Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Water SA
On-line version ISSN 1816-7950
Print version ISSN 0378-4738
Water SA vol.37 n.2 Pretoria Apr. 2011
Removal of Escherichia coli from biological effluents using natural and artificial mineral aggregates
M Miranda-RíosI; VM Luna-PabelloI,*; MT Orta de VelásquezII; JA Barrera-GodínezI
IFacultad de Química, Universidad Nacional Autónoma de México, Ciudad Universitaria, México D. F. 04510, México
IIInstituto de Ingeniería, Universidad Nacional Autónoma de México, Ciudad Universitaria, México D. F. 04510, México
ABSTRACT
Ability for disinfecting sterile biological effluents inoculated with Escherichia coli ATCC 25922 at concentrations of 105 CFU/mℓ, using a natural mineral aggregate (NMA) and artificial mineral aggregates (AMA's) consisting of individual oxides as FeO, CuO y Ag2O and combined oxides as Fe2O3-Cu2O, Fe2O3-Ag2O, Cu2O-Ag2O, Fe2O3-Cu2O-Ag2O, contained alginate beads, was compared. The results indicate that Ag2O and Fe2O3-Ag2O, Cu2O-Ag2O combinations, as well as NMA, inactivated 100% of E. coli in 30 min, whereas the oxides mixture, Fe2O3-Cu2O-Ag2O, took 13 min. It was observed that redox potential values were closely related to the disinfection level achieved. The advantage resulting from using alginate beads was that these allow the formation of AMA, which has higher disinfectant ability relative to NMA.
Keywords: disinfection, biological effluent, Fe2O3, Cu2O and Ag2O, alginate beads, Escherichia coli, natural mineral aggregate, artificial mineral aggregate
Introduction
Biological effluents from domestic wastewater treatment are required to be disinfected before reuse (Liberti et al., 2000) because they still contain microorganisms of intestinal origin, such as helminth ova and faecal coliform bacteria. Escherichia coli is a bacterium of enteric origin whose occurrence and abundance allows for its use in defining the sanitary quality of water and wastewater. The World Health Organization (WHO, 1989) has established a maximum level of 1 000 faecal coliforms unit (FCU)/100 mℓ for Category A water quality. Chlorination is the most widely used wastewater disinfection method, even though it has a drawback due to the formation of trihalomethanes and organochlorinated compounds which are carcinogens. An alternative disinfection method is the use of some metals, either alone or combined, such as Fe, Cu or Ag in the solid state (Davies and Etris, 1997; You et al., 2005), in ionic form (Craig, 2001; Jiang et al., 2006a; Silva-Martínez et al., 2004; Silvestry-Rodriguez et al., 2007), in combination with UV light (Kim et al., 2008) or as formulations where metal ions of Al, Cu or Ag are added to a solid matrix like zeolites (Rivera-Garza et al., 2000; De La Rosa-Gómez et al., 2008), ceramic material (Kim et al., 2004), silicates (Kawashita et al., 2003), colloids and metal nanoparticles (Chaloupka et al., 2010; Cho et al., 2005; Choi et al., 2008; Li et al., 2008), polymers (Lukhele et al., 2010) or biopolymers (Yi et al., 2003). However, experiences in using metals for disinfecting wastewater have been few, and mainly consist of using metal ions in combination with other chemical disinfectants, such as chlorine, hydrogen peroxide or peracetic acid (PAA). These combinations of disinfectants have been applied to influents from advanced primary treatment (APT), biological effluents or raw water (Pedahzur et al., 1995; Orta de Velásquez et al., 2008; Luna-Pabello et al., 2009). In most cases, for achieving total inactivation of test microorganisms, contact time tends to be large, i.e. up to 2 h (Table 1).
Mineral aggregates present an opportunity for improvement. Mineral aggregates may contain metals such as Fe, Cu and Ag at their different oxidation states, thus increasing their germicidal effect. These metals contribute to inhibition of the cellular respiration process, due to the inactivation of -SH radicals of respiration enzymes, the interruption of the electrontransfer chain and DNA and RNA disruption (Davies and Etris, 1997; Silva-Martínez et al., 2004; Holt and Bard, 2005; Sharma et al., 2005; Yamanaka et al., 2005; Silvestry-Rodriguez et al., 2007; Park et al., 2009; Chaloupka et al., 2010). Natural mineral aggregates (NMAs) have shown germicidal activity but they exhibit drawbacks such as not having a homogeneous composition and containing undesirable metals such as As and Pb (Miranda-Ríos and Luna-Pabello, 2002-2003).
A possible matrix to make artificial mineral aggregates is sodium alginate, which is a natural ionic polysaccharide having many applications in the food and pharmaceutical industries (Braccini and Pérez, 2001). Alginate has been used for immobilising biomolecules and also is a strong chelating agent for metals. With most divalent cations, it produces gels that are heat irreversible (Park et al., 2007).
Based on the above, the objective of this study was to determine the contact time required to disinfect a biological effluent containing E. coli, at initial concentrations of 105 CFU/ mℓ, using silver shot, copper shot, natural mineral aggregates (NMA's) and artificial mineral aggregates (AMA's) formed with Fe2O3Cu2O, Ag2O, separated or combined.
Experimental
Sterile biological effluent: The biological effluent was obtained from Ciudad Universitaria UNAM, located at the southern zone of Mexico City. Two hundred litres were collected from the effluent of the activated sludge system, before it passes through the sand filter, and was subjected to physicochemical analysis as described by Eaton et al. (2005), and then sterilised by autoclaving at 1.1 kg/cm2, 120ºC for 15 min.
Inoculum: Escherichia coli ATCC 25922 bacteria preserved on nutritive agar (BBL) were inoculated in an Erlenmeyer flask containing 100.0 mℓ of sterile nutritive broth (BBL) and were placed overnight in an incubator with orbital agita tion (G24 New Brunswick) at 37ºC and 250.0 r/min. After 18 h of incubation the inoculum was adjusted with nutritive broth at OD of 1.4 at 600 nm wavelength using an UV-VIS spectrophotometer (Pharmacia Biotech, Ultrospec 3000). Subsequently, 10.0 mℓ of inoculum were diluted in 99.0 mℓ of sterile distilled water. One millilitre from this decimal dilution was pre-adapted in a flask containing 99.0 mℓ of the sterile biological effluent. The flask was again placed in the incubator with orbital agitation for 24 h.
Escherichia coli presence is the most reliable indicator of faecal bacterial contamination of surface waters in different countries. An appropriate health-based indicator of microbial pathogens should possess several characteristics (Arana et al., 2000). The indicator should always be present when pathogens are present and should not be detected when the pathogens are absent; it should have a life span similar to that of the pathogens of concern; it should be present in large numbers and should be readily detected by simple and inexpensive methods; and it should not multiply in the environment once it has been shed by the host. Based on these conditions, if the indicator is isolated from the water under examination the pathogenic organisms could still be present; if the indicator is absent, pathogenic organisms are also probably absent (Eaton et al., 2005; Kim et al., 2008).
Water for testing: Water for testing was prepared by adding 5.0 mℓ of pre-adapted E. coli at a concentration of 106 CFU/ mℓ for each 95.0 mℓ of sterile biological effluent, to obtain a concentration of 105 CFU/mℓ.
Preparation of alginate beads: Sodium alginate solution was prepared dissolving 7.5 g of sodium alginate (Sigma), 3 500 mPa·s and 2.5 g sodium alginate (Sigma), 14 000 mPa·s, in 400 mℓ of distilled water. This solution was sterilised by autoclaving, was allowed to cool and then powdered Fe2O3, Cu2O (J.T. Baker) and Ag2O (Merck) were added to it. The solution was homogenised with a magnetic agitator until it was completely dissolved. The addition of each oxide was calculated for obtain ing the same percentage by weight as that of NMA (see Table 2). The above mixture was taken by a 3.0 mℓ sterile syringe and then was added drop by drop to a 2.0% calcium chloride solution (J.T. Baker), thus forming alginate beads which contain metals. These beads, which have a diameter of 2.00 mm, are filtered off from the CaCl2 solution and are allowed to dry. As a blank, alginate beads with no added metals were prepared.

Microbiological analysis: The concentration of E. coli was determined by the dilutions method and by the technique of membrane filter using sterile nitrocellulose filters (Millipore, Bedford, MA, USA) with a pore size of 0.45 µm and a diameter of 47 mm and agar M-FC (BBL) added with rosolic acid (Hycel de México, S.A. de C.V.) at 2% in NaOH (Merck) (Eaton et al., 2005). Petri dishes were placed in a water jacketed incubator (Ac-Lab) for 24 h at a temperature of 44.5ºC.
Physicochemical characterisation of NMA: A natural mineral aggregate (NMA) with a particle size ranging between 2.0 to 3.36 nm, was obtained from a mine located in Zacatecas State, Mexico. This material was characterised by X-ray diffraction techniques and inductively-coupled plasma atomic emission spectroscopy (ICP-OES) (Eaton et al., 2005).
Disinfection tests: To evaluate disinfecting capacity of NMA and alginate beads containing separate oxides, 4 tests were carried out:
• NMA
• Alginate beads containing separate oxides
• Oxide pairs: Fe2O3-Cu2O, Fe2O3-Ag2O and Cu2O-Ag2O
• Triple combination Fe2O3-Cu2O-Ag2O
From each flask, 100.0 mℓ of water for testing was taken, and for each test either 1 g of NMA or 1 g of alginate beads was added, as shown in Table 3. Flasks were placed in an incubator with orbital agitation, at 37ºC and 250 r/min. At pre-established contact times of 0.0, 30.0 and 60.0 min or 0, 15.0 and 30.0 min, the concentration of surviving bacteria was determined, as well as pH, dissolved O2 and redox potential. The disinfectant activity of alginate beads containing metal oxides and NMA was stopped by adding a neutralising solution prepared with 280.0 mℓ of Tween 80 (Sigma), 40.0 g of soy lecithin and 1.25 mℓ of phosphate buffer solution, making the volume up to 1 ℓ with distilled water. Once homogenised, the solution was sterilised by autoclaving at 1.1 kg/cm2, 120ºC for 15 min (Bloomfield, 1991). This neutralising solution was used either applying 1.0 mℓ on the membrane, where direct seeding is carried out, or in amounts of 9.0 mℓ applied into the assay tubes used for preparing the 10-1 decimal dilution, for seeding by diluting. The experiments were conducted in triplicate and the results presented are mean values. The results were analysed statistically using SPSS 15.0 software. Statistical analysis consisting of a factorial analysis of variance (ANOVA) was performed on the entire data set to determine if significant differences existed between the results obtained using different types of disinfectant (NMA or AMA) and contact time. The differences between treatments were analysed by Tukey's HSD (Honestly Significantly Different) test at P<0.05.

Kinetics of disinfection - The kinetics of disinfection was established according to the Hom equation (Gyürék and Finch, 1998). In this model, the concentration of disinfectant remains unchanged during the disinfection process.

where:
t = time (minutes)
Nt/N0 = quantity of surviving microorganisms,
k* =Constant, time -1, this constant includes the die-off coefficient and the disinfectant doses Cn (when n=1) and m without change.
Results and discussion
The results of microbiological and physicochemical charac terisation of the effluent of the activated sludge system before it was sterilised, are shown in Table 4. The data indicate that it corresponds to a typical secondary effluent (Metcalf and Eddy, 2004; Orta de Velásquez et al., 2008; Luna-Pabello et al., 2009).

The results of physicochemical characterisation of NMA by the x-ray diffraction technique indicate that is comprised of quartz, sanidine, nymite, montmorillonite, calcite and Fe oxide (III). ICP-OES analysis indicated that NMA contains Fe (1.78 % w/w) in a higher proportion than other metals, such as Cu (0.0103% w/w), Zn (0.0743% w/w), As (0.0037 %w/w), Ag (0.0101% w/w) and Pb (0.0448% w/w). Despite the concentrations of As and Pb being detected in low proportions, it is desirable that they would not be present in a disinfected effluent.
X-ray analysis of the bulk flotation product indicated that pyrite (FeS2) is the main form of Fe and indicated the presence of calcite (CaCO3) in the NMA. The calcite in the NMA would neutralise any H2SO4 that might form from a possible pyrite oxidation, freeing carbonate anions. Also, as a result of prevalent acidity conditions, soluble Pb and As species would form their carbonates and would adsorb onto cemented layers or precipitate on the calcite, and thus reduce the aqueous availability of these metals (Armienta and González Hernández, 2007; Smedley, 2008; Romero et al., 2008; Espinosa et al., 2009).
In the NMA experiments, redox potential in test water was oxidant (132.6±0.85 mV). Since the pH values were around 8.5 ±0.03 units, As and Pb could not be incorporated into the final effluent.. After 30 min of contact between test water and NMA, Fe, Cu, As, Ag and Pb concentrations were lower than the detection limit in the test water, and Zn had a concentration of 0.58 mg/ℓ.
The tests conducted with NMA indicated that almost 100% of 105 CFU/mℓ E. coli may be removed during 30 min of contact (Fig. 1), whereas with copper shot and silver shot 90 min are needed (Miranda-Ríos and Luna-Pabello, 2002-2003). NMA requires from 15 to 30 min to obtain an effluent that would be considered by WHO as Category A, that is, suitable to for reuse in agricultural irrigation (less than 1 000 FCU/100 mℓ), while a zeolite containing silver at a concentration of 1.4% w/w needs 110 min to achieve the same disinfection level (De La Rosa-Gómez et al., 2008). Consequently, the combination of metals in NMA exerts a synergistic effect on disinfection because it requires 80 min less than silver zeolite and 60 min less than either copper shot or silver shot to achieve the same results. A Tukey HSD test (P<0.05) showed statistically significant differences between the disinfection % at 15 min of contact time achieved with use of NMA (99.679%) and AMA with Ag (i.e. Ag2O (99.990%), Fe2O3-Ag2O (99.997%), Cu2O Ag2O (99.999%) and Fe3O-Cu2O-Ag2O (100.000%) alginate beads) versus AMA without Ag (i.e. Fe2O3 (76.010%), Cu2O (79.940%) and Fe2O3-Cu2O (49.820%) alginate beads).

In Fig. 2 and Fig. 3 it can be seen that both alginate beads containing Ag2O and those formed with Fe2O3-Ag2O or Cu2O Ag2O combinations require less than 30 min contact time to achieve the total removal of bacteria. In a similar time period, beads with Fe2O3 and those containing Cu2O reduce the initial content of E. coli by less than 1 base-10 logarithmic unit (log10). Moreover, after a contact time of 15 min, beads with Fe2O3-Cu2O combination reduce the content of E. coli only by 0.32 log, whereas with beads containing Fe2O3-Ag2O o Cu2O-Ag2O the concentration decreases 0.48 and 0.17 log10 units, respectively. Finally, for the beads containing Ag2O, the concentration was reduced to 1.33 log10 units, resulting in a survival of more than 1 log10.
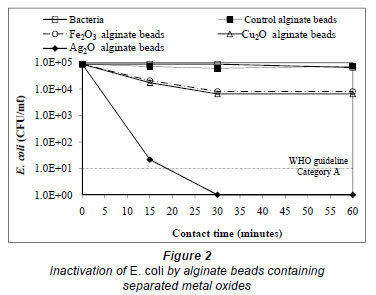

The value corresponding to a Category A effluent is achieved at a contact time from 15 to 30 min for Ag2O beads and from 10 to 15 min for Fe2O3-Ag2O and Cu2O- Ag2O beads. As shown in Figs. 2 and 3, the effect produced by the union between Ag2O and Fe2O3 or Cu2O is an increase in the disin fecting capacity of silver oxide, requiring a shorter contact time to remove the E. coli added, due to the synergistic effect of these oxides on each other. Similar observations for an increase in disinfecting capacity were reported for the union of electrolytically-produced Cu and Ag ions (Rohr et al., 2000; Silva-Martínez et al., 2004) or Cu and Ag oxides contained in a ceramic matrix (Kim et al., 2004), for water from cooling towers; however, a synergistic effect on disinfection due to Fe2O3 and Ag2O has not been reported.
Figure 4 shows that alginate beads containing the 3 oxides, Fe2O3, Cu2O and Ag2O, require a contact time of 13 min to completely remove E. coli, and from 5 to 10 min to reach the values established by WHO for a Category A effluent. The increase in removal of E. coli is again attributed to the synergism of the 3 metals. As observed in Fig. 3, the disinfecting capacity of the Fe2O3-Cu2O combination is minimal; therefore the increase in the disinfecting capacity with the combination of the 3 oxides is not only attributable to the metals involved, but to the presence of oxygen in the medium. In this sense, an increase in bacterial inactivation has been demonstrated by the production of oxygen-reactive species by silver (Davies and Etris, 1997), both in the matrix containing it (Inoue et al., 2002) or by intracellular production as a consequence of the damage that silver causes by forming insoluble complexes with DNA and cellular RNA (Park et al., 2009). This causes the interruption of growth, metabolism and reproduction in the affected cell, as well as changes in structure and permeability of the cell membrane, disturbing the interchange of material between cell and its environment. Silver causes the cytoplasmic membrane to shrink and to separate from the cell wall (Feng et al., 2000).

An increase in the bacterial inactivation effect produced by disinfectants generating reactive oxygen species like H2O2 and PAA, and Cu and Ag in their ionic states, was observed for APT and biological treatment effluents (Orta de Velásquez et al., 2008; Luna-Pabello et al., 2009). In these experiments, the time required to obtain Category A effluents was 30 min for H2O2-Cu (50.0-1.0 mg/ℓ), H2O2-Ag (200.0-1.0 mg/ℓ) or PAA-Ag (7.5-1.0 mg/ℓ) combinations and was reduced by 10 min for Cu-Ag-PAA (0.1-1.0 -20.0 mg/ℓ) combination. That is, these contact times were higher than those required for the combination of the 3 metallic oxides. This supports the theory that oxygen contained in the oxides of the 3 metals used plays an active role in disinfection.
In Fig. 5, an increase in disinfection by using the combination of the 3 metals is observed, where a contact time lower than 5 min. was needed for removing 105 CFU/mℓ E. coli, while NMA requires 30 min (Fig. 1). Consequently, combination of the 3 metals is a good option in disinfection of effluents from biological treatment systems, without the presence of undesirable metals such as Pb and As (Miranda-Ríos and Luna-Pabello, 2002-2003).

NMA required a contact time of 30 min (Fig. 1) and AMA required 13 min (Fig. 4). The E. coli inactivation kinetics followed the Hom equation using either the NMA or the Fe2O3-Cu2O-Ag2O AMA and the corresponding equations were: Nt/ N0= exp(-0.1555×t1.3327) and Nt/N0= exp(-0.669×t 1.204), respectively. In both cases, it was observed that the inactivation rate increases with the contact time due to the fact that m is greater than 1.0. The rate constant is more than 2 times greater for the AMA than for the NMA, according to its greater disinfectant power.
Also, the NMA rate constant is very close to that measured for the Cu-Ag PAA (k*=-0.1612) (Luna-Pabello et al., 2009). In both cases, the silver concentration was 1.0 mg/ℓ and the initial bacteria concentration was 105 CFU/100 mℓ.
In the case of the use of chloramine, at a concentration level of 2.4 mg/ℓ, to disinfect a biological effluent, a rate constant of -0.361 was measured, which is close to that found for the Fe2O3-Cu2O-Ag2O beads. However, the m exponent for chloramine disinfection (m=0.715) is lower than 1.0 (Pretorius and Pretorius, 1999); that is, the rate decreases with the contact time, which implies that chloramine is in fact consumed during the disinfection. This is in contrast to disinfection with Fe2O3-Cu2O-Ag2O beads, for which no metal consumption has been observed.
Moreover, for all tests performed, the pH was maintained at about 8.5, whereas in the case of dissolved oxygen there was a slight tendency to decrease from 5.6 to 5.1 mg/ℓ O2. With regard to redox potential, the value increased by 18.0 mV in assays where Ag2O y Fe2O3-Ag2O was used; by 20 mV for Cu2O- Ag2O beads; by 34 mV for FeO-Cu2O- Ag2O beads; and by 24 mV for NMA. This increase in redox potential, where the environment becomes more oxidising, can be related to E. coli removal, as a greater increase produces a smaller contact time to inactivate the bacteria. For flasks containing alginate beads comprising Cu2O, Fe3O and Fe2O3-Cu2O combination, redox potential was maintained about 8 mV. These beads showed very low disinfecting capacity (less than 1 base-10 logarithmic unit).
In Mexico, wastewater is a valuable (and sometimes the only) resource available for crop irrigation, but is frequently reused in agriculture without any proper application of disinfection measures. This inevitably poses a grave risk to health. Therefore, the importance of this type of study lies in the priority it attaches to treating the high levels of microbial contamination which exist in wastewater, when said wastewater is destined for reuse in agriculture (Orta de Velásquez et al., 2008).
Chlorination is the wastewater disinfection method more widely used, even though it might lead to the formation of trihalomethanes and organochlorinated compounds which are carcinogens. The main alternatives to chlorination are ozona tion, and the use of ultraviolet light. According to estimates carried out by Collivignarelli et al. (2000), the investment cost for the disinfection of wastewater previously treated by a biological system varies, depending on the size of the plant, as follows (in South African Rands): Chlorine dioxide = ZAR138 427 to 1 993 343; ozone = ZAR346 067 to 5 613 198; ultraviolet light = ZAR263 010 to 7 336 609. Another possible technical alternative for the disinfection of raw or partially treated wastewater is the use of metals such as silver (Ag) and copper (Cu). However, there is little information available on this subject.
It should be noted that as the wastewater moves forward in the treatment train its faecal coliform content as well as its nutritional content diminishes. For this reason, if the adequate disinfection of wastewater at the early stages of the treatment is achieved using low concentrations of metals, it would be possible to preserve the nutrients and this would represent an advantage when used in agricultural irrigation, while avoiding the creation of carcinogenic compounds associated with the addition of chlorine (Keraita et al., 2008; Luna-Pabello et al., 2009). Before widespread application can be recommended, however, economic feasibility studies need to be conducted. Nevertheless, the alternative remains potentially interesting for developing countries.
Conclusions
In the case of NMA, for alginate beads containing Ag2O and combinations of Fe2O3-Ag2O and Cu2O-Ag2O, 30 min of contact time were required for inactivating 100% of E. coli at a concentration of 105 CFU/mℓ. For beads containing the Fe2O3-Cu2O-Ag2O mixture 13 min were required. In order to attain Category A water quality, a contact time of 15 to 30 min was required for NMA and Ag2O beads, whereas 10 to 15 min was required for Fe2O3-Ag2O y Cu2OAg2O beads. It was observed that redox potential values are closely related to the disinfection level achieved. The need to use less time to achieve the desired disinfection level is closely related to the synergistic effect of the metals present. The observed sequence of decreasing bacterial inactivation effect was as follows: Fe2O3-Cu2O-Ag2O>Fe2O3-Ag2O=Cu2OAg2O>Ag2O=NMA>Fe2O3=Cu2O=Fe2O3-Cu2O. The advantage of using alginate beads is that it allows the formation of AMA, which has a greater disinfecting capacity than NMA.
Acknowledgements
The authors acknowledge the financial support of the DGAPA (UNAM) through PAPIIT Project IN215006 and IN107209. Graduate scholarship for MMR was provided by CONACYT. The authors thank the anonymous reviewers for their valuable suggestions that greatly improved the manuscript.
References
ARANA I, SANTORUM A, MUELA A. and BARCINA I (2000) Effect of disinfection upon dissolved organic carbon (DOC) in wastewater: bacterial biossays. Letters in Appl. Microbiol. 31 157-162. [ Links ]
ARMIENTA MA and GONZÁLEZ-HERNÁNDEZ G (2007) Solid phase control on the mobility of potentially toxic elements in an abandoned lead/zinc mine tailings impoundment, Taxco, Mexico. Appl. Geochem. 22 (1) 109-127. [ Links ]
BLOOMFIELD SF (1991) Methods for assessing antimicrobial activ ity. In: Denyer SP and Hugo WB (eds.) Mechanisms of Action of Chemical Biocides. Their Study and Exploitation. Society for Applied Bacteriology Technical Series No 27. Blackwell Scientific Publications, Oxford. 1-21. [ Links ]
BRACCINI I and PEREZ S (2001) Molecular basis of Ca2+-induced gelation in alginates and pectins: The egg-box model revisited. Biomacromolecules 2 1089-1096. [ Links ]
CASSELLS JM, YAHYA MT, GERBA CP and ROSE JB (1995) Efficacy of combined system of copper and silver and free chlorine for inactivation of Naegleria fowleri amoebas in water. Water Sci. Technol. 31 119-122. [ Links ]
CHALOUPKA K, MALAM Y and SEIFALIAN AM (2010) Nano silver as a new generation of nanoproduct in biomedical applica tions. Trends Biotechnol. 28 (11) 580-588. [ Links ]
CHO KH, PARK JE, OSAKA T and PARK SG (2005) The study of antimicrobial activity and preservative effects of nanosilver ingre dient. Electrochim. Acta 51 (5) 956-960. [ Links ]
CHOI O, DENG KK, KIM NJ, ROSS L, SURAMPALLI RY and HU Z (2008) The inhibitory effects of silver nanoparticles, silver ions, and silver chloride colloids on microbial growth. Water Res. 42 3066-3072. [ Links ]
COLLIVIGNARELLI C, BERTANZA G and PEDRAZZANI R (2000) A comparison among different wastewater disinfection systems: Experimental results. Environ. Technol. 21 1-16. [ Links ]
CRAIG M W (2001) Coping with resistance to copper/silver disinfection. Water Eng. Manage. 148 (11) 25-27. [ Links ]
DAVIES RL and ETRIS SF (1997) The development and functions of silver in water purification and disease control. Catalysis Today 36 107-114. [ Links ]
DE LA ROSA-GÓMEZ I, OLGUÍN M and ALCÁNTARA TD (2008) Bactericides of coliform microorganisms from wastewater using silver-clinoptilolite rich tuffs. Appl. Clay. Sci. 45-53. [ Links ]
EATON AD, CLESCERI LS, RICE EW, GREENBERG AE and FRANSON AH (2005) Standard Methods for the Examination of Water and Wastewater, 21st edn. American Public Health Association, Washington DC, USA. 1332 pp. [ Links ]
ESPINOSA E, ARMIENTA MA, CRUZ O, AGUAYO A and CENICEROS N (2009) Geochemical distribution of arsenic, cadmium, lead and zinc in river sediments affected by tailings in Zimapán, a historical polymetalic mining zone of México. Environ. Geol. 58 1467-1477. [ Links ]
FENG QL, WU J, CHEN GQ, CUI FZ, KIM TN and KIM JO (2000) A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J. Biomed. Mater. Res. A. 52 (4) 664-668. [ Links ]
GYÜRÉK LL and FINCH GR (1998) Modeling water treatment chemical disinfection kinetics. J. Environ. Eng. 124 (9) 783-793. [ Links ]
GANGADHARAN D, HARSHVARDAN K, GNANASEKAR G, DIXIT D, POPAT KM and ANAND PS (2010) Polymeric microspheres containing silver nanoparticles as a bacterial agent for water disinfection. Water Res. 44 5481-5487. [ Links ]
HOLT KB and BARD AJ (2005) Interaction of Silver (I) ions with the respiratory chain of Escherichia coli: An electrochemical and scanning electrochemical microscopy study of the antimicrobial mechanims of micromolar Ag+. Biochem. 44 13214-13223. [ Links ]
INOUE Y, HOSHINO M, TAKAHASHI H, NOGUCHI T, MURATA T KANZAKI Y, HAMASHIMA H and SASATSU M (2002) Bactericidal activity of Ag-zeolite mediated by reactive oxygen species under aerated conditions. J. Inorg. Biochem. 92 37-42. [ Links ]
JIANG JQ, WANG S and PANAGOULOPOULOS A (2006a) The explotation of potassium ferrate (VI) as a disinfectant/coagulant in water and wastewater treatment. Chemosphere. 63 212-219. [ Links ]
JIANG JQ, PANAGOULOPOULOS A, BAUER M and PEARCE P (2006b) The application of potassium ferrate for sewage treatment. J Environ. Manage. 79 215-220. [ Links ]
JIANG JQ, WANG S and PANAGOULOPOULOS A (2007) The role of potassium ferrate (VI) in the inactivation of Escherichia coli and in the reduction of COD for water remediation. Desalination 210 266-273. [ Links ]
KAWASHITA M, TODA S, KIM HM, KOKUBO T and MASUDA N (2003) Preparation of antibacterial silver-doped silica glass micro spheres. J. Biomed. Mater. Res. 66A (2) 266-274. [ Links ]
KIM J, CHO M, OH B, CHOI S and YOON J (2004) Control of bacterial growth in water using synthesized inorganic disinfectant. Chemosphere 55 775-780. [ Links ]
KIM JY, LEE C, CHO M and YOON J (2008) Enhanced inactivation of E. coli and MS-2 phage by silver ions combined with UV-A and visible light irradiation. Water Res. 42 356-362. [ Links ]
KERAITA B, JIMENEZ B and DRECHSEL P (2008) Extent and implications of agricultural reuse of untreated, partly treated and diluted wastewater in developing countries. CAB Reviews: Perspect. Agric., Vet. Sci., Nutr. Nat. Resourc. 3 (58) 1-15. [ Links ]
LIBERTI L, LOPEZ A, NOTARNICOLA M, BARNEA N, PEDAH-ZUR R and FATTAL B (2000) Comparison of advanced disinfecting methods for municipal wastewater reuse in agriculture. Water Sci. Technol. 42 215-220. [ Links ]
LI Q, MAHENDRA S, LYON DY, BRUNET L, LIGA VM, LI D and ALVAREZ PJJ (2008) Antimicrobial nanomaterials for water disinfection and microbial control: Potential applications and implications. Water Res. 42 4591-4602. [ Links ]
LUKHELE LP, KRAUSEI RWM, MAMBA BB and MOMBA MNB (2010) Synthesis of silver impregnated carbon nanotubes and cyclodextrin polyurethanes for the disinfection of water. Water SA 36 (4) 433-436. [ Links ]
LUNA-PABELLO VM, MIRANDA-RÍOS M, JIMÉNEZ B and ORTA T (2009) Effectiveness of the use of Ag, Cu and PAA to disinfect municipal wastewater. Environ. Technol. 30 (2) 129-139. [ Links ]
METCALF E and EDDY C (2004) Wastewater Engineering: Treatment and Reuse (4th edn.). McGraw Hill, Washington DC, USA. 1819 pp. [ Links ]
MIRANDA-RÍOS M and LUNA-PABELLO VM (2002-2003) Eliminación de Escherichia coli de aguas residuales pretratadas biológicamente empleando Terreros de minas de plata. Anuario Latinoamericano de Educación Química (ALDEQ). San Luis Argentina. XVI (XVI) 247- 250. [ Links ]
MILAN, Z, DE LAS POZAS C, CRUZ M, BORJA R, SANCHEZ E, ILANGOVAN K, ESPINOSA Y and LUNA B (2001) The removal of bacteria by modified natural zeolites. J Environ. Sci. Health A 36 (6) 1073-1087. [ Links ]
ORTA DE VELÁSQUEZ MT, YÁÑEZ-NOGUEZ I, JIMÉNEZ-CISNEROS B and LUNA-PABELLO VM (2008) Adding silver and copper to hydrogen peroxide and peracetic acid in the disinfection of an advanced primary treatment effluent. Environ. Technol. 29 (11)1209-1217. [ Links ]
PARK HG, KIM TW, CHAE MY and YOO IK (2007) Activated carbon-containing alginate adsorbent for the simultaneous removal of heavy metals and toxic organics. Process Biochem. 42 1371-1377. [ Links ]
PARK HJ, KIM JY, KIM J, LEE JH, HAHN JS, GU M B and YOON J (2009) Silver-ion-mediated reactive oxygen species generation affecting bactericidal activity. Water Res. 43 1027-1032. [ Links ]
PEDAHZUR R, LEV O, FATTAL B and SHUVAL HI (1995) the interaction of silver ions and hydrogen peroxide in the inactivation of E. coli: A preliminary evaluation of a new long acting residual drinking water disinfectant. Water Sci. Technol. 31 123-129. [ Links ]
PRETORIUS PC and PRETORIUS WA (1999) Disinfection of purified sewage effluent with monochloramine. Water SA 25 (4) 463-47. [ Links ]
RIVERA-GARZA M, OLGUÍN MT, GARCÍA-SOSA I, ALCÁN-TARA D and RODRÍGUEZ-FUENTES G (2000) Silver supported on natural Mexican zeolite as an antibacterial material. Microporous Mesoporous Mater. 39 431-444. [ Links ]
ROHR U, WEBER S, SELENKA F and WILHELM M (2000) Impact of silver and copper on the survival of amoebae and ciliated protozoa in vitro. Int. J. Hyg. Environ. Health 203 (1) 87-89. [ Links ]
ROMERO FM, ARMIENTA MA, GUTIÉRREZ ME and VILLA-SEÑOR G (2008) Factores geológicos y climáticos que determinan la peligrosidad y el impacto ambiental de jales mineros. Rev. Int. Contam. Ambient. 24 (2) 43-54. [ Links ]
SCHINK T and WAITE TD (1980) Inactivation of f2 virus with Ferrate (VI). Water Res. 14 1705-1717. [ Links ]
SHARMA KV, KAZAMA F, JIANGYONG H and RAY AK (2005) Ferrates (iron(VI) and iron (V)): Environmentally friendly oxi dants and disinfectants. J. Water Health. 3 (11) 45-58. [ Links ]
SILVA-MARTÍNEZ S, ALVAREZ-GALLEGOS A and MARTÍNEZ E (2004) Electrolytically generated silver and copper ions to treat cooling water: an environmentally friendly novel alternative. Int. J. Hydrogen Energ. 29 921-932. [ Links ]
SMEDLEY PL (2008) Sources and distribution of arsenic in groundwater and aquifers. In: Appelo T (ed.) Arsenic in Groundwater: A World Problem. Proc. Seminar, Utrecht, 29 November 2006. Netherlands National Committee of the IAH (International Association of Hydrogeologists). 4-32. [ Links ]
SILVESTRY-RODRIGUEZ N, SICAIROS-RUELAS EE, GERBA P CH and BRIGHT KR (2007) Silver as a disinfectant. Rev. Environ. Contam. Toxicol. 191 23-45. [ Links ]
WHO (1989) Health Guidelines for the Use of Wastewater in Agriculture and Aquaculture, Technical Report Series No. 778. World Health Organization, Geneva. [ Links ]
YAMANAKA M, HARA K and KUDO J (2005) Bactericidal actions of silver ion solution on Escherichia coli, studied by energyfiltering transmission electron microscopy and proteomic analysis. Appl. Environ. Microb. 71 (11) 7589-7593. [ Links ]
YI Y, WANG Y and LIU H (2003) Preparation of new crosslinked chitosan with crown ether and their adsorption for silver ion anti bacterial activities. Carbohydr. Polym. 53 425-430. [ Links ]
YOU Y, HAN H, CHIU PC and JIN Y (2005) Removal and inactivation of waterbone viruses using zerovalent iron. Environ. Sci. Technol. 39 9263-9269. [ Links ]
Received 16 January 2010; accepted in revised form 1 February 2011.
* To whomall correspondence should be addressed. + (52) 56 22 37 63; fax: + (52) 56 22 37 63; e-mail: victormlp@yahoo.com














