Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
Water SA
versão On-line ISSN 1816-7950
versão impressa ISSN 0378-4738
Water SA vol.37 no.3 Pretoria Jul. 2011
Biosorptive removal of Pb2+, Cd2+ and Zn2+ ions from water by Lagenaria vulgaris shell
Dragana-Linda Mitic-StojanovicI; Aleksandra ZarubicaII; Milovan PurenovicII; Danijela BojicII; Tatjana AndjelkovicII; Aleksandar Lj. BojicII,*
IPublic waterworks enterprise 'VODOVOD', Vranje, Serbia
IIDepartment of Chemistry, Faculty of Science and Mathematics, University of Nis, 33 Višegradska Street Serbia
ABSTRACT
Lagenaria vulgaris (LV) shell was used as a biosorbent for the removal of heavy metal ions, Pb2+, Cd2+ and Zn2+, from aqueous solutions. Experiments were carried out under batch conditions. The effects of contact time, initial pH, temperature and stirring speed on removal efficiency are presented. Sorption of the investigated metals was fast, reaching equilibrium after about 5 to 10 min, depending on the metal. Biosorption was highly pH-dependent, and the optimal pH for investigated metals was in the range of 4.5 to 6.0. The effects of temperature demonstrated that biosorption of the metals is a chemical process. SEM analysis revealed interesting morphological changes after acid refinement of the raw biosorbent and metal uptake that is related to the chemical nature of the biosorption process. EDX analysis of Lagenaria vulgaris biosorbent (LVB) before and after metal sorption revealed that the ion exchange mechanism was the principal sorption process. Fourier transform infrared spectroscopy (FTIR) analysis has shown that major functional groups (carboxyl and hydroxyl) on the biosorbent surface took part in the metal ion uptake process as active sites. The results obtained showed that Lagenaria vulgaris based biosorbent could be used as an effective and low-cost pre-treatment step for removal of toxic metals from wastewaters.
Keywords: Lagenaria vulgaris, heavy metals, biosorption, contact time, pH, temperature, FTIR, SEM-EDX
Introduction
Toxic heavy metals are released into the biosphere through industrial activities and spread into the environment. Their presence in the environment can be detrimental to people, plants and animals. These inorganic micro-pollutants are non-biodegradable, highly toxic and have a carcinogenic (Cd) and suspected carcinogenic (Pb) effect (Cimino et al., 2000). They can accumulate in water, soil, plants and living tissues, thus becoming concentrated throughout the food chain.
The efficiency of heavy metal absorption by plants is affected by numerous factors such as: plant species, natural habitat of the plants, pH of the soil, organic matter content, and whether the habitat is naturally rich in toxic metals or is anthropogenically polluted. A variety of industries are responsible for the release of toxic heavy metals into the environment through their wastewaters. These include: manufacturing of paper, pesticides and batteries, iron production, and many other industries, such as metal finishing, electroplating, printed circuit and non-ferrous metal works (Friedman and Waiss, 1972; Kjellstrom et al., 1977; Forstner and Wittman, 1981; Kabata-Pendias and Pendias, 2001; Pastircakova, 2004; Celik and Demirbas, 2005). Therefore, it is desirable that their levels be considerably reduced in industrial and municipal effluents to meet regulatory standards.
Conventional methods have been used to remove metal ions from water effluents, such as: lime precipitation (Esalah et al., 2000), or oxidation/reduction process, coagulation and flotation (Zouboulis et al., 1997) and effective, but expensive, processes such as ion exchange, reverse osmosis (Dang et al., 2009), electrodialysis (Canet et al., 2002), ultra-filtration, electrolytic removal, etc. (Gardea-Torresdey et al., 1998; Zhang et al., 1998; Patterson, 1985; Inglezakis et al., 2003). However, these conventional techniques have their own inherent limitations such as lower efficiency, sensitive operating conditions, and secondary sludge production (Ahluwalia and Goyal, 2005). The most widely used method for removing heavy metals from water is precipitation as insoluble hydroxide in weak alkaline medium (Patterson, 1985). However, a major problem with this method is the disposal of the precipitated hydroxide. Another powerful technology is the adsorption of heavy metals from domestic and industrial wastewaters using activated carbon (Horikoshi et al., 1981; Hosea et al., 1986; Ravindran et al., 1999; Toles and Volesky, 2000; Marshall, 2002). However, the high cost of the activated carbon and its loss during regeneration restrict its application (Dhiraj, 2008).
For the past 15 years an alternative method for removal of toxic metal ions from wastewater has been considered, based on the sorption capacity of waste materials, both organic and inorganic (Volesky and Holan, 1995). The materials that have been investigated as biosorbents include aquatic plants such as seaweeds (Williams and Edyvean, 1997), sugarcane bagasse, mould, yeast, and other microbial and agricultural products such as wool, rice, straw, banana peel, coconut husk, hazelnut husk, tree bark, peat moss, peanut skins, tea leaves, etc. (Brauckmann, 1990; Tan et al., 1993; Volesky and Holan, 1995; Williams et al., 1998; Cimino et al., 2000; Johnson et al., 2002). These biomaterials, which are by-products or wastes from various industrial activities and agricultural waste materials, have wide application. The major advantages of biosorption over conventional treatment methods include low cost, high efficiency, minimisation of chemical or biological sludge and possibility of biosorbent regeneration (Zhao et al., 1999; Cimino et al., 2000). Agricultural waste materials, either in their natural form or after some physical or chemical modification, have all been shown to complex and sequester heavy metals (Bailey et al., 1999; Hashem et al., 2005; Dhiraj, 2008), due to their structural composition (they mainly contain cellulose, hemi-cellulose, lignin, proteins, lipids, simple sugars, hydrocarbons) and numerous functional groups (carboxyl, carbonyl, hydroxyl, phenyl, ester, acetamido, amido, amino, sulphydryl). Biosorption takes place in a heterogeneous system, which involves solid biosorbent and liquid phase (solution) with dissolved species. The mechanism of biosorption is rather complex and involves many different processes: chemisorption, complexation, ion-exchange, chelation, and physical adsorption, which are dependent on the diffusion process (Sud et al., 2008.). Depending on the biomass chemical structure and/or functional groups, some of the above mentioned mechanisms are favoured. As an example, in the case of ligno-cellulosis biosorbents, the functional groups of which are mostly presented by carboxyl, carbonyl and hydroxyl groups, predominant biosorption mechanisms are ion-exchange, complexation and chelation (Volesky and Holan, 1995; Dang et al., 2009; Iqbal et al., 2009).
This study deals with the possibility of using Lagenaria vulgaris biomass as a biosorbent for removal of toxic ions, such as Pb2+, Cd2+ and Zn2+ from aqueous solutions. The effects of contact time, pH, temperature and stirring speed were monitored to optimise the sorption process and to quantify removal efficacy for the investigated heavy metals. Scanning electron microscopy (SEM), energy dispersive X-ray analysis (EDX) and Fourier transform infrared spectroscopy (FTIR) were conducted to investigate the metals' interaction with the Lagenaria vulgaris biosorbent.
Experimental
Materials and methods
Lagenaria vulgaris (Cucurbitaceae family) is a creeping, hardy plant (Decker-Walters et al., 2004). This plant is found growing mainly on alluvial sandy soil and red loam, on flat areas and moderate slopes, and in higher-lying areas, and needs light and warmth (Okoli and Nyanayo, 1998; Horsfall and Spiff, 2005). The outer shell is hard and ligneous, covering the spongy white pith characterised by its bitter taste. The experiments in this study were carried out using the shell of Lagenaria vulgaris grown in the southern area of Serbia without irrigation and fertilisation, at an altitude of 700 m.
Biosorbent preparation
Freshly-harvested fruits were manually emptied, crushed into 2 to 3 cm pieces, washed with deionised water to remove dust and soluble impurities, and dried for 24 h in hot air at 55 ± 1ºC. The residual material was then ground in a crusher mill, dried as in the previous procedure to a constant weight and sieved to fractionise a size range from 1.00 to 1.25 mm, using successive sieving through octagon sieves (OCT-DIDGITAL 4527-01). A general description of this material is given in Table 1. The raw biomass was then refined, in order to remove all metals bio-accumulated in the plant during its growth, by soaking in an excess of 0.3 M HNO3 with stirring for 24 h. After the acid washing, the biosorbent was rinsed with deionised water and neutralised using 0.1 M NaOH solution. It was then washed thoroughly to remove all other soluble materials until the pH of the washed water became neutral. The biomaterial was centrifuged at 3 000 r·min-1 and oven-dried at 55±1ºC to a constant weight.
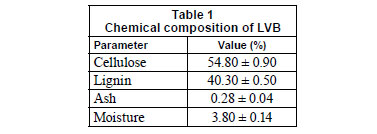
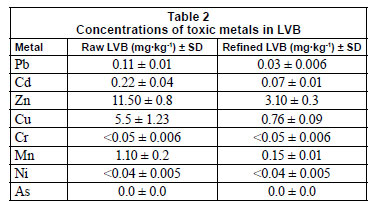
Biosorbent was analysed for heavy metals content, in its raw form and after refinement. Results are presented in Table 2. The concentrations of Pb, Cd, Zn, Cu and Mn were found to be much higher before refinement. The concentrations of Cr, Ni and As were below the detection limit in both cases. These data are in accordance with characteristics of numerous plants from the genus Lagenaria to accumulate heavy metals from soil during growth (Singh et al., 2010).
Reagents
All chemicals were of reagent grade and used without further refinement. HNO3, NaOH, and Pb, Cd and Zn beads were purchased from Merck (Germany). H2O2 was purchased from Carlo Erba (Italy). Deionised water was used for all experiments. Standard metal stock solutions were prepared by dissolving given amounts of pure metals (Pb, Cd, Zn) in HNO3 (1:3). All standard solutions were stored in a refrigerator at +4ºC. Working standard solutions were prepared just before use by the appropriate dilution of the stock solutions.
Analysis of biosorbent
The concentrations of Pb2+, Cd2+ and Zn2+ ions in samples of model solutions and biosorbent were determined using the GBC Avanta (Australia) flame atomic absorption spectrophotometer (FAAS) by standard methods (APHA, 1998). Before analysis, the prepared biomass had been acid digested (HNO3 + H2O2) in the microwave oven (MWD) (ETHOS, Milestone, Italy) (Wu et al., 1997; Araujo et al., 2002). Structural components had been determined using standard methods (Horwitz and Latimer, 2006). The pH values of solutions were monitored during treatment by a pH-meter HACH SenIon 3 (HACH, USA).
Batch biosorption experiments
All biosorption experiments were carried out under the batch conditions. Model solutions of each metal at different concentrations were obtained by dilution of the stock solutions. The initial pH of solutions was adjusted to the defined pH value, pH-metrically with HNO3 or NaOH solutions (0.1, 0.01 M), without buffering. No further adjustment of pH was done during the experiments. Experiments were conducted in 400 mℓ Erlenmeyer flasks, using 250 mℓ of the metal model solution. Flasks were stirred at 250 r·min-1 at the required temperature. Temperature was maintained at desired values using a temperature-controlled water bath. The accuracy of the temperature measurements was ± 0.5ºC. The solution was stirred for 5 min in order to ensure that it was well mixed, and the defined dose of Lagenaria vulgaris biosorbent (LVB) was added. Aliquots of solutions (5.0 mℓ) were withdrawn at preset time intervals; the biosolids were removed immediately by filtration through a 0.45 µm regenerated cellulose membrane filter (Agilent Technologies, Germany) and preserved with 0.10 mℓ of concentrated HNO3. Filtrates were analysed for residual metal concentrations using FAAS. Kinetic experiments were performed by taking samples at defined intervals up to 180 min. In order to evaluate the effect of solution pH on the biosorption process, experiments were carried out at different initial pH values, from 2.0 to 7.0, with all other parameters kept constant. Biosorption at pH above 7.0 was not carried out to avoid any possible interference of metal precipitation.
Scanning electron microscopy with combined energy dispersive x-ray analysis
The surface morphology and elemental composition of the biomass samples, before and after heavy metal loading, were investigated using a scanning electron microscope (SEM) JEOL JSM-6460LV with combined energy dispersive x-ray (EDX) analyser at a voltage of 25 keV. Biomass samples were firstly attached to 10 mm alumina-based mounts using double-sided tape, sputter-coated with gold using a 30 mA current for 180 s, at working distance 50 mm (BAL-TEC-SCD005, Balzers, Germany), under vacuum in an argon atmosphere, in order to achieve their surface conductivity. An accelerating voltage of 25 kV for primary electrons as well as working distance of 10 mm proved to be satisfactory. Spot sizes varied from 25 to 40 nm depending on the applied magnification. Speed 3, offered by software as 'fine' scan, was chosen because of the occasional problems with Speed 4, known as 'super fine' scan rate, manifested as 'floating' of images at higher magnifications. The EDX technique was used to determine the elemental composition of the biomass surface before and after metal uptake.
FTIR characterisation
The infrared spectra of the biomass samples before and after metal uptake were recorded, using a BOMEM MB-100 (Hartmann & Braun, Canada) Fourier transform infrared spectrometer operating in the range 4 000-400 cm-1. FTIR characterisation was performed in order to identify chemical functional groups present on the LVB that might be involved in the metal uptake procedure. The obtained FTIR spectra were analysed using Win Bomem Easy software.
Data presentation and analysis
In all experiments, solution samples were taken in triplicate for the measurement of the metal ion concentrations. Average values ± SD (error bars) are presented in all graphs and tables.
The standard deviation from the means was calculated using the following equation:

where:
n = the number of experimental data points
xi = the value of the individual data point
x = the average of the data points
Kinetics of process was expressed using pseudo-first-order rate model:

This gives, by integrating and rearranging the resultant linear equation:
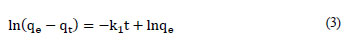
where:
k1= biosorption rate constant (min-1)
qτ , qe = amounts of metal ions adsorbed on the LVB at a given time τ and at equilibrium (mg·g-1)
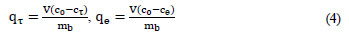
where:
V = volume of solution (ℓ)
mb = dry weight of biosorbent (g)
c0, cτ and ce = the initial concentration and residual concentrations of metal ions at the sampling time and at equilibrium (mg·ℓ-1)
The removal efficiency (RE) of metal ions by biosorbent was calculated using the equation:

Statistical analysis, calculation of the data and linear least square fitting was carried out using OriginPro 8.0 (OriginLab Corporation, USA) software.
Results and discussion
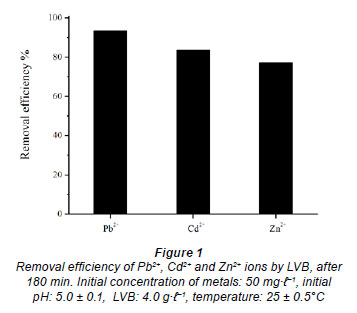
The effects of different experimental parameters on Pb2+, Cd2+ and Zn2+ ion biosorption using Lagenaria vulgaris biosorbent is described in detail below. Results of the investigated metal ions' adsorption at 25 ± 0.5ºC, with an initial metal concentration of 50.0 mg·ℓ-1, LVB at 4.0 g·ℓ-1 and initial pH 5.0, are shown in Fig. 1. Removal efficiency occurred in the following order: Pb2+ > Cd2+ > Zn2+.
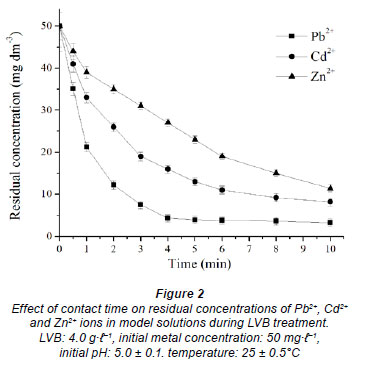
Effect of contact time
To determine the dependence of metal sorption on time, 1.0 g of LVB was exposed to a 250 mℓ single metal model solution of lead, cadmium or zinc ions with initial concentration 50.0 g·ℓ-1, at 25 ± 0.5ºC and pH 5.0, in the batch process. The residual metal concentration in aqueous solution was determined after a contact time of 0.5, 1, 2, 3, 4, 5, 6, 8, 10, 15, 20, 40, 60, 90, 120 and 180 min (Fig. 2) (Ajmal et al., 2001; Horsfall and Spiff, 2005). Metal uptake, as a function of contact time, was noticed to occur in 2 phases. The first phase was extremely rapid in the case of all investigated metals, regardless of the influence of metal nature. The maximum adsorption efficiency was observed in the first 5 min of sorbent-sorbate contact, which was followed by a slow phase of metal removal spread over a longer period until equilibrium was reached (Fig. 2). The sorption equilibrium was attained after 5, 8 and 10 min of contact time, when 92.5, 81.6 and 77.2% of total Pb2+, Cd2+ and Zn2+ ions, respectively, were removed. After 180 min of contact time, removal efficiency was slightly greater: 96.0, 88.6 and 83.0%, respectively (Fig. 1.). A constant (k1) relating to the rate of Pb2+ removal from aqueous model solution, determined from Eq. (3), has the highest value of 0.73 min-1 (r2 = 0.97). The kinetic rate constants for Cd and Zn are proportionally less, with values of k1 = 0.29 min-1 (r2 = 0.98) and k1 = 0.17 min-1 (r2 = 0.97), respectively. The results were similar to those reported in the literature (Kahraman et al., 2005; Goyal et al., 2008; Pandey et al., 2008). High initial metal concentration in the solution and the abundant availability of the active sites on the surface area at the beginning of the treatment has positive effects on the rate of the heterogeneous process. It is especially expressed in the case of LVB, because this material has a significant amount of micro-pores and/or meso-pores responsible for an efficient diffusion to micro-pores. The lower metal concentration and the gradual occupancy of the active sites results in a weaker driving force and a less efficient sorption process, the rate of which depends on ion diffusion through the pores of the biosorbent. The rate of metal sorption on LVB, i.e. removal efficiency from the water phase, depends on the nature of the metal. This relates to the ability for making complexes with functional groups on the LVB surface, as well as to the stability in aqueous system. Lead shows a higher tendency for binding to oxygen functional groups of LVB; therefore, it is the metal which is most efficiently removed from the water phase. Zinc and cadmium have similar characteristics, and the decline in concentration throughout time and the rate constants for the process provide evidence for this finding. In addition, differential sorption of the ions may be ascribed to the differences in their ionic radii. The ionic radius of Pb2+ is 119 pm, while that of Cd2+ is 95 pm, and that of Zn2+ is 74 pm. The smaller the ionic radius, the greater its tendency to hydrolyse, which further leads to reduced sorption (Wulfsberg, 1987). After 10 min of the process, metal concentrations in the solution remain unchanged, or changes are negligible within 3 h (results not shown).
Effect of initial pH
Solution pH is a significant control parameter affecting the biosorption process. Batch experiments were conducted at different initial pH values ranging from 2.0 to 7.0. Metal solutions (50 mg·ℓ-1) were brought into contact with the biosorbent (4.0 g·ℓ-1) at 25 ± 0.5ºC for 10 min. The effect of pH on the removal efficiency of Pb2+, Cd2+ and Zn2+ ions is presented in Fig. 3. The results indicate that relatively little biosorption took place at the initial pH of 2 and only 27.5% of Pb2+, 12.3% of Cd2+ and 6.5% of Zn2+ ions were removed. The increase in pH of the solutions from 2 up to the range 5-6, depending on the metal, causes a rapid increase in the removal efficiency from aqueous model solutions for the investigated metal ions. With a further increase in pH to 7, the amount of metal ion removed by LVB starts to decrease, which is in agreement with the results of similar studies (Zhao et al., 1999; Goyal et al., 2008; Panda et al., 2008; Iqbal et al., 2009). Adsorption of the investigated metals reached its maximum at the following pH values: 4.5 for Pb2+ (93.5%), 5.0 for Cd2+ (83.6%) and 6.0 for Zn2+ (77.2%), in accordance with hydrolysis constants. At a pH higher than the presented optimal values, several hydroxyl low-soluble species may be formed, such as: Pb(OH)+, Pb(OH)2 for lead; Cd(OH)2, Cd(OH)3- for cadmium and Zn(OH)+, Zn(OH)2, Zn(OH)3- (Sag et al., 1998; Liu and Liu, 2003; Lodeiro et al., 2006; Romera et al., 2007; Goyal et al., 2008) in the case of zinc. In order to avoid the precipitation of metal ions no experiments were carried out at pH above 7.0.
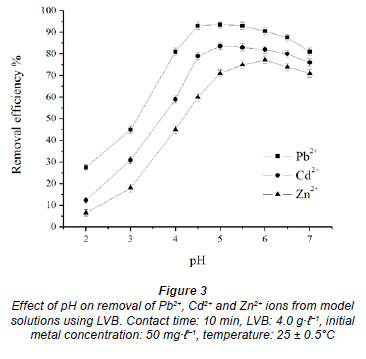
Many studies have shown that the pH of aqueous model solutions affects both the solubility of metal ions and the ionisation states of biosorbent functional groups (Cimino et al., 2000; Saygideger et al., 2005; Goyal et al., 2008; Iftikhar et al., 2009). Due to its chemical composition (cellulose, lignin), LV biomass has different surface functional groups, such as carboxyl, carbonyl, hydroxyl, phenyl groups. These different functional groups show a high affinity towards heavy metals; therefore they can complex the metal ions (Dhiraj, 2008; Iftikhar et al., 2009). Heavy metal cations seemed to be mostly unsorbed at low pH values. Minimal adsorption at high solution acidity could be explained by a high concentration and mobility of H+ ions. That is why, in comparison to lead, cadmium and zinc ions, hydrogen ions were adsorbed first on the sorption sites. An increase in metal ion desorption capacity with an increase in H+ ion concentration indicated that the adsorption process was probably carried out via an ion exchange mechanism (Fig. 1). At decreased acid concentration, metal ions in solution entered into competition with H+ ions for the sorption sites, according not only to the mass transfer law but also to their chemical and physical properties. Adsorption capacity of the investigated metal ions increased with pH ranging from 2 up to 5 or 6, since the negative charge on LV biomass increased. A number of protons will then compete with metal ions for the ligands and thereby increase the interaction of metal ions with the active sites (Sag et al., 1998; Cimino et al., 2000; Inglezakis et al., 2003). At a higher pH range from 5-6 to 7; the existence of counterpart neutral and negatively charged ions might result in lower adsorption efficiency. Possibly some precipitation of metal could occur at the mentioned pH values.
Effect of temperature
The effect of temperature on metal removal efficiency was studied at 10, 20, 25, 30, 40 and 50ºC, at initial pH 5, initial metal concentration 50 mg·ℓ-1 and 4.0 g·ℓ-1 LVB. The results of removal efficiency for 10 min of contact time vs. temperature are given in Fig. 4. An increase in temperature from 10 to 30ºC leads to an increase in the removal efficiency of all investigated metals, with rates of 0.26, 0.56 and 0.75ºC-1 for Pb2+, Cd2+ and Zn2+ ions, respectively. This may indicate that biosorption of heavy metals onto LVB is a chemical process. Biosorption of zinc using LVB is assumed to be that which is most affected by temperature, due to its low removal rate (Fig. 2).
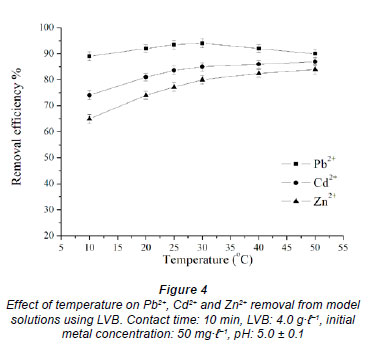
With a further increase in temperature from 30 to 50ºC the removal efficiency of Pb2+ starts to decrease, while in the case of Cd2+ and Zn2+ it continuously increases, but at a slightly lower rate. It can be seen that sorption of Pb2+ is less affected by temperature and is characterized by a different dependence relative to cadmium and zinc. The decrease in removal efficacy at temperatures above 30ºC indicates that the biosorption of lead is an exothermic process, while the sorption of Cd2+ and Zn2+ is an endothermic process. Also, below an equilibrium time for the investigated metals an increase in temperature leads to increase in the biosorption rate, suggesting a kinetically-controlled process. After equilibrium is reached the biosorption of Pb2+ decreases and that of Cd2+ and Zn2+ increases (results not shown). This effect may be explained by an increase in active sites formed on biosorbent at higher temperatures. The obtained results are in agreement with the study of Sawalha et al. (2007), which shows that biosorption of lead by leaves of saltbush is a spontaneous process with a negative value of Gibbs free energy, as opposed to that of cadmium and zinc which present a positive ΔG.
Effect of stirring speed
The biosorbent based on Lagenaria vulgaris shell has a low specific weight, and floats on the surface, in contact with the solution. Therefore, stirring of the solution is necessary, in order to achieve contact between sorbent and water phase. The effect of stirring speed on Pb2+, Cd2+ and Zn2+ removal efficiency was studied at 50, 100, 150, 200, 250, 300, 400 and 500 r·min-1, at initial pH 5, initial metal concentration 50 mg·ℓ-1 and 4.0 g·ℓ-1 LVB. Results show that the removal efficiency increased with an increase of stirring speed, in the case of all investigated metals (Fig. 5). Maximum removal efficiency was achieved at 200-250 r·min-1. At rates beyond 400 r·min-1, sorption of metals partly declines.
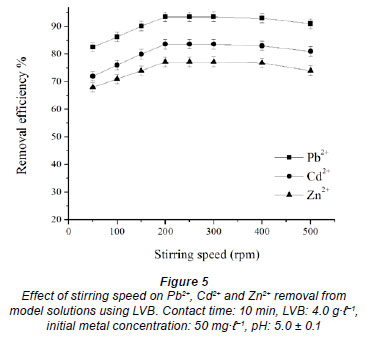
It is obvious that with an increase in stirring speed, the rate of diffusion of metal ions towards the surface of the biosorbent increases. Since the sorption of metals in the range from 200 to 400 r·min-1 was found to be more or less the same, the stirring speed of 250 r·min-1 was selected as optimal for all experiments. A decrease in removal efficiency with further increases of speed up to 500 r·min-1 could be the consequence of vortex phenomena, which reduce the contact surface between phases.
Morphological characterisation of the LV biomass
The SEM image (Fig. 6a) shows a highly porous morphology of the raw biomaterial with pores of more or less different shapes and sizes. This figure also reveals the existence of plant vessels of horizontal as well as vertical orientations, and a variety of cavities on the external surface. These may contribute to the relatively high surface area of the biomaterial. Such biomaterial refined with nitric acid, and further playing the role of biomass in the adsorption process (Fig. 6b), exhibits somewhat changed morphology with extended surface arrangement regarding the repetition of structures, plant vessel orientations, the subsistence of pores of comparable shapes and sizes, and the smallest openings becoming more available for reactants. One of the reasons for this change may be the removal of the alkaline and alkaline earth metals during refinement, which could contribute to the changes in morphology. Dimensions of the smallest pores are comparable in both samples, suggesting that the refinement procedure has its main impact on the uniformity of the biomass structure. The authors suggest that the mentioned pores represent active sites of the adsorption process. Similar observations concerning morphological properties but dealing exclusively with the issue of pores of different sizes and shapes, have been reported in papers dealing with the modified biomaterials, such as acid-treated olive stone, widely studied as biomass; treated olive cake, and a cornelian cherry (Demirbas et al., 2004; Cimino et al., 2005; Aziz et al., 2009).
In the procedures investigated here, the effect of metal binding to the biomass surface caused some changes in the surface morphology in the case when refined biomass was used (Fig. 6c). The edge of each microstructure looks less obvious and the diameters of pores are larger in comparison with that before biosorption took place. This is in accordance with a number of authors who have identified surface morphological changes before and after metal loading (Chen et al., 2002; Demirbas et al., 2004) through SEM. This may imply that metal binding is strongly related to the plant chemical composition, which is additionally confirmed by FTIR analysis, as described below.
The refined biomass samples displayed apparently regularly arranged active coal-like structures of a prismatic type characterised by a rough appearance (Fig. 6b). It seems that a metal could easily penetrate into the inside of the biomass through pores or channels and further be adsorbed at interior sites/acidic centres (Murphy et al., 2009). A more porous biomass structure would definitely favour the diffusion of metal species to the centre or into the bulk of the used biomass, as has similarly been observed in other studies. Moreover, remarkably porous material having a high specific surface area should theoretically be able to play an important role in heavy metal and basic dye removal from aqueous solutions by low-cost agricultural waste materials (Liu and Xu, 2007). SEM micrographs also reveal that morphological changes of biomass after loading by the investigated metals do not depend on metal species or their ionic radii (results not shown).
Changes in the structure and morphology of the biomass surface, as discussed here, may be caused by binding of metals onto active centres of LVB, which further reduces the probability of hydrogen bridge generation between carboxyl and hydroxyl groups (and also other groups), resulting in a more regularly arranged structure (and surface appearance) of the biomass. In addition, refining the biosorbent with acid may cause some alkaline and alkaline earth metals to be removed, resulting in a more regularly arranged structure as compared to that in the raw state.
The EDX analysis (Fig. 7) suggests that C and O contributed the 2 major elements of the biomass, with addition of the major metal constituents (K and Ca) on the biomass surface. This is in agreement with the chemical composition of the biomass (Table 1), where cellulose represents the main ingredient while proteins are not evident (also confirmed by the absence of N in EDX spectra). The registered alkali (K) and alkali earth (Ca) metal peaks originate from the raw plant stage normally characterised by the presence of these metals. Yang et al. (2008) suggested Ca continuation in forms of CaCO3, CaO or Ca(OH)2. It is also supposed that the observed Cl content may be attributed to salts of naturally occurring plant elements. A negligible amount of Cu, probably of anthropogenic origin, observed in the raw biomass sample is eliminated after the refinement procedure, Table 3 and Fig. 7.
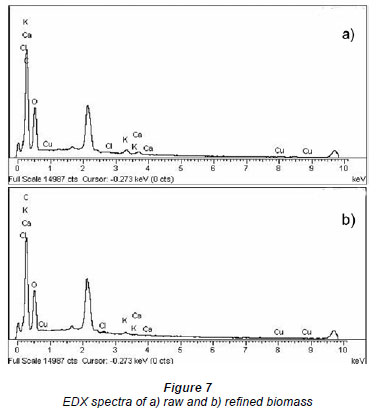
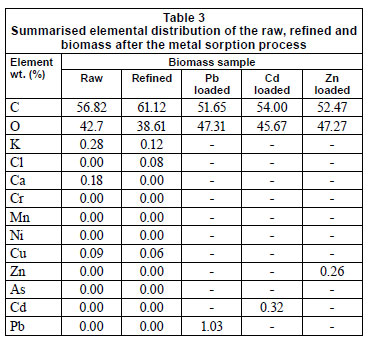
Summarised quantitative results obtained using the EDX method (Table 3) give an insight into the chemical composition of the biomass (further explained in the section discussing the FTIR results). The EDX spectra depict the peaks of C, O (H could not be measured), K and Ca, which are the main constituents of cellulose and lignin present in the biomass (Fig. 7 and Table 1). These peaks point out the polymer types representing available functionalities on the biomass, e.g. -COOH and -OH.
A reduced peak for O (Fig. 7), recorded in the refined biomass samples, may indicate some chemical transformations or variations in polymer chains. In addition, the carboxylic groups (Aziz et al., 2009) may confer ion exchange properties to the treated biomaterial in the metal elimination process, as claimed earlier. Variation in the quantity of the particular acidic binding sites was earlier stated as a major influence on metal binding to the biomass surface (Murphy et al., 2008). Similarly, it was previously estimated that the areas that have higher metal uptake could have higher content of COO- groups that easily formed metal-organic surface complexes (Yang and Chen, 2008). Therefore, in the current study, differences in C and O content could also be connected with a variation in quantity of the available acidic centres, further affecting the adsorption process.
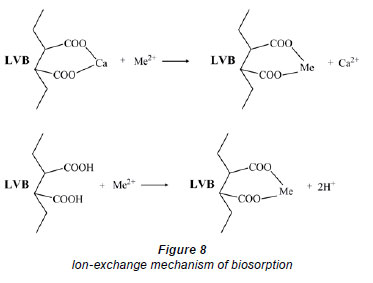
What is more important to underline is a reduction or complete elimination of Ca, naturally occurring in the plant, after refinement and adsorption processes; Ca could be removed during heavy metal elimination via an ion-exchange mechanism (Fig. 8). In this way, treated aqueous solutions become richer in Ca content in comparison to the natural level (results not shown). In some earlier studies, the ion-exchange mechanism was established as a dominant or even significant one (Chen et al., 2002; Iqbal et al., 2009). Surface uptake is highest for Pb, among the heavy metals investigated for adsorption with LVB. (Table 3), a result which is expected given the results for removal efficiency (Fig. 1) and effect of contact time (Fig. 2).
FTIR spectral analysis
The quality and quantity of sorption/chemisorption of metals onto the biomass surface is strongly determined by functional groups present on the plant material (Krishnani et al., 2008; Panda et al., 2008). Therefore, FTIR characterisation was carried out to analyse the major functional groups which exist in the biosorbent (Fig. 9).
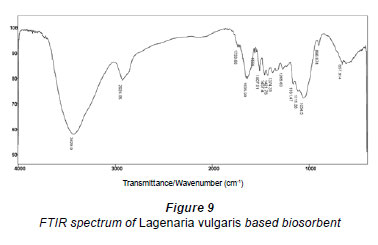
Peaks appearing in the FTIR spectrum of Lagenaria vulgaris based biosorbent are assigned to various groups and bonds in accordance with their respective wavenumbers (cm-1) as reported in the literature (Krishnani et al., 2008; Panda et al., 2008; Yao et al., 2009; Iqbal et al., 2009). The broad and intense peak at 3 429 cm-1 (ranging from 3 200 to 3 600 cm-1) is assigned to the stretching of O-H groups due to inter- and intra-molecular hydrogen bonding of polymeric compounds, such as alcohols, occasionally phenols, and carboxylic acids present in the cellulose and lignin (Fig. 9 and Table 1). The stretching of O-H bonds results in vibrations within a range of frequencies/wavenumbers which specify the presence of O-H bonds of carboxylic acids and/or free hydroxyl groups (Gnanasambandam and Protor, 2000; Iqbal et al., 2009). The peaks at 1 653 and 1 636 cm-1 may be attributed to C=O and N-H stretching vibrations, respectively. This observation concerning the presence of amine groups may be questionable due to the fact that nitrogen was not evident from physicochemical characterisation of the biomaterial (by EDX analysis) suggesting that those bands should be assigned to other groups.
The clear band at 2 924 cm-1 indicates symmetric or asymmetric C-H stretching vibration of aliphatic acids, while the peak registered at 1 733 cm-1 additionally represents the stretching vibration of C=O bonds, which originates from non-ionic carboxyl groups (-COOH, -COOCH3), and may be denoted by carboxylic acids or corresponding esters (Li et al., 2007). Moreover, the band at 1 054 cm-1 can be connected to the existence of stretching vibrations of C-O of alcohol groups and carboxylic acids (Guibaud et al., 2003). It is worth underlining that the FTIR spectrum of raw Lagenaria vulgaris based biosorbent (Fig. 9) shows an abundance of carboxyl and hydroxyl groups. These groups present in the biomass may act in de-protonated forms as key sites in coordination of heavy metals (Ashkenazy et al., 1997).
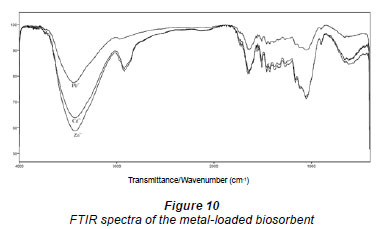
FTIR spectra of metal (Cd2+, Pb2+ and Zn2+) loaded LVB are more or less similar to those before treatment (Figs. 9 and 10), but characterised by changes in intensity and shift in position of the peaks due to metal sorption. The first observed change was the attenuation of the intensity and shift of the peaks in the region of 3 500-3 200 cm-1 and 1 100-900 cm-1, indicating a decrease of the free hydroxyl group on the biomass. The minor shift of the peaks 1 733, 1 653 and 1 054 cm-1 to lower wavenumbers also suggests the involvement of the C-O group in metal binding. These changes may be explained by metal ions associated with carboxylate and hydroxylate anions, revealing that carboxyl and hydroxyl groups are dominant in the process of metal ion uptake (Ashkenazy et al., 1997). Effects were most intensive in the case of lead (Fig. 10), due to the highest sorption efficiency occurring for this metal, in comparison with cadmium and zinc.
Conclusion
The present study reported the characteristics and important parameters that determine the process of heavy metal sorption, and the possible sorption mechanism, in using a new biosorbent based on the shell of Lagenaria vulgaris (typically grown in the Southeast area of Serbia). Sorption of metal ions (Pb2+, Cd2+ and Zn2+) was dependent on the following experimental conditions: sorbate-sorbent contact time, initial pH, temperature and stirring speed. Metal ion biosorption was extraordinarily fast, reaching maximal efficiency in the first 5 (Pb2+), 8 (Cd2+) and 10 (Zn2+) min. Initial pH strongly affects the sorption process and it was concluded that adsorption is favoured by an increase of pH, with an optimal pH value of about 5. It was also revealed that different pH-dependent sorption profiles for various heavy metals could be related to the nature of chemical interactions for each metal. Additionally, it is important to emphasize that the existence of relatively large numbers of hydrogen ions may change the direction of reversible ion exchange equilibrium, which indicates that the presented sorption process was probably tailored via an ion exchange mechanism. Biosorption on LVB is temperature dependent, which may indicate that it is chemical process. Sorption of lead should be an exothermic process, while the sorption of the other 2 metals is an endothermic process. SEM images have shown that the smallest pores on LVB represent active sites of the adsorption process, and changes in the surface morphology after metal uptake, which are explained by metal binding are also strongly related to the chemical composition of the plant. In addition, EDX spectra of LVB, before and after metal sorption, support the proposal that an ion exchange mechanism is involved in the sorption procedure. FTIR spectra revealed that major functional groups playing a role as active sites on the biosorbent surface in the metal ion uptake process included carboxyl and hydroxyl groups as dominant ones. Based on the reported observations, LVB, as a highly porous material, is recommended for use as an efficient and low-cost agricultural material for the removal of heavy metals from effluents.
Acknowledgements
The authors would like to acknowledge the financial support of the Ministry of Science and Technological Development of the Republic of Serbia (Project TR34008).
References
AHLUWALIA SS and GOYAL D (2005) Removal of heavy metals from waste tea leaves from aqueous solution. Eng. Life Sci. 5 158-162. [ Links ]
AJMAL M, RAO R, AHMAD R, AHMAD J and RAO L (2001) Removal and recovery of heavy metals from electroplating wastewater by using Kyanite as an adsorbent. J. Hazard. Mater. B 87 127-137. [ Links ]
APHA (1998) Standard Methods for the Examination of Water and Wastewater (20th edn.). American Publishers Health Association, Washington, DC. [ Links ]
ARAUJO GCL, GONZALEZ MH, FERREIRA AG, NOGUEIRA ARA and NOBREGA JA (2002) Effect of acid concentration on closed-vessel microwave-assisted digestion of plant materials. Spectrochim. Acta B 57 2121-2132. [ Links ]
ASHKENAZY R, GOTTLIEB L and YANNAI S (1997) Characterization of acetone-washed yeast biomass functional involved in lead biosorption. Biotechnol. Bioeng. 55 1-10. [ Links ]
AZIZ A, OUALI MS, ELANDALOUSSI EH, DE MENORVAL LC and LINDHEIMER M (2009) Chemically modified olive stone: A low-cost sorbent for heavy metals and basic dyes removal from aqueous solutions. J. Hazard. Mater. 163 441-447. [ Links ]
BAILEY SE, OLIN TJ, BRICKA RM and ADRIAN DD (1999) A review of potentially low-cost sorbents for heavy metals. Water Res. 33 2469-2479. [ Links ]
BRAUCKMANN BM (1990) Industrial solution amenable to biosorption. In: Volesky B (ed.) Biosorption. CRC Press, Boca Raton, FL. [ Links ]
CANET L, ILPIDE M and SETA P (2002) Efficient facilitated transport of lead, cadmium, zinc and silver across a flat sheet-supported liquid membrane mediated by lasalocid A. Separ. Sci. Technol. 37(8)1851-1860. [ Links ]
CELIK A and DEMIRBAS A (2005) Removal of heavy metal ions from aqueous solutions via adsorption onto modified lignin from pulping wastes. Energ. Source. 27 1167-1177. [ Links ]
CHEN JP, HONG LA, WU SN and WANG L (2002) Elucidation of interactions between metal ions and Ca alginate-based ion-exchange resin by spectroscopic analysis and modeling simulation. Langmuir 18 9413-9421. [ Links ]
CIMINO G, CAPPELLO RM, CARISTI C and TOSCANO G (2005) Characterization of carbons from olive cake by sorption of wastewater pollutants. Chemosphere 61 947-955. [ Links ]
CIMINO G, PASSERINI A and TOSCANO G (2000) Removal of toxic cations and Cr(VI) from aqueous solution by hazelnut shell. Water Res. 34 2955-2962. [ Links ]
DANG VBH, DOAN HD, DANG-VU T and LOHI A (2009) Equilibrium and kinetics of biosorption of cadmium(II) and copper(II) ions by wheat straw. Bioresour. Technol. 100 211-219. [ Links ]
DECKER-WALTERS DS, WILKINS-ELLERT M, CHUNG S-M and STAUB JE (2004) Discovery and genetic assessment of wild bottle gourd [Lagenaria siceraria (Mol.) Standley, Cucurbitaceae] from Zimbabwe. Econ. Bot. 58 501-508. [ Links ]
DEMIRBAS E, KOBYA M, SENTIRK E and OZKAN T (2004) Adsorption kinetics for the removal of chromium(VI) from aqueous solutions on the activated carbons prepared from agricultural wastes. Water SA 30 533-539. [ Links ]
DHIRAJ S, MAHAJAN G and KAUR MP (2008) Agricultural waste material as potential adsorbent for sequestering heavy metal ions from aqueous solutions - A review. Bioresour. Technol. 99 6017-6027. [ Links ]
ESALAH OJ, WEBER ME and VERA JH (2000) Removal of lead, cadmium and zinc from aqueous solutions by precipitation with sodium di-(n-octyl) phosphinate. Can. J. Chem. Eng. 78 948-954. [ Links ]
FORSTNER U and WITTMAN CTW (1981) Metal Pollution in the Aquatic Environment. Springer, New York. [ Links ]
FRIEDMAN M and WAISS AC Jr. (1972) Mercury uptake by selected agricultural products and by-products. Environ. Sci. Technol. 6 457-458. [ Links ]
GARDEA-TORRESDEY JL, GONZALEZ JH, TIEMANN KJ, RODRIGUEZ O and GAMEZ G (1998) Phytofilteration of hazardous cadmium, chromium, lead, and zinc ions by biomass of Medicago sativa (alfalfa). J. Hazard. Mater. 57 29-39. [ Links ]
GNANASAMBANDAM R and PROTOR A (2000) Determination of pectin degree of esterification by diffuse reflectance Fourier transform infrared spectroscopy. Food Chem. 68 327-332. [ Links ]
GOYAL P, SHARMA P, SRIVASTAVA S and SRIVASTAVA MM (2008) Saraca indica leaf powder for decontamination of Pb: removal, recovery, adsorbent characterization and equilibrium modeling. J. Environ. Sci. Tech. 5(1)27-34. [ Links ]
GUIBAUD G, TIXIER N, BOUJU A and BAUDU M (2003) Relation between extracellular polymer's composition and its ability to complex Cd, Cu and Pb. Chemosphere 52 1701-1710. [ Links ]
HASHEM A, AKASHA RA, GHITH A and HUSSEIN DA (2005) Adsorbent based on agricultural wastes for heavy metal and dye removal: A review. Energ. Edu. Sci. Technol. 19 69-86. [ Links ]
HORIKOSHI T, NAKAJIMA A and SAKAGUCHI T (1981) Studies on the accumulation of heavy metal elements in biological systems, XIX: accumulation of uranium by microorganisms. Eur. J. Appl. Microbiol. Biotechnol. 12 90-96. [ Links ]
HORSFALL M JNR and SPIFF AI (2005) Equilibrium sorption study of Al3+, Co2+ and Ag+ in aqueous solutions by fluted pumpkin (Telfairia Occidentalis HOOK f) waste biomass. Acta Chim. Slov. 52 174-181. [ Links ]
HORWITZ W and LATIMER GW (2006) Official Methods of Analysis of AOAC International (18th edn.). Association of Official Analytical Chemists, Arlington, VA. [ Links ]
HOSEA M, GREENE B, MCPHERSON R, HENZL M, ALEXANDER MD and DARNALL DW (1986) Accumulation of elemental gold on alga Chlorella vulgaris. Inorg. Chim. Acta 123 161-165. [ Links ]
IFTIKHAR AR, BHATTI HN, HANIF MA and NADEEM R (2009) Kinetic and thermodynamic aspects of Cu(II) and Cr(III) removal from aqueous solutions using rose waste biomass. J. Hazard. Mater. 161(2-3)941-947. [ Links ]
INGLEZAKIS VJ, LOIZIDOU MD and GRIGOROPOULOU HP (2003) Ion exchange of Pb2+, Cu2+, Fe3+, and Cr3+ on natural clinoptilolite: selectivity determination and influence of acidity on metal uptake. J. Colloid. Interf. Sci. 261 49-54. [ Links ]
IQBAL M, SAEED A and ZAFAR SI (2009) FTIR spectrophotometry, kinetics and adsorption isotherms modeling, ion exchange, and EDX analysis for understanding the mechanism of Cd2+ and Pb2+ by mango peel waste. J. Hazard. Mater. 164 161-171. [ Links ]
JOHNSON PD, WATSON MA, BROWN J and JEFCOAT IA (2002) Peanut hull pellets as a single use sorbent for the capture of Cu(II) from wastewater. Waste Manage. 22 471-480. [ Links ]
KABATA-PENDIAS A and PENDIAS H (2001) Trace Elements in Soils and Plants. CRC Press Inc., Boca Raton. [ Links ]
KAHRAMAN S, ASMA (HAMAMCI) D, ERDEMOGLU S and YESILADA O (2005) Biosorption of Copper(II) by live and dried biomass of the white rot fungi Phanerochaete chrysosporium and Funalia trogii. Eng. Life Sci. 5(1)72-77. [ Links ]
KJELLSTROM T, SHIROISHI K and ERWIN PE (1977) Urinary beta./sub 2/-microglobulin excretion among people exposed to cadmium in the general environment. Environ. Res. 13 318-344. [ Links ]
KRISHNANI KK, XIAOGUANG MX, CHRISTODOULATOS C and BODDU VM (2008) Biosorption mechanism of nine different heavy metals onto biomatrix from rice husk. J. Hazard. Mater. 153 1222-1234. [ Links ]
LI FT, YANG H, ZHAO Y and XU R (2007) Novel modified pectin for heavy metal adsorption. Chin. Chem. Lett. 18 325-328. [ Links ]
LIU Q and LIU Y (2003) Distribution of Pb(II) species in aqueous solutions. J. Colloid. Interf. Sci. 268 266-269. [ Links ]
LIU Y and XU H (2007) Equilibrium, thermodynamics and mechanisms of Ni2+ biosorption by aerobic granules. Biochem. Eng. J. 35 174-182. [ Links ]
LODEIRO P, BARRIADA JL, HERRERO R and SASTRE DE VICENTE ME (2006) The marine microalgae Cystoseira baccata as biosorbent for cadmium(II) and lead(II) removal: kinetic and equilibrium studies. Environ. Pollut. 142 264-273. [ Links ]
MURPHY V, HUGHES H and McLOUGHLIN P (2008) Comparative study of chromium biosorption by red, green and brown seaweed biomass. Chemosphere 70 1128-1134. [ Links ]
MURPHY V, TOFAIL SAM, HUGHES H and MCLOUGHLIN P (2009) A novel study of hexavalent chromium detoxification by selected seaweed species using SEM-EDX and XPS analysis. Chem. Eng. J. 148 425-433. [ Links ]
OKOLI BE and NYANAYO BL (1998) Polynology of Telfairia L. (Cucurbitacae). Folia Geobot. Phytotx. 23 281-286. [ Links ]
PANDA GC, DAS SK and GUHA AK (2008) Biosorption of cadmium and nickel by functionalized husk of Lathyrus sativus. Colloid Surf. B 62 173-179. [ Links ]
PANDEY PK, VERMA Y, CHOUBEY S, PANDEY M and CHANDRASEKHAR K (2008) Biosorptive removal of cadmium from contaminated groundwater and industrial effluents. Bioresource Technol. 99 4420-4427. [ Links ]
PASTIRCAKOVA K (2004) Determination of trace metal concentrations in ashes from various biomass materials. Energ. Edu. Sci. Technol. 13 97-104. [ Links ]
PATTERSON JW (1985) Industrial Wastewater Treatment Technology (2nd edn.). Butterworth Publisher, Stoneham, MA. [ Links ]
RAVINDRAN V, STEVENS MR, BADRIYHA BN and PIRBAZARI M (1999) Modeling the sorption of toxic metals on chelant-impregnated adsorbent. Am. Inst. Chem. Eng. J. 45(5)1135-1146. [ Links ]
ROMERA E, GONZALEZ F, BALLESTER A, BLAZQUEZ ML and MUNOZ JA (2007) Comparative study of biosorption of heavy metals using different types of algae. Bioresour. Technol. 98 3344-3353. [ Links ]
SAG Y, KAYA A and KUTSAL T (1998) The simultaneous biosorption of Cu(II)/ and Zn on Rhizopus arrhizus: application of the adsorption models. Hydromet. 50 297-314. [ Links ]
SAWALHA MF, PERALTA-VIDEA JR, ROMERO-GONZALEZ J, DUARTE-GARDEA M and GARDEA-TORRESDEY JL (2007) Thermodynamic and isotherm studies of the biosorption of Cu(II), Pb(II) and Zn(II) by leaves of saltbush (Atriplex canescens). J. Chem. Thermodynamics 39 488-492. [ Links ]
SAYGIDEGER S, GULNAZ O, ISTIFLI ES and YUCEL N (2005) Adsorption of Cd(II), Cu(II) and Ni(II) ions by Lemna minor L.: effect of physicochemical environment. J. Hazard. Mater. 126B 96-104. [ Links ]
SINGH A, SHARMA RK, AGRAWAL M and MARSHALL FM (2010) Risk assessment of heavy metal toxicity through contaminated vegetables from waste water irrigated area of Varanasi, India. Trop. Ecol. 51(2S)375-387. [ Links ]
SUD D, MAHAJAN G and KAUR MP (2008) Agricultural waste material as potential adsorbent for sequestering heavy metal ions from aqueous solutions - A review. Bioresour. Technol. 99 6017-6027. [ Links ]
TAN WT, OOI ST and LEE CK (1993) Removal of Cr(VI) from solution by coconut husk and palm pressed fibers. Environ. Technol. 14 277-283. [ Links ]
TOLES CA and MARSHALL WE (2002) Copper ion removal by almond shell carbons and commercial carbons: batch and column studies. Sep. Sci. Technol. 37(10)2369-2383. [ Links ]
VOLESKY B (2000) Biosorption of Heavy Metals. CRC Press, Boca Raton, FL. [ Links ]
VOLESKY B AND HOLAN ZR (1995) Biosorption of heavy metals. Biotechnol. Progr. 11 235-250. [ Links ]
WILLIAMS CJ and EDYVEAN RGJ (1997) Optimization of metal adsorption by seaweeds and seaweed derivatives. Process. Saf. Environ. 75(B) 19-26. [ Links ]
WILLIAMS CJ, ADERHOLD D and EDYVEAN RGJ (1998) Comparison between biosorbents for the removal of metal ions from aqueous solutions. Water Res. 32 216-224. [ Links ]
WU SL, FENG XB and WITTMEIER A (1997) Microwave digestion of plant and grain reference materials in nitric acid or a mixture of nitric acid and hydrogen peroxide for the determination of multi-elements by inductively coupled mass spectrometry. J. Anal. At. Spectrom. 12 797-806. [ Links ]
WULFSBERG G (1987) Principles of Descriptive Chemistry. Brooks/Cole Publishing, Monterey CA. [ Links ]
YANG L and CHEN JP (2008) Biosorption of hexavalent chromium onto raw and chemically modified Sargassum sp. Biores. Technol. 99 297-307. [ Links ]
YAO L, YE ZF, TONG MP, LAI P and NI JR (2009) Removal of Cr3+ from aqueous solution by biosorption with aerobic granules. J. Hazard. Mater. 165 250-255. [ Links ]
ZHANG L, ZHAO L, YU Y and CHEN C R (1998) Removal of lead from aqueous solution by non-living Rhizopus nigricans. Water Res. 32 1437-1444. [ Links ]
ZHAO M, DUNCAN JR and VAN HILLE RP (1999) Removal and recovery of zinc from solution and electroplating effluent using Azolla filiculoides. Water Res. 33(6)1516-1522. [ Links ]
ZOUBOULIS AI, MATIS KA, LANARA BG and LOOS-NESKOVIC C (1997) Removal of cadmium from dilute solutions by hydroxyapatite. II. Floatation studies. Sep. Sci. Technol. 32(10)1755-1767. [ Links ]
Received 20 September 2010; accepted in revised form 21 April 2011.
* To whom all correspondence should be addressed. +381 63 106 40 16; fax: +381 18 533 014; e-mail: bojica@pmf.ni.ac.rs














