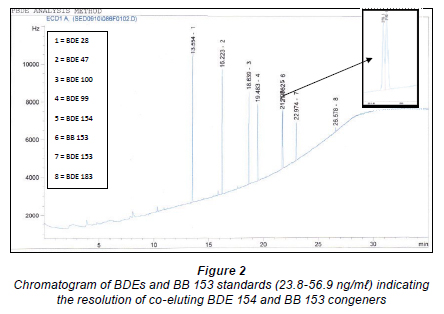
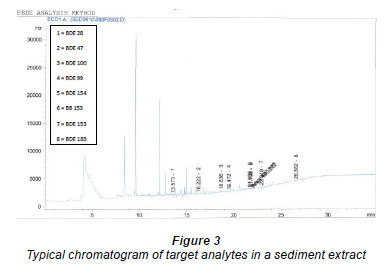
Koryt'ar et al. (2005) reported the co-elution of BDE 154 with BB 153 on DB-1, DB-5 and CP-Sil 19 columns. They were able to achieve a complete baseline separation of these co-eluting compounds on HT-5, DB-17, DB-XLB (30 m x 0.25 mm x 0.25 µm) and HT-8 (25 m x 0.22 mm x 0.25 µm) GC columns. The development of analytical procedure for the evaluation of BFRs must therefore take into consideration the co-elution of these congeners. This is because most environmental matrices investigated, particularly in the North American environment, have been found to contain relatively high levels of BB 153 (Yun et al., 2008), which might produce unexpectedly high values for BDE 154 if not separated. A good analytical technique which is capable of discriminating amongst these co-eluting congeners is essential. In this study, it was possible to demonstrate the applicability of narrow-bore GC capillary column (0.1 µm film thickness) for the resolution of these co-eluting congeners. Secondly, the major setback associated with the use of longer capillary GC columns, i.e. prolonged analysis time, was overcome. A complete chromatographic separation of all target compounds was achieved within 30 min (Fig. 3), thus making the proposed method rapid, efficient and cost-effective.
Performance evaluation of the proposed method
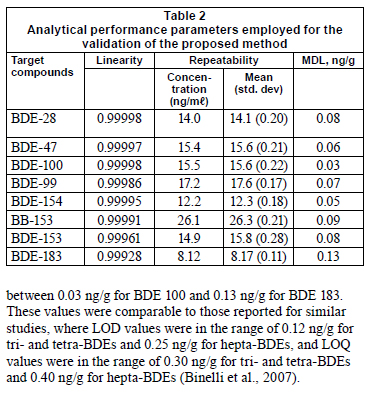
The suitability of the proposed method was assessed by evaluating linearity, repeatability and accuracy, as well as the limits of detection of each compound. The calibration study was performed using certified reference standards produced by CIL laboratories, Andover, MA (USA). The concentration range of each compound was between 1 and 100 ng/mℓ, with the exception of BDE 183 which had a range of 5 to 100 ng/mℓ. Triplicate analyses of the calibration standards were performed. As can be seen in Table 2, the correlation coefficient (R2) values were higher than 0.9992 for all of the target compounds, showing a good response of the electron capture detector to the target compounds. The precision of the method was evaluated by performing repeatability tests over a range of low concentration levels of the target compounds. This was done by estimating the relative standard deviation (RSD) of 7 replicates of each level. At this concentration range the RSD values were between 0.8% at 26 ng/mℓ and 1.78% at 15.75 ng/mℓ for BDE 153. Evaluation of the proposed method's accuracy was verified by recovery studies. As shown in Table 3, recoveries of all congeners varied between 84.4 for BDE 183 and 110% for BDE 28 (with RSD < 12%), falling within the acceptable limits recommended for PBDE analysis (USEPA, 2007). The method detection limits (MDLs) were estimated based on a matrix spiking experiment where pre-extracted sediment were spiked with a low concentration, 2.5 ng/mℓ, with the exception of BDE 183, the concentration of which was 5.0 ng/mℓ. A value of 3 times the standard deviation of 7 replicates of low-level spiked sediment samples was used as the method detection limit. The MDL values obtained ranged between 0.03 ng/g for BDE 100 and 0.13 ng/g for BDE 183. These values were comparable to those reported for similar studies, where LOD values were in the range of 0.12 ng/g for tri- and tetra-BDEs and 0.25 ng/g for hepta-BDEs, and LOQ values were in the range of 0.30 ng/g for tri- and tetra-BDEs and 0.40 ng/g for hepta-BDEs (Binelli et al., 2007).
Application of the proposed method to field samples
The developed method was applied to 19 sediment samples collected from a stream and river within the City of Cape Town. The control site was a stream located within a popular botanical garden within the city. The other site was the Black River, which receives treated effluent from the second-largest WWTP in the city, with a present capacity of 99.5Mℓ/d, corresponding to 800 000 population equivalents. The mean concentrations of the investigated BFRs in sediment samples collected from these locations is presented in Table 4. The occurrence of most of the analytes in the control site is indicative of the ubiquitous nature of these contaminants.
For most of the congeners, the concentrations found at the control site were slightly higher than those found in the bottom sediment of the river investigated. Since there are no discharges into the stream, except via atmospheric precipitation, it could be inferred that other possible sources of PBDEs might be responsible for the observed levels. Hale et al. (2008) recently conducted a study to assess PBDE levels in remote regions of Antarctica. The presence of these contaminants in most matrices monitored was suggestive of inputs from local sources. A notable possible source of these contaminants in the control site is the use of compost manure usually derived from WWTP sludge. These were extensively used for various ornamental works within the botanical garden. Secondly, the botanical garden is situated on a highland, where fast-moving water during rainfall can possibly erode deposited contaminants into nearby water bodies.
The levels of both PBDEs and BB 153 found in the upstream and point of discharge samples were generally low. The majority of the congeners were below the calculated MDLs for these regions. However, all of the congeners were detected in quantifiable amounts in the downstream region. The low levels of contaminants found in the upstream, and particularly in the point of discharge regions, were expected.
For instance, the river is very shallow around the point of discharge; thus effluent discharges from the WWTP generate significant turbulence in this region. This results in the gradual accumulation of very coarse sandy sediment containing relatively small amounts of organic matter. Consequently, the suspended particulate-bound contaminants, which are largely non-settleable in the water column in this region, are transferred further downstream. The prolonged deposition of these contaminants might be responsible for the relatively high contaminant levels observed downstream for the investigated river. Detectable levels of all of the PBDE congeners, as well as BB 153, were found in all of the sediment samples analysed. Of these congeners, BDE 183 and BDE 99 were the most dominant congeners found at the point of discharge and downstream regions. The observed trend suggests that the effluents (municipal and industrial) received by the WWTP might contain penta- as well as octa-BDE formulations, thus implicating WWTPs as important sources of PBDEs in receiving water bodies (Samara et al., 2006). BDE 209, which is a major congener of deca-BDE formulation, was not included in this analytical method development for PBDE analysis, because its analysis often requires a separate analytical procedure for its determination. Since BDE 209 contributes significantly to the overall PBDE levels in most studies, it will be necessary to exclude this congener from the summed PBDE values before a suitable comparison can be made. In general, the results obtained in this preliminary study, the first to report PBDE levels in sediment samples in South Africa, were much lower than those from North American and European studies, which reported total PBDE concentrations as high as 148 ng/g (Samara et al., 2006), 212 ng/g (Oros et al., 2005) and up to 200 ng/g (Sellstrom et al., 1999), and were comparable with those reported in some Asian studies 0.16-94.6 ng/g (Chen et al., 2006), 0.04-94.7 ng/g (Mai et al., 2005).
Conclusions
A simple analytical procedure for the simultaneous determination of PBDEs and BB 153 was developed. The mechanical shaking technique employed for the isolation of target analytes in sediment samples was optimised. Of the 3 extraction solvent combinations tested, the use of n-hexane:acetone (2:1, v/v) gave the best recovery of all of the target analytes. Extract purification was done on Pasteur pipette columns containing layers of different forms of silica gel. The chromatographic separation of these analytes was achieved using a long narrow bore capillary column with moderate polarity. With the optimum GC conditions, the rapid chromatographic separation of target analytes was achieved. The overall performance of the method was evaluated using various analytical performance parameters, including linearity, sensitivity, repeatability and recovery tests. Unfortunately, the analytical recoveries of target analytes in sediment samples were not assessed with the use of a surrogate standard. The results of the matrix spike experiment conducted under the optimum conditions gave acceptable recoveries of all of the target analytes. One interesting outcome of this study was that effluent discharges from the WWTP are potential sources of PBDEs and BB 153 contamination in the river investigated.
Acknowledgements
The authors would like to thank the National Research Foundation (NRF) for financial support and the allocation of a grantholder-linked D.Tech bursary to AP Daso. We also thank the Cape Peninsula University of Technology for providing logistics and laboratory facilities.
References
AWOFOLU OR, MBOLEKWA Z, MTSHEMLA V and FATOKI OS (2005) Levels of trace metals in water and sediment from Tyume River and its effects on an irrigated farmland. Water SA 31(1)87-94. [ Links ]
BINELLI A, SARKAR SK, CHATTERJEE M, RIVA C, PAROLINI M, BHATTACHARYA B, BHATTACHARYA AK and SATPATHY KK (2007) Concentration of polybrominated diphenyl ethers (PBDEs) in sediment cores of Sundarban mangrove wetland, northeastern part of Bay of Bengal (India). Mar. Pollut. Bull. 54 1220-1229. [ Links ]
BINNING K and BAIRD D (2001) Survey of heavy metals in the sediments of the Swartkops River estuary, Port Elizabeth, South Africa. Water SA 27(4)461-466. [ Links ]
BRANCHI I, ALLEVA E and COSTA LG (2002) Effects of perinatal exposure to a polybrominated diphenyl ether (PBDE 99) on mouse neurobehavioural development. Neurotoxicol. 23(3)375-384. [ Links ]
BROWN C and MAGOBA R (2009) Rivers and wetlands of Cape Town - Caring for our rich aquatic heritage. WRC Report No. TT 376/08. Water Research Commission, Pretoria. [ Links ]
CHEN SJ, GUO XJ, MAI BX, CHEN ZM, LUO XJ, SHENG GY, FU JM and ZENG EY (2006) Polybrominated diphenyl ethers in surface sediments of the Yangtze River Delta: levels, distribution and potential hydrodynamic influence. Environ. Pollut. 144 951- 957. [ Links ]
COVACI A, VOORSPOELS S and DE BOER J (2003) Determination of brominated flame retardants, with emphasis on polybrominated diphenyl ethers (PBDEs) in environmental and human samples: a review. Environ. Int. 29 735-756. [ Links ]
DALVIE MA, CAIRNCROSS E, SOLOMON A and LONDON L (2003) Contamination of rural surface and ground water by endosulfan in farming areas of the Western Cape, South Africa. Environ. Health: A Global Access Science Source 2 1-15. [ Links ]
DASO AP, FATOKI OS, ODENDAAL JP and OKONKWO JO (2010) A review on sources of brominated flame retardants and routes of human exposure with emphasis on polybrominated diphenyl ethers. Environ. Rev. 18 239-254. [ Links ]
DE BOER J, ALLCHIN C, LAW R, ZEGERS B and BOON JP (2001) Method for the analysis of polybrominated diphenyl ethers in sediments and biota. Trends Anal. Chem. 20 591-599. [ Links ]
DUFAULT C, POLES G and DRISCOLL LL (2005) Brief postnatal PBDE exposure alters learning and the cholinergic modulation of attention in rats. Toxicol Sci. 88(1)172-180. [ Links ]
DWA (Department of Water Affairs, South Africa) (2007) National aquatic ecosystem health monitoring programme (NAEHMP). E-Communication. November 2007. URL:www.dwa.gov.za/rhp/ecommunication/rhp_e-com_Nov2007.pdf (Accessed 25 August 2010). [ Links ]
FATOKI OS and AWOFOLU OR (2003a) Levels of Cd, Hg and Zn in some surface waters from the Eastern Cape Province, South Africa. Water SA 29(4)375-380. [ Links ]
FATOKI OS and AWOFOLU OR (2003b) Persistent organochlorine pesticide residues in freshwater systems and sediments from the Eastern Cape, South Africa. Water SA 29(3)323-330. [ Links ]
FATOKI OS, BORNMAN M, RAVANDHALALA L, CHIMUKA L, GENTHE B and ADENIYI A (2010) Phthalate ester plasticizers in freshwater systems of Venda, South Africa and potential health effects. Water SA 36(1)117-125. [ Links ]
FONTANA AR, SILVA MF, MARTINEZ LD, WUILLOUD RG and ALTAMIRANO JC (2009) Determination of polybrominated diphenyl ethers in water and soil samples by cloud point extraction-ultrasound-assisted back-extraction-gas-chromatography-mass spectrometry. J. Chromatography A. 1216 4339-4346. [ Links ]
GEVAO B, AL-BAHLOUL M, AL-GHADBAN AN, AL-OMAIR A, ALI L, ZAFAR J and HELALEH M (2006) House dust as a source of human exposure to polybrominated diphenyl ethers in Kuwait. Chemosphere 64 603-608. [ Links ]
GEVAO B, MUZAINI S and HELALEH M (2008) Occurrence and concentrations of polybrominated diphenyl ethers in sewage sludge from wastewater treatment plants in Kuwait. Chemosphere 71 242-247. [ Links ]
GUZZELLA L, ROSCIOLI C and BINELLI A (2008) Contamination by polybrominated diphenyl ethers of sediments from the Lake Maggiore basin (Italy and Switzerland). Chemosphere 73 1684-1691. [ Links ]
HALE RC, KIM SL, HARVEY E, LA GUARDIA MJ, MAINOR TM, BUSH EO and JACOBS EM (2008) Antarctica research bases: local sources of polybrominated diphenyl ether (PBDE) flame retardants. Environ. Sci. Technol. 42 1452-1457. [ Links ]
HALLGREN S and DARNERUD PO (2002) Polybrominated diphenyl ethers (PBDEs), polychlorinated biphenyls (PCBs) and chlorinated paraffins (CPs) in rats-testing interactions and mechanisms for thyroid hormone effects. Toxicol. 177(2-3)227-243. [ Links ]
HARDY ML (2002) The toxicology of the three commercial polybrominated diphenyl oxide (ether) flame retardants. Chemosphere 46 757-777. [ Links ]
HARLEY KG, MARKS AR, CHEVRIER J, BRADMAN A, SJODJIN A and ESKENAZI B (2010) PBDE concentrations in women's serum and fecundability. Environ. Health Perspect. 118(5)699-704. [ Links ]
HARRAD S, IBARRA C, DIAMOND M, MELYMUK L, ROBSON M, DOUWES J, ROOSENS L, DIRTU AC and COVACI A (2008) Polybrominated diphenyl ethers in domestic indoor dust from Canada, New Zealand, United Kingdom and United States. Environ. Int. 34 232-238. [ Links ]
JOHANSSON N, VIBERG H, FREDRIKSSON A and ERIKSSON P (2008) Neonatal exposure to deca-brominated diphenyl ether (PBDE 209) causes dose-response changes in spontaneous behaviour and cholinergic susceptibility in adult mice. Neurotoxicol. 29(6)911-919. [ Links ]
JOHNSON A and OLSON N (2001) Analysis and occurrence of polybrominated diphenyl ethers in Washington State freshwater fish. Arch. Environ. Contam. Toxicol. 41 339-344. [ Links ]
KNOTH W, MANN W, MEYER R and NEBHUTH J (2007) Polybrominated diphenyl ether in sewage sludge in Germany. Chemosphere 67 1831-1837. [ Links ]
KORYT´AR P, COVACI A, DE BOER J, GELBIN A and BRINKMAN UAT (2005) Retention-time database of 126 polybrominated diphenyl ether congeners and two Bromkal technical mixtures on seven capillary gas chromatographic columns. J. Chromatography A. 1065 239-249. [ Links ]
KUPPER T, FELIPPE DE ALENCASTRO L, GATSIGAZI R, FURRER R, GRANDJEAN D and TARRADELLAS J (2008) Concentrations and specific loads of brominated flame retardants in sewage sludge. Chemosphere 71 1173-1180. [ Links ]
KURIYAMA SN, TALSNESS CE, GROTE K and CHAHOUD I (2005) Developmental exposure to low dose PBDE 99: 1- effects on male fertility and neurobehaviour in rat offspring. Environ. Health Perspect. 113 149-154. [ Links ]
LAGALANTE AF and OSWALD TD (2008) Analysis of polybrominated diphenyl ethers (PBDEs) by liquid chromatography with negative-ion atmospheric pressure photoionization tandem mass spectrometry (LC/NI-APPI/MS/MS): application to house dust. Anal. Bioanal. Chem. 391 2249-2256. [ Links ]
LI K, FU S, YANG ZZ and XU XB (2008) Composition, distribution and characterisation of polybrominated diphenyl ethers (PBDEs) in the soil in Taiyuan, China. Bull. Environ. Contam. Toxicol. 81 588-593. [ Links ]
LORBER M (2008) Exposure of Americans to polybrominated diphenyl ethers. J. Exposure Sci. Environ. Epidemiol. 18 2-19. [ Links ]
MAI BX, CHEN SJ, LUO XJ, CHEN LG, YANG QS, SHENG GY, PENG PA, FU JM and ZENG EY (2005) Distribution of polybrominated diphenyl ethers in sediments of the Pearl River Delta and adjacent South China Sea. Environ. Sci. Technol. 39 3521-3527. [ Links ]
McDONALD TA (2002) A perspective on the potential health risks of PBDEs. Chemosphere 46 745 -755. [ Links ]
OKONKWO JO and MOTHIBA M (2004) Physico-chemical characteristics and pollution levels of heavy metals in the rivers in Thohoyandou, South Africa. J. Hydrol. 308 122-127. [ Links ]
ODUSANYA DO, OKONKWO JO and BOTHA B (2009) Polybrominated diphenyls in leachates from selected landfill sites in South Africa. Waste Manage. 29(1)96-102. [ Links ]
OROS DR, HOOVER D, RODIGARI F, CRANE D and SERICANO J (2005) Levels and distribution of polybrominated diphenyl ethers in water, surface sediments and bivalves from the San Francisco estuary. Environ. Sci. Technol. 39(1)33-41. [ Links ]
RAAB U, PREISS U, ALBRECHT M, SHAHIN N, PARLAR H and FROMME H (2008) Concentrations of polybrominated diphenyl ethers, organochlorine compounds and nitro musks in mother's milk from Germany (Bavaria). Chemosphere 72 87-94. [ Links ]
SAITO I, ONUKI A and SETO H (2007) Indoor organophosphate and polybrominated flame retardants in Tokyo. Indoor Air 17 28-36. [ Links ]
SAMARA F, TSAI CW and AGA DS (2006) Determination of potential sources of PCBs and PBDEs in sediments of the Niagara River. Environ. Pollut. 139 489-497. [ Links ]
SELLSTROM U, KIERKEGAARD A, ALSBERG T, JONSSON P, WAHLBERG C and DE WIT C (1999) Brominated flame retardants in sediments from European estuaries, the Baltic Sea and sewage sludge. Organohalogen Compounds 40 383-386. [ Links ]
SEREDA BL and MEINHARDT HR (2005) Contamination of the water environment in malaria endemic areas of KwaZulu-Natal, South Africa by DDT and its metabolites. Bull. Environ. Contam. Toxicol. 75(3)538-545. [ Links ]
SHAW SD and KANNAN K (2009) Polybrominated diphenyl ethers in marine ecosystems of the American continents: Foresight from current knowledge. Rev. Environ. Health 24(3)157-229. [ Links ]
STAPLETON HM (2006) Instrumental methods and challenges in quantifying polybrominated diphenyl ethers in environmental extracts: a review. Anal. Bioanal. Chem. 386 807-817. [ Links ]
STOKER TE, LAWS SC, CROFTON KM, HEDGE JM, FERRELL JM and COOPER RL (2004) Assessment of DE-71, a commercial polybrominated diphenyl ether (PBDE) mixture, in the EDSP male and female pubertal protocols. Toxicol. Sci. 78(1)144-155. [ Links ]
USEPA (UNITED STATES ENVIRONMENTAL PROTECTION AGENCY) (2007) Method 1614: Brominated diphenyl ethers in water, soil, sediment and tissue by HRGC/HRMS. Washington DC, USA. [ Links ]
VAN AARDT WJ and ERDMANN R (2004) Heavy metals (Cd, Pb, Cu, Zn) in mudfish and sediments from three hard-water dams of the Mooi River catchment, South Africa. Water SA 30(2)211-218. [ Links ]
WANG D, CAI Z, JIANG G, LEUNG A, WONG MH and WONG WK (2005a) Determination of polybrominated diphenyl ethers in soil and sediment from an electronic waste recycling facility. Chemosphere 60 810-816. [ Links ]
WANG X, DING X, MAI B, XIE Z, XIANG C, FU J and ZENG EY (2005b) Polybrominated diphenyl ethers in airborne particulates collected during a research expedition from Bohai Sea to the Arctic. Environ. Sci. Technol. 39 7803-7809. [ Links ]
WANIA F and DUGANI CB (2003) Assessing the long-range transport potential of polybrominated diphenyl ethers: a comparison of four multimedia models. Environ. Toxicol. Chem. 22(6)1252-1261. [ Links ]
WEBSTER L, RUSSELL M, WALSHAM P, PHILLIPS LA, PACKER G, HUSSY I, SCURFIELD J A, DALGARNO EJ and MOFFAT CF (2009) An assessment of persistent organic pollutants (POPs) in wild and rope grown blue mussels (Mytilius edulis) from Scottish coastal waters. J. Environ. Monit. 11 1169-1184. [ Links ]
WILFORD BH, SHOEIB M, HARNER T, ZHU J and JONES KC (2005) Polybrominated diphenyl ethers in indoor dust in Ottawa, Canada: Implications for sources and exposure. Environ. Sci. Technol. 39 7027-7035. [ Links ]
YUN SH, ADDINK R, McCABE JM, OSTASZEWSKI A, MACKENZIE-TAYLOR D, TAYLOR AD and KANNAN K (2008) Polybrominated diphenyl ethers and polybrominated biphenyls in sediment and floodplain soils of the Saginaw River watershed, Michigan, USA. Arch. Environ. Contam. Toxicol. 55 1-10. [ Links ]
ZHOU SN, REINER EJ, MARVIN C, HELM P, RIDDELL N, DORMAN F, MISSELWITZ M, SHEN L, CROZIER P, MACPHERSON K and BRINDLE ID (2010) Development of liquid chromatography atmospheric pressure chemical ionisation tandem mass spectrometry for analysis of halogenated flame retardants in wastewater. Anal. Bioanal. Chem. 396 1311-1320. [ Links ]
Received 1 October 2010; accepted in revised form 30 May 2011.
* To whom all correspondence should be addressed. +2778 902-7213; fax: +2721 460 3905; e-mail: adegbenrop@yahoo.com














