Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
Water SA
versão On-line ISSN 1816-7950
versão impressa ISSN 0378-4738
Water SA vol.37 no.3 Pretoria Jul. 2011
Dimethylglyoxime based ion-imprinted polymer for the determination of Ni(II) ions from aqueous samples
Modise Rammika*; Godfred Darko; Zenixole Tshentu; Joyce Sewry; Nelson Torto
Department of Chemistry, Rhodes University, PO Box 94, Grahamstown 6140, South Africa
ABSTRACT
A Ni(II)-dimethylglyoxime ion-imprinted polymer {Ni(II)-DMG IIP} was synthesised by the bulk polymerisation method. The morphology of the Ni(II)-DMG IIP and non-imprinted polymer were observed by scanning electron microscopy and the chemical structures were evaluated by infrared spectroscopy. Selectivity of the Ni(II)-DMG IIP was studied by analysing, using an inductively coupled plasma-optical emission spectrometer, for Ni(II) ions that were spiked with varying concentrations of Co(II), Cu(II), Zn(II), Pd(II), Fe(II), Ca(II), Mg(II), Na(I) and K(I) in aqueous samples. The studies revealed Ni(II) recoveries ranging from 93 to 100% in aqueous solutions with minimal interference from competing ions. Enrichment factors ranged from 2 to 18 with a binding capacity of 120 µg·g-1. Co(II) was the only ion found to slightly interfere with the determination of Ni(II). Selectivity studies confirmed that the Ni(II)-DMG IIP had very good selectivity, characterised by %RSD of less than 5%. The limits of detection and quantification were 3x10-4 µg·mℓ-1 and 9x10-4 µg·mℓ-1, respectively. The accuracy of the method was validated by analysing a custom solution of certified reference material (SEP-3) and the concentration of Ni(II) obtained was in close agreement with the certified one. The Ni(II)-DMG IIP was successfully employed to trap Ni(II) ions from a matrix of sea, river and sewage water. It is believed that the Ni(II)-DMG IIP has potential to be used as sorbent material for pre-concentration of Ni(II) ions from aqueous solutions by solid-phase extraction.
Keywords: nickel, polymer, dimethylglyoxime, interference
Introduction
Heavy metals are stable and cannot be degraded or destroyed easily (Zheng. et al., 2007; Prechthai et al., 2008; Yao et al., 2009). They therefore tend to accumulate in the environment, from where they eventually get into the human food chain. The human body needs some of these metals at trace levels but further accumulation in the body results in various health effects (Singh et al., 2010; Wang et al., 2005; Khan et al., 2008). It is therefore important to determine their levels in the environment. This is especially important for water, where heavy metal contamination is prevalent (Momodu and Anyakora, 2010; Mansour and Sidky, 2002; Rico et al., 1989; Miller et al., 2004; Dhakate and Singh, 2008). However, the determination of heavy metals in water samples is rather challenging as they occur at low concentration levels in complex matrices (Ubalua et al., 2007; Hlavay et al., 2004; Namieśnik, 2002; Winkler et al., 1991; Dheri et al., 2007). Hence, there is the need to remove the matrices or develop pre-concentration strategies that can enhance selectivity prior to their determination in water samples (Tan and Huang, 2009; Li and Zhou, 2006).
Various pre-concentration techniques, such as normal digestion (Balcerzak, 2002; Rechcigl and Payne, 1989), microwave digestion (Rechcigl and Payne, 1989;Sarin et al., 2004), slurry techniques (Lima et al., 2000), chemical precipitation (Massoumi and Hedrick, 1969), electro-deposition (Ruotolo and Gubulin, 2002), cementation (Chang et al., 2007), ultra-filtration (Hong et al., 1998), ion exchange (Shao et al., 1991), activated carbon adsorption (Wilson et al., 2006), liquid-liquid extraction (Sarma and Reddy, 2002) and solid-phase extraction (SPE) (Hennion, 1999), have been used to abstract trace metals from matrices. These methods have the limitation of poor selectivity as one sorbent normally picks up several metal ions at once (Pourreza et al., 2010). SPE can be combined with ion-imprinted polymers (IIPs) in order to improve selectivity of ions (analytes) of interest from environmental samples (Ramakrishnan and Rao, 2006; Otero et al., 2009; Romaní et al., 2009a; Romaní et al., 2009b; Zhu et al., 2009; Krishna et al., 2004).
Ion-imprinted polymers (IIPs) are highly selective cross-linked polymeric materials, synthesised by complexing a template (metal ion) and a functional monomer in the presence of a crosslinker (Nishide et al., 1976). The reaction proceeds via a free radical initiator in an appropriate solvent normally referred to as a porogen. Removal of the template creates cavities that are complementary in size and charge to the metal of interest. The memory is a result of the effect of the size and charge of the metal ion that was present in the cavity during polymerisation. Hence the cavities have a high recognition/selectivity for the metal ion only. The functional mechanism is similar to that of antibodies or enzymes. However, IIPs have advantages over antibodies in that they are cheap and can be used in or with a variety of solvents at high temperature, pressure and pH, which antibodies or enzymes would not tolerate.
Due to their high selectivity, IIPs have been used as sorbents in SPE (Rao et al., 2004). Such sorbents have an advantage over conventional SPE sorbents in that they can be used in a variety of solvents at high temperature and pressure, and over a wide pH range, without compromising on their efficiencies. IIP-SPE combines the advantages of SPE, such as low cost, speed, simplicity and flexibility, with the high selectivity of IIPs (Wilson et al., 2006). Of all the methods used in preparing IIPs, the trapping method is the most commonly used (Romaní et al., 2008; Praveen et al., 2005; Metilda et al., 2007; James et al., 2009; Daniel et al., 2003; Romaní et al., 2009a; Romaní et al., 2009b; Uguzdogan et al., 2010; Saraji and Yousefi, 2009). This is because other methods require the use of a complexing ligand having vinyl groups (Ersoz et al., 2004; Li et al., 2005; Su et al., 2007; Jiang et al., 2006; Chen et al., 2009), which are scarce.
Nickel is one of the most widely imprinted ions. It is imprinted by different methods of polymerisation. Most nickel ion-imprinting studies have been carried out using the trapping method (Romaní et al., 2008; Praveen et al., 2005; Daniel et al., 2003; Romaní et al., 2009a, Romaní et al., 2009b,; Jiang et al., 2006; Singh and Mishra, 2010), with different chelating agents such as hydroxyquinoline (Otero et al., 2009; Romaní et al., 2009a; Zhu et al., 2009, Romaní et al., 2008) or modified hydroxyquinoline (Romaní et al., 2009) and its derivatives (mainly 5,7-dichloroquinoline) (Praveen et al., 2005), and dithizone (Saraji and Yousefi, 2009). Other methods, such as surface imprinting and chemical immobilisation, have also been employed (Su et al., 2007; Jiang et al., 2006; Chen et al., 2009; Vijaya et al., 2008). However, dimethylglyoxime (DMG) has rarely been used as a ligand in these ion-imprinting polymerisation reactions. DMG has been used for the determination of nickel using colourimetric and gravimetric techniques (Gazda et al., 2004; Ali et al., 1999; Willard, 1943; Qureshi et al., 2008; Korolczuk, 2000, Van Den Berg and Nimmo, 1987). Daniel et al., 2003, designed a Pd(II)-DMG IIP for the selective uptake of palladium ions from dilute aqueous solutions. The total time needed to prepare the polymer was more than 2 days. Therefore, new methods are needed to reduce this period of time if DMG-based ion-imprinted polymers are to be successfully used as SPE sorbents for the determination of Ni(II).
In this paper, we report the synthesis of a DMG-based Ni(II) ion-imprinted polymer {Ni(II)-DMG IIP} that can be used to selectively trap Ni(II) ions in aqueous solutions in the presence of competing cations. To the best of our knowledge, no Ni(II) ion imprinting work has been reported using DMG as the trapped ligand.
Experimental
Chemicals and reagents
Analytical grade sodium hydroxide pellets, hydrochloric acid/potassium chloride buffer solution, disodium hydrogen phosphate buffer solution, acetic acid (glacial), sodium acetate buffer solution, 4-vinylpyridine, 2,2'-azobisisobutyronitrile (AIBN), 2-methoxy ethanol, styrene, nickel(II) sulfate hexahyrate (NiSO4.6H2O), divinyl benzene (DVB), dimethylglyoxime (DMG), trace metal grade nitric acid, hydrochloric acid and stock solutions of Ni(II), Cu(II), and Co(II) were obtained from Sigma Aldrich (Steinheim, Germany). Pd(II) was obtained from BDH laboratories Chemical Division (London, UK). Standard solutions of Ca(II), Fe(II), Mg(II), Zn(II), Na(I), and K(I) were freshly prepared from their nitrate salts also obtained from Sigma Aldrich. Filter paper was acquired from Whatman (Maidstone, UK). An A10 milli-Q system from Millipore RiOs (Bedford, USA) was used to generate ultrapure water. A custom solution of certified reference material (CRM), SEP-3 was obtained from Inorganic Ventures (Christiansburg, USA).
Instrumentation
Scanning electron microscopy (SEM) images were acquired by a TS5136ML Digital Vega Microscope from Tescan (Brno, Czech Republic). FTIR (400-4 000 cm-1) spectra were recorded on a PerkinElmer Spectrum 100 spectrometer (Massachusetts, USA) equipped with a universal ATR sampling accessory. Concentrations of metals were determined using an iCAP 6000 series inductively coupled plasma-optical emission spectrometer (ICP-OES) from Thermo Electron Corporation (Cheshire, United Kingdom). The solution pH was measured using the Jenway 3510 pH meter (Essex, UK).
Preparation of the Ni(II)-DMG IIP
Ni(II)-DMG IIP was prepared by mixing NiSO4·6H2O (0.263 g), 4-VP (0.23 mℓ) and DMG (0.465 g) in 2-methoxy ethanol (10.0 mℓ) with stirring for 10 min, to form a ternary complex. Subsequently, DVB (1.78 mℓ), styrene (1.15 mℓ) and AIBN (50.0 mg) were added. The mixture was cooled to 0ºC and purged with nitrogen gas for 10 min. Polymerisation was carried out by heating in an oil bath at 70ºC for 3 h. The red-coloured Ni(II)-DMG IIP was then homogenised using a pestle and mortar to obtain a fine powder. The powder was sieved through a 45 µm mesh before it was subsequently washed 3 times with 50% HCl and then 3 times with ultrapure water. The leached Ni(II)-DMG IIP was then dried at 55ºC for 12 h. The NIP was prepared by the same procedure except that NiSO4·6H2O was omitted.
Sample collection and preparation
Sea, river, untreated sewage and treated sewage water samples were collected in polyethylene containers by a grab sampling method. 100.0 mℓ portions of water samples doused with 2.0 mℓ conc. HNO3 and 5.0 mℓ conc. HCl were digested by heating on a hot plate at 90ºC until the volume was reduced to 20.0 mℓ. The solution was then filtered through Whatman No. 1 filter paper, after allowing it to cool, and diluted to 100.0 mℓ with ultrapure water prior to ICP-OES analysis (Method 3005A, 1992).
Optimisation of pH
The concentration of hydrogen ions is very important in IIPs because, as mentioned earlier, the selectivity of IIPs is based on size and charge. Therefore, high concentrations of the hydrogen ions interfere with the rebinding of the metal ion (Kempe and Kempe, 2010) to the cavity, as the hydrogen ions also bind with the cavity, and a low concentration of hydrogen ions means there are a lot of hydroxides, which usually form precipitates with metals even under slightly acidic conditions (Athikomrattanakul et al., 2009). Hence it is very important to optimise pH in order to have maximum rebinding of metal ions on the cavity. A set of 3 replicates of 30.0 mℓ portions of 10 µg·mℓ-1 Ni(II) solutions were prepared and their pH adjusted from 1.0 to 12.0. From pH 1 to 3, the hydrochloric acid/potassium chloride buffer was used, for pH 4 to 6, sodium acetate buffer was used, and for pH 7 to 12, the disodium hydrogen phosphate buffer was used. Then 40.0 mg of Ni(II)-DMG IIP was added into each solution and stirred for 5 min.
Optimisation of mass
Sorbents or imprinted polymers have loading capacities (Genhua et al., 2010) - the concentration of metal ions per mass of sorbent. Therefore, it is important to know the mass of sorbent needed per volume of sample, in order to avoid using too much of sorbent or wasting sorbent when a low mass can be used. However, adding a low mass of sorbent will compromise the results obtained. For mass optimisation, 10.0 to 100.0 mg of leached Ni(II)-DMG IIP were weighed into each solution and stirred for 30 min.
Optimisation of time
Time is important in rebinding studies as sufficient time should be allowed for the metal ion to rebind to the sites. This is mainly because the binding sites are sometimes not on the surface; hence time is required for metal ions to move towards binding sites (Piletsky et al., 2010). To optimise for time needed for Ni(II) rebinding, equal volumes of Ni solutions (30.0 mℓ) were used and adjusted to pH 8.0. Times for rebinding of Ni(II), ranging from 15 s to 1 800 s, were evaluated.
Recyclability
To test for reusability of the IIP, 3 replicates of 30.0 mℓ portions containing 10 µg·mℓ-1 Ni(II) were taken, buffered with disodium hydrogen phosphate and their pH adjusted to 8.0. Then 50.0 mg of a leached Ni(II)-DMG IIP was weighed into each solution and stirred for 5 min. The Ni(II)-DMG IIP was evaluated for reuse 6 times.
In all of the experiments, the amount of Ni(II) absorbed by Ni(II)-DMG IIP was desorbed with 50% HCl and the concentration of Ni(II) quantified by ICP-OES. This value was in all cases not significantly different from the one obtained by taking the reduction in concentration of the Ni(II) solution as the amount trapped by the Ni(II)-DMG IIP. The percentage extraction efficiency was then calculated as shown in Eq. (1):

where:
Ci is the initial solution concentration (µg·mℓ-1) and
Cs is the solution concentration after adsorption.
Selectivity and interference studies
For selectivity, 50.0 mg of leached Ni(II)-DMG IIP or NIP material was placed into 30.0 mℓ portions of 10 µg·mℓ-1 solutions of Ni(II), Mg(II), Ca(II), Na(I), K(I), Co(II), Cu(II), Zn(II), Fe(II) and Pd(II), which were then stirred for 5 min. The Ni(II)-DMG IIP was filtered off and the change in metal concentration was taken as the amount trapped by the Ni(II)-DMG IIP or NIP. The recoveries of each metal were calculated after quantification with ICP-OES. For interference, 50.0 mg Ni(II)-DMG IIP was placed into 30.0 mℓ aliquots of each of 10 µg·mℓ-1 Ni(II), Mg(II), Ca(II), Na(I), K(I), Co(II), Pd(II), Cu(II), Zn(II) and Fe(II), which were then stirred for 5 min. The concentration of Ni(II) was kept at 10 µg·mℓ-1, while the concentrations of Mg(II), Ca(II), Na(I), K(I), Co(II), Pd(II), Cu(II), Zn(II) and Fe(II) were varied from 10 to 20 µg·mℓ-1 in successive experiments. A mixture containing 10 µg·mℓ-1 of Mg(II), Ca(II), Na(I) and K(I) was also prepared and the interference on Ni(II) recovery at 10 µg·mℓ-1 and 20 µg·mℓ-1of these metal ions was evaluated. Lastly, a solution containing all of the above elements was prepared at 10 µg·mℓ-1and at 20 µg·mℓ-1and the interference of the elements in this solution with recovery of Ni(II) was tested at these levels. The effect of Co(II) and Cu(II) on the recovery of Ni(II) and the interaction between the two were evaluated using the scheme outlined by Montgomery et al. (2005).
Results and discussion
Characterisation of the morphology of the polymers
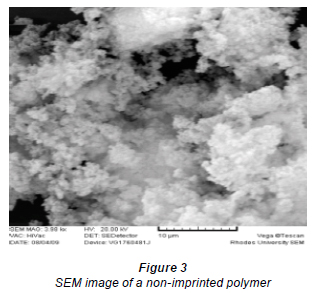
Figure 1 shows the SEM images of the Ni(II)-dimethylglyoxime complex, Fig. 2 is Ni(II)-DMG IIP, Fig. 3 is NIP and Fig. 4 is leached DMG IIP. The morphology of the Ni(II)-DMG complex was fibrous while that of Ni(II)-DMG IIP had more agglomerates; the NIP was spongier and the leached Ni(II)-DMG IIP had a more porous morphology. Literature has shown that IIPs prepared by bulk polymerisation are large-sized swollen irregular agglomerates, whereas the NIP consists of agglomerates and also spherical monodisperse particles, whilst the leached IIP is expected to have some cracks and pores on the surface (Romaní et al., 2008; Romaní et al., 2009a Uguzdogan et al., 2010; Vijaya et al., 2008; Tokalioglu et al., 2009; Arani et al., 2008; Esen et al., 2009; Daniel et al., 2005). In this case, the spongier morphology of the NIP shows that it is 'relaxed' and has the ability to absorb Ni(II) ions and become 'stiff' like Ni(II)-DMG IIP. The morphology of the Ni(II)-DMG complex is expected to show needle-like structures with a diameter of about 200 nm and a length of up to several micrometers (Ni et al., 2006; Kordatos et al., 2009), which is what was observed.



IR studies
The IR spectrum of Ni(II)-DMG IIP in Fig. 5C indicates that Ni(II) is bound through the nitrogen atoms due to the change in (C═N) from around 1 636 cm-1 and 1 638 cm-1, respectively, in the free DMG (as shown in Fig. 5A) and NIP (as shown in Fig. 5B) to 1 628 cm-1 in the Ni(II)-DMG IIP (as shown in Fig. 5C). The IR spectrum of the Ni(II)-DMG IIP (as shown in Fig. 5C) also shows the presence of an OH deformation band at 1 737 cm-1, indicating the formation of a hydrogen bridge (Burger et al., 1965; Cardoso et al., 2009; Nyquist et al., 2001; Panja et al., 1991). This hydrogen bridge confirms the formation of the red square planar Ni(II)-DMG IIP complex and is absent in the DMG and in the NIP (as shown in Figs. 5A and 5B).

Optimisation of pH
At pH 0 to 4.0 the recoveries ranged from 13 to 24%; good recoveries (72-89%) were recorded in the pH range of 6.0 to 10.0, and beyond pH 10.0 the recovery dropped (Fig. 6). The pH range determined for optimal rebinding of Ni(II) is in agreement with that reported in literature (Otero et al. 2009; Romaní et al., 2009a; Romaní et al., 2008; Romaní et al., 2009b; Praveen et al., 2005; Uguzdogan et al., 2010; Saraji and Yousefi, 2009; Ersoz et al., 2004; Chen et al., 2009; Gazda et al., 2004; Panja et al., 1991). Ni(II) is better adsorbed by DMG at higher pH values. This is because it is the conjugate base of DMG which complexes the metal ions. At lower pH values, DMG is protonated and cannot bind effectively with metals. Its binding capacity is enhanced when it is deprotonated at higher pH values.

Optimisation of mass
50.0 mg of leached ion-imprinted polymer was found to be the optimal mass of the Ni(II)-DMG IIP needed to achieve the maximum recovery of 98% (as shown in Fig. 7), and therefore 50.0 mg was used in this study, making up a polymer-to-solution ratio of 5:3. The Ni(II)-DMG IIP performed exceptionally well, compared to some other IIPs, as it used lower quantities of polymer per volume of sample (Romaní et al., 2008; Romaní et al., 2009a; Ersoz et al., 2004; Singh and Mishra, 2010), taking into consideration the higher concentrations of samples that were used in this study. The concentration (µg·mℓ-1) of a metal ion bound to the IIP, referred to as binding capacity, Q, is calculated as shown in Eq. (2):

where:
V is the volume of the solution (mℓ)
Ci is the initial solution concentration (µg·mℓ-1)
Cs is the solution concentration after adsorption
W is the mass(g) of the polymer used for extraction.

The exact value for the Ni(II)-DMG IIP was found to be 120 µg·g-1.
Reusability and kinetics of the ion-imprinted polymer
Although some IIPs have been reported to be reusable (Otero et al., 2009; Romaní et al., 2009a; Rao et al., 2004; Daniel et al., 2003), the percentage recovery of Ni(II)-DMG IIP dropped from 98% to 44% upon the sixth usage (as shown in Fig. 8). This is, however, not a major limitation as the solid-phase extraction sorbents, which are the ultimate goal of synthesising this IIP, are used only once. A maximum of 1 min was the time needed to rebind all the nickel ions onto imprinted sites of the Ni(II)-DMG IIP. Considering the time taken by other polymers, i.e. 2 min (Otero et al., 2009), 5 min (Praveen et al., 2005; Jiang et al., 2006) and 20 min (Uguzdogan et al., 2010), this Ni(II)-DMG IIP showed good kinetics of rebinding.

Selectivity and interference studies
The recovery of Ni(II) was much higher than that of Co(II), Cu(II), Zn(II), Pd(II), Ca(II), Mg(II), Na(I) and K(I), demonstrating that the cavities in the Ni(II)-DMG IIP had higher affinity for Ni(II), as shown in Table 1. The %EE was calculated using Eq. (1). Despite the fact that Fe(II), Co(II) and Cu(II) (Zen and Lee, 1993; Ershova and Ivanov, 2000; De Sousa and Korn, 2001) are known to interfere with the complexation of nickel with DMG, these metals did not show any interference with the extraction efficiency of Ni(II) when the Ni(II)-DMG IIP was used. Although DMG is selective for both Pd(II) and Ni(II), it seems that in this case Ni(II)-specific cavities were created. This was evidenced by the lower extraction efficiencies recorded for Co(II), Cu(II), Zn(II) and Pd(II), which have about the same size and charge as Ni(II). Fe(II) had a reasonably high recovery but did not interfere with the determination of Ni(II).
The selectivity of the Ni(II)-DMG IIP for Ni(II) in this study was much better than that previously reported for some polymers (Romaní et al., 2009a; Praveen et al., 2005; Romaní et al., 2009b). Zn(II), Pd(II), Fe(II), Ca(II), Mg(II), Na(I) and K(I) in the water samples did not interfere with the binding of Ni(II) on the IIP, as shown in Table 2. To compute the main effect of Cu(II) on Ni(II) for the 2 factorial design experiments, the average response in all runs with Cu(II) at the high setting was computed and the average response of all runs with Cu(II) at the low setting were subtracted (Montgomery, 2005). The same procedure was followed to evaluate for the main effect of Co(II). Cu(II) and Co(II) interfered with the rebinding of Ni(II); the results of interaction studies performed with these ions are shown in Table 2 (the last 4 entrants). Co(II) interfered slightly with the determination of Ni(II) and the interference of Cu(II) on Ni(II) was low. According to Montgomery (2005), for an effect to be taken as significant, it has to be greater than 5 - the effect of Co(II) on Ni(II) was less than 5 (at -3.5). Most Ni(II) ion imprinting work does not have these data, which can help to evaluate the effect of Co(II) on rebinding of Ni(II), as the 2 ions compete severely (Griffing et al., 1947; Yang and Black, 1994; Kumbasar and Sahin, 2008).

Limit of detection and limit of quantification.
The evaluated limit of detection (LOD) and limit of quantification (LOQ) were rather low, 3 x 10-4 and 9 x 10-4 µg·mℓ-1, respectively, and very low compared to some previously reported values, as shown in Table 3. Those with both lower LOD and LOQ than the Ni(II)-DMG IIP had lower selectivity for Ni(II) or higher interference from closely-related ions or high %RSD values (Romaní et al., 2009a; Praveen et al., 2005; Romaní et al., 2009b).

Analysis of CRM
The developed method was validated by analysing a custom solution of CRM of water (SEP-3), as shown in Tables 4 and 5. The results showed that the value obtained from the method is within the error of the CRM. The %RSD was found to be 4.29. Accuracy of the determinations, expressed as relative error between the certified and observed values of the reference material, were <0.2%. The precision of these measurements, expressed as RSD for 5 independent determinations, was also satisfactory, being lower than 3% in all cases. The LOD of the Ni(II)-DMG IIP was found to be 0.0003 ± 0.0001 mg·ℓ-1 while the LOQ was 0.0009 mg·ℓ-1. The recoveries of the spiked CRM samples were very good (99-100%).
Analysis of real samples
The Ni(II)-DMG IIP was then used to determine the concentration of Ni(II) in water samples (Table 6). EFs were calculated as shown in Eq. 3:

where:
c is the Ni(II) conc. value obtained when using Ni(II)-DMG IIP (µg·mℓ-1) and
b is the Ni(II) conc. value obtained without digestion (µg·mℓ-1).
The complexity of the water sample was estimated from the ratio of concentrations obtained with and without digestion. The recoveries were very good given the complexity of the matrices, because the ratios of the Ni(II) concentration values in the sample obtained with digestion to the values of Ni(II) obtained without digestion was low. In all of the water samples the concentrations of Cu(II) and Zn(II) were higher than that of Ni(II) (as shown in Table 7), but they did not interfere with the rebinding of Ni(II) on the Ni(II)-DMG IIP. Major elements found in water, Ca(II), Mg(II), Na(I) and K(I), also did not affect the recoveries of Ni(II) using the IIP. The enrichment factor was found to range from 2 to 18. This shows that the 18-fold improvement in recoveries was achieved when using the Ni(II)-DMG IIP, a considerable improvement in terms of access to the Ni(II) by Ni(II)-DMG IIP.
Conclusions
The synthesised Ni(II)-DMG IIP was prepared for the selective determination of Ni(II) from other closely-related metal ions in complex aqueous matrices. High Ni(II) recoveries with good %RSD were obtained in the presence of closely-related cations such as Co(II), Cu(II), Zn(II), Pd(II) and Fe(II). Low LOQ and LOD, short contact time required for rebinding of the Ni(II), short time period required for preparation of the IIP, lower volume/mass of reagents used to prepare the IIP, simplicity of the synthesis of the IIP, good enrichment factors, very low interference from closely-related metal ions and small polymer mass to solution volume required for optimal uptake of Ni(II), are some of the positive characteristics that this polymer offered. The Ni(II)-DMG IIP was successfully used to trap Ni(II) ions from sea, river and sewage water, and the results were validated by analysing a CRM. Based on the results presented in this work, the Ni(II)-DMG IIP offers a good opportunity to be used as sorbent in solid-phase extraction of aqueous samples.
Acknowledgements
The authors wish to thank the Strengthening Capacity in Agricultural Research and Development in Africa (SCARDA) programme and the National Research Foundation (NRF) of South Africa, for financial support, the Department of Agricultural Research (Botswana), for granting study leave to Modise Rammika, and Prof Catherine Ngila for valuable discussions.
References
ALI A, YE Y, XU G, YIN X and ZHANG T (1999) Determination of nickel after online sorbent preconcentration by FI-FAAS using dimethylglyoxime as a complexing agent. Microchem. J. 63(3)365-373. [ Links ]
ARANI SS, AHMADI SJ, SAMANI AB and MARAGHEH MG (2008) Synthesis of nano-pore samarium (III)-imprinted polymer for preconcentrative separation of samarium ions from other lanthanide ions via solid phase extraction. Anal. Chim. Acta 623(1)82-88. [ Links ]
ATHIKOMRATTANAKUL U, KATTERLE M, EICHELMANN NG and SCHELLER FW (2009) Development of molecularly imprinted polymers for the binding of nitrofurantoin. Biosens. Bioelectro. 25(1)82-87. [ Links ]
BALCERZAK M (2002) Sample digestion methods for the determination of traces of precious metals by spectrometric techniques. Anal. Sci. 18(7)737-750. [ Links ]
BHASKARA SPVR and REDDY BR (2002) Liquid-liquid extraction of nickel at macro-level concentration from sulphate/chloride solutions using phosphoric acid based extractants. Miner. Eng. 15(6)461-464. [ Links ]
BURGER K, RUFF I and RUFF F (1965) Some theoretical and practical problems in the use of organic reagents in chemical analysis-IV: Infra-red and ultra-violet spectrophotometric study of the dimethylglyoxime complexes of transition metals. J. Inorg. Nucl. Chem. 27(1)179-190. [ Links ]
CARDOSO WS, DIAS VLN, COSTA WM, RODRIGUES IA, MARQUES EP, SOUSA AG, BOAVENTURA J, BERERRA CWB, SONG C, LIU H, ZHANG J and MARQUES ALB (2009) Nickel-dimethylglyoxime complex modified graphite and carbon paste electrodes: preparation and catalytic activity towards methanol/ethanol oxidation. J. Appl. Electrochem. 39(1)55-64. [ Links ]
CHANG FC, LO SL and KO CH (2007) Recovery of copper and chelating agents from sludge extracting solutions. Sep. Purif. Technol. 53(1)49-56. [ Links ]
CHEN AH, YANG CY, CHEN CY, CHEN CY and CHEN CW (2009) The chemically crosslinked metal-complexed chitosans for comparative adsorptions of Cu(II), Zn(II), Ni(II) and Pb(II) ions in aqueous medium. J. Hazard Mater. 163(2-3)1068-1075. [ Links ]
DANIEL S, GLADIS JM and RAO TP (2003) Synthesis of imprinted polymer material with palladium ion nanopores and its analytical application. Anal. Chim. Acta 488(2)173-182. [ Links ]
DANIEL S, PRABHAKARA RAO P and PRASADA RAO T (2005) Investigation of different polymerization methods on the analytical performance of palladium(II) ion imprinted polymer materials. Anal. Chim. Acta 536(1-2)197-206. [ Links ]
DE SOUSA CSD and KORN M (2001) Effects of ultrasonic irradiation on the spectrophotometric determination of nickel with dimethylglyoxime. Anal. Chim. Acta 444(2)309-315. [ Links ]
DHAKATE R and SINGH VS (2008) Heavy metal contamination in groundwater due to mining activities in Sukinda valley, Orissa - A case study. J. Geog. Reg. Plan. 1(4)058-067. [ Links ]
DHERI GS, BRAR MS and MALHI SS (2007) Heavy-metal concentration of sewage-contaminated water and its impact on underground water, soil, and crop plants in alluvial soils of northwestern India. Commun. Soil Sci. Plant Anal. 38(9-10)1353-1370. [ Links ]
ERSHOVA NI and IVANOV VM (2000) Improvement of direct determination of trace nickel in environmental samples by diffuse reflection spectroscopy using chromaticity characteristics. J. Anal. Chem. 367(2)210-211. [ Links ]
ERSOZ A, SAY R and DENIZLI A (2004) Ni(II) ion-imprinted solid-phase extraction and preconcentration in aqueous solutions by packed-bed columns. Anal. Chim. Acta 502(1)91-97. [ Links ]
ESEN C, ANDAC M, BERELI N, SAY R, HENDEN E and DENZIL A (2009) Highly selective ion-imprinted particles for solid-phase extraction of Pb2+ ions. Mater. Sci. Eng. C 29 (8) 2464-2470. [ Links ]
GAZDA DB, FRITZ JS and PORTER MD (2004) Determination of nickel(II) as the nickel dimethylglyoxime complex using colorimetric solid phase extraction. Anal. Chim. Acta 508(1)53-59. [ Links ]
GENHUA W, GUOCHENG S, DAYU W, YOYUNG S, ZHUQING W and CHIYANG H (2010) Synthesis of ion-imprinted mesoporous silica gel sorbent for selective adsorption of copper ions in aqueous media. Microchim. Acta 171(1-2)203-209. [ Links ]
GRIFFING M, DE VRIES T and MELLON MG (1947) Spectrographic examination of organic precipitates. Anal. Chem. 19(9)654-655. [ Links ]
HENNION MC (1999) Solid-phase extraction: Method development, sorbents and coupling with liquid chromatography. J. Chromatogr. A 856(1-2)3-54. [ Links ]
HLAVAY J, PROHASKA T, WEISZ M, WENZEL WW and STINGEDER GJ (2004) Determination of trace elements bound to soil and sediment fractions. Pure Appl. Chem. 76(2)415-442. [ Links ]
HONG JJ, YANG SM, LEE CH, CHOI YK and KAJIUCHI T (1998) Ultrafiltration of divalent metal cations from aqueous solution using polycarboxylic acid type biosurfactant. J. Colloid Interface Sci. 202(1)63-73. [ Links ]
JAMES D, VENKATESWARAN G and RAO TP (2009) Removal of uranium from mining industry feed simulant solutions using trapped amidoxime functionality within a mesoporous imprinted polymer material. Microporous Mesoporous Mater. 119(1-3)165-170. [ Links ]
JIANG N, CHANG X, ZHENG H, HEA Q and HU Z (2006) Selective solid-phase extraction of nickel(II) using a surface-imprinted silica gel sorbent. Anal. Chim. Acta 577(2)225-231. [ Links ]
KEMPE H and KEMPE M (2010) Influence of salt ions on binding to molecularly imprinted polymers. Anal. Bioanal. Chem. 396 (4) 1599-1606. [ Links ]
KHAN S, CAO Q, ZHENG YM, HAUNG YZ and ZHU YG (2008) Health risks of heavy metals in contaminated soils and food crops irrigated with wastewater in Beijing. China Environ. Poll. 152(3)686-692. [ Links ]
KORDATOS K, VLASOPOULOS AD, STRIKOS S, NTZIOUNI A, GAVELA S, TRASOBARES S and RIGOPOULOU VK (2009) Synthesis of carbon nanotubes by pyrolysis of solid Ni(dmg)2. Electrochim. Acta 54(9)2466-2472. [ Links ]
KOROLCZUK M (2000) Voltammetric method for direct determination of nickel in natural waters in the presence of surfactants. Talanta 53(3)679-686. [ Links ]
KRISHNA PG, GLADIS JM, RAO TP and NAIDU GR (2004) Selective recognition of neodymium (III) using ion imprinted polymer particles. J Mol. Recognit. 18(1)109-116. [ Links ]
KUMBASAR RA and SAHIN I (2008) Separation and concentration of cobalt from ammoniacal solutions containing cobalt and nickel by emulsion liquid membranes using 5,7-dibromo-8-hydroxyquinoline (DBHQ). J. Membr. Sci. 325(2)712-718. [ Links ]
LI Q, SU H, LI J and TAN T (2005) Application of surface molecular imprinting adsorbent in expanded bed for the adsorption of Ni2+ and adsorption model. J. Environ. Manage. 85(4)900-907. [ Links ]
LI Z and ZHOU L (2006) Interferences removal for cadmium determination in samples with complex matrices by hydride generation coupled with non-dispersive atomic fluorescence spectrometry. Anal. Sci. 22 (1) 123-129. [ Links ]
LIMA EC, BARBOSA F, KRUG FJ, SILVA MM and VALE MGR (2000) Comparison of ultrasound-assisted extraction, slurry sampling and microwave-assisted digestion for cadmium, copper and lead determination in biological and sediment samples by electrothermal atomic absorption spectrometry. J. Anal. At. Spectrom. 15(8)995-1000. [ Links ]
MANSOUR SA and SIDKY MM (2002) Ecotoxicological Studies. 3. Heavy metals contaminating water and fish from Fayoum Governorate, Egypt. Food Chem. 78(1)15-22. [ Links ]
MASSOUMI A and HEDRICK CE (1969) Solvent extraction of metal ions and surface active agents from saturated sodium chloride brine. J. Chem. Eng. 14(1)52-54. [ Links ]
METHOD 3005A (1992) Acid digestion of waters for total recoverable or dissolved metals for analysis by FLAA or ICP spectroscopy, Rev. 1. EPA, Woodrow Wilson Plaza, USA. [ Links ]
METILDA P, PRASAD K, KALA R, GLADIS JM, RAO TP and NAIDU GRK (2007) Ion imprinted polymer based sensor for monitoring toxic uranium in environmental samples. Anal. Chim. Acta 582(1)147-153. [ Links ]
MILLER JR, HUDSON-EDWARDS KA, LECHLER PJ, PRESTON D and MACKLIN MG (2004) Heavy metal contamination of water, soil and produce within riverine communities of the Río Pilcomayo basin, Bolivia. Sci. Total Environ. 320(2-3)189-209. [ Links ]
MOMODU MA and ANYAKORA CA (2010) Heavy metal contamination of ground water: The Surulere case study. Res. J. Environ. Earth Sci. 2(1)39-43. [ Links ]
MONTGOMERY DC (2005) Design and Analysis of Experiments (6th edn.). John Wiley and Sons, New York, USA. [ Links ]
NAMIEŚNIK J (2002) Trace analysis - Challenges and problems. Crit. Rev. Anal. Chem. 32(4)271-300. [ Links ]
NI X, ZHAO Q, ZHOU F, ZHENG H, CHENG J and LI B (2006) Synthesis and characterization of NiO strips from a single source. J. Cryst. Growth 289(1)299-302. [ Links ]
NISHIDE H, DEGUCHI J and TSUCHIDA E (1976) Selective adsorption of metal ions on crosslinked poly(vinylpyridine) resin prepared with a metal ion as a template. Chem. Lett. 5(2)169-174. [ Links ]
NYQUIST RA (2001) Interpreting Infrared, Raman and Nuclear Magnetic Resonance Spectroscopy, Vol. 1. Academic Press, Florida, USA. [ Links ]
OTERO NG, VALIÑO CT, ROMANÍ JO, VÁZQUEZ EP, PIÑEIRO AM and BARRERA PB (2009) On-line ionic imprinted polymer selective solid-phase extraction of nickel and lead from seawater and their determination by inductively coupled plasma-optical emission spectrometry. Anal. Bioanal. Chem. 395(4)1107-1115. [ Links ]
PANJA PK, BALA S, PAL C AND GOSH PN (1991) Infrared spectroscopic studies of dimethylglyoxime chelates of nickel, cobalt, copper, palladium and platinum. J. Mol. Struct. 249(2-4)277-283. [ Links ]
PILETSKY SA, MATUSCHEWSKI H, SCHEDLER U, WILPERT A, PILETSKA EV, THIELE TA and ULBRICHT M (2000) Surface functionalization of porous polypropylene membranes with molecularly imprinted polymers by photo graft copolymerization in water. Macromolecules 33(8)3092-3098. [ Links ]
POURREZA N, ZOLGHARNEIN J, KIASAT AR and DASTYAR T (2010) Silica gel-polyethylene glycol as a new adsorbent for solid phase extraction of cobalt and nickel and determination by flame atomic absorption spectrometry. Talanta 81(3)773-777. [ Links ]
PRAVEEN RS, DANIEL S and RAO TP (2005) Solid phase extraction preconcentration of cobalt and nickel with 5,7-dichloroquinone-8-ol embedded styrene-ethylene glycol dimethacrylate polymer particles and determination by flame atomic absorption spectrometry (FAAS). Talanta 66 (2) 513-520. [ Links ]
PRECHTHAI T, PARKPIAN P and VISVANATHAN C (2008) A spectral algorithm for solving the relativistic Vlasov-Maxwell equations. J Hazard Mater. 156(1)86-94. [ Links ]
QURESHI A, SINGH NL, SHAH S, KULRIYA P, SINGH F and AVASTHI DK (2008) Modification of polymer composite films using 120 MeV Ni10+ ions. Nucl. Instrum. Methods Phys. Res. 266(8)1775-1779. [ Links ]
RAMAKRISHNAN K and RAO TP (2006) Ion imprinted polymer solid phase extraction (IIP-SPE) for preconcentrative separation of erbium(III) from adjacent lanthanides and yttrium. Sep. Sci. Technol. 41(2)233-246. [ Links ]
RAO TP, DANIEL S and GLADIS JM (2004) Tailored materials for preconcentration or separation of metals by ion-imprinted polymers for solid-phase extraction (IIP-SPE). TrAC Trends Anal. Chem. 23(1)28-35. [ Links ]
RECHCIGL JE and PAYNE GG (1989) Poster presented at 1989 annual meetings of American Society of Agronomy, Crop Science Society of America and Soil Society of America, Las Vegas, Nevada, 1989. [ Links ]
RICO MA C, HERNÁNDEZ LM and GONZÁLEZ MA J (1989) Water contamination by heavy metals (Hg, Cd, Pb, Cu and Zn) in Doñana National Park (Spain). Bull. Environ. Contam. Toxicol. 42(42)582-588. [ Links ]
ROMANÍ JO, PI˜NEIRO AM, BARRERA PB and ESTEBAN AM (2009a) Inductively coupled plasma-optical emission spectrometry/mass spectrometry for the determination of Cu, Ni, Pb and Zn in seawater after ionic imprinted polymer based solid phase extraction. Talanta 79(3)723-729.
ROMANÍ JO, PI˜NEIRO AM, BARRERA PB and ESTEBAN AM (2009b) Ionic imprinted polymer for nickel recognition by using the bi-functionalized 5-vinyl-8-hydroxyquinoline as a monomer: Application as a new solid phase extraction support. Microchem. J. 93(2)225-231.
ROMANÍ JO, PI˜NEIRO AM, BARRERA PB and ESTEBAN AM (2008) Synthesis, characterization and evaluation of ionic-imprinted polymers for solid-phase extraction of nickel from seawater. Anal. Chim. Acta 630(1)1-9.
RUOTOLO LAM and GUBULIN JC (2002) Electrodeposition of copper ions on fixed bed electrodes: Kinetic and hydrodynamic study. Braz. J. Chem. Eng. 19(1)105-118. [ Links ]
SARAJI M and YOUSEFI H (2009) Selective solid-phase extraction of Ni(II) by an ion-imprinted polymer from water samples. J. Hazard Mater. 167(1-3)1152-1157. [ Links ]
SARIN RK, SRIVASTAVA S, SRIVASTAVA AK, ANIL G and REDDY MRP (2004) Multielement determination in gum opium by microwave digestion and inductively coupled plasma optical emission spectroscopy. Chem. Pap. 58(2)101-103. [ Links ]
SHAO X, HU S and GOVIND R (1991) Continuous membrane dialysis using ion-exchange resin suspension for extracting metal ions. Ind. Eng. Chem. Res. 30 (6) 1231-1239. [ Links ]
SINGH A, SHARMA RK, AGRAWAI M and MARSHALL FM (2010) Health risk assessment of heavy metals via dietary intake of foodstuffs from the wastewater irrigated site of a dry tropical area of India. Food Chem. Toxicol. 48(2)611-619. [ Links ]
SINGH DK and MISHRA S (2010) Synthesis, characterization and analytical applications of Ni(II)-ion imprinted polymer. Appl. Surf. Sci. 256(24)7632-7637. [ Links ]
SU H, LI J and TAN T (2007) Adsorption mechanism for imprinted ion (Ni2+) of the surface molecular imprinting adsorbent (SMIA). Biochem. Eng. J. 39(3)503-509. [ Links ]
TAN CH and HUANG XG (2009) Trace lead measurement and online removal of matrix interference in geosamples by ion-exchange coupled with flow injection and hydride generation atomic fluorescence spectrometry. J Autom. Methods Manage. Chem. 2009, Article ID 101679, doi:10.1155/2009/101679. [ Links ]
TOKALIOĞLU S, YILMAZ V, KARTAL S, DELIBAŞ A and SOYKAN C (2009) Synthesis of a novel chelating resin and its use for selective separation and preconcentration of some trace metals in water samples. J Hazard Mater. 169(1-3)593-598. [ Links ]
UBALUA AO, CHIJIOKE UC and EZERONYE OU (2007) Determination and assessment of heavy metal content in fish and shellfish in Aba river, Abia State, Nigeria. KMITL Sci. Tech. J. 7(1)16-23. [ Links ]
UGUZDOGAN E, DENKBAS EB and KABASAKAL OS (2010) The use of polyethyleneglycolmethacrylate-co-vinylimidazole (PEGMA-co-VI) microspheres for the removal of nickel(II) and chromium(VI) ions. J. Hazard Mater. 177(1-3)119-125. [ Links ]
VAN DEN BERG CMG and NIMMO M (1987) Determination of interactions of nickel with dissolved organic material in seawater using cathodic stripping voltammetry. Sci. Total Environ. 60 185-195. [ Links ]
VIJAYA Y, POPURI SR, BODDU VM and KRISHNAIAH A (2008) Modified chitosan and calcium alginate biopolymer sorbents for removal of nickel (II) through adsorption. Carbohydr. Polym. 72(2)261-271. [ Links ]
WANG X, SATO T, XING B and TAO S (2005) Health risks of heavy metals to the general public in Tianjin, China via consumption of vegetables and fish. Sci. Total Environ. 350(1-3)28-37. [ Links ]
WILLARD H (1943) Advanced Quantitative Analysis. D. Van Nostrand Company, USA, [ Links ].
WILSON K, YANG H, SEO CW and MARSHALL WE (2006) Select metal adsorption by activated carbon made from peanut shells. Bioresour. Technol. 97(18)2266-2270. [ Links ]
WINKLER P, SCHULZ M and DANNECKER W (1991) Problems with the determination of heavy metals in precipitation. Fresenius J. Anal. Chem. 340(9)575-579. [ Links ]
YANG J and BLACK J (1994) Competitive binding of chromium, cobalt and nickel to serum proteins. Biomaterials 15 (4) 262-268. [ Links ]
YAO FX, MACIAS F, VIRGEL S, BIANCO F, JIANG X and ARBESTAIN MC (2009) Chemical changes in heavy metals in the leachates from technosols. Chemosphere 77(1)29-35. [ Links ]
ZEN JM and LEE ML (1993) Determination of traces of nickel(II) at a perfluorinated ionomer/dimethylglyoxime mercury film electrode. Anal. Chem. 65(22)3238-3243. [ Links ]
ZHENG GD, GAO D, CHEN TB and LU W (2007) Stabilization of nickel and chromium in sewage sludge during aerobic composting. J. Hazard Mater. 142(1-2)216-221. [ Links ]
ZHU X, CUI Y, ZOU X and LI Z (2009) Selective solid-phase extraction of lead(II) from biological and natural water samples using surface-grafted lead(II)-imprinted polymers. Microchim. Acta 164(1-2)125-132. [ Links ]
Received 18 November 2010; accepted in revised form 30 May 2011.
* To whom all correspondence should be addressed. +267 71414353 ; fax: +267 3928965; e-mail: modiserammika@yahoo.co.uk














