Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Water SA
On-line version ISSN 1816-7950
Print version ISSN 0378-4738
Water SA vol.36 n.5 Pretoria Oct. 2010
Sonochemical degradation of the antibiotic cephalexin in aqueous solution
Weilin GuoI,*; Hongzhi WangI; Yahui ShiI; Guangyou ZhangII
ISchool of Resources and Environment, University of Jinan, Jinan 250022, China
IISchool of Chemistry and Chemical Engineering, University of Jinan, Jinan 250022, China
ABSTRACT
The degradation of cephalexin in aqueous solution under ultrasound irradiation was investigated. Biodegradability of the solution was evaluated by the BOD5/COD ratio, which was raised from zero to 0.36 after ultrasound treatment, indicating that the ultrasound irradiation process is a successful pre-treatment step to improve the biodegradability of cephalexin solution. The influences of ultrasound power and pH value on the degradation of cephalexin were studied. It was found that the optimal ultrasound power for cephalexin degradation in the system was 200 W and the rate of cephalexin degradation was maximal in the pH range of 6.5 to 8.5. The degradation kinetics of cephalexin in aqueous solution under various operational conditions was also investigated. It was found that the degradation of cephalexin follows a pseudo-first order kinetics.
Keywords: antibiotic, biodegradability, cephalexin, degradation, ultrasound
Introduction
The wide application of antibiotics in human and veterinary medicine has led to large-scale dissemination of refractory and even toxic pollutants in the environment. In many countries, a multitude of extremely resistant antibiotics have been found in treated sewage, industrial effluent, the aquatic environment and even in drinking water (Klavarioti et al., 2009). They are extremely resistant to biological degradation processes and because of their continuous input they could remain in the environment for a long time; their presence in the environment is considered dangerous in both low and high concentrations.
Recently, a variety of treatment methods like advanced oxidation processes (AOPs) (Klavarioti et al., 2009; Isariebel and Carine 2009), anaerobic treatment (Amin et al., 2006), powdered activated carbon adsorption (Choi et al., 2008), etc., have been suggested for the treatment of most industrial effluents containing toxic organic chemicals. The subject of this paper is the use of ultrasound as an AOP for the degradation of antibiotics in wastewater. Sonolysis has proven to be an effective method for degrading organic effluents into less toxic compounds and in many cases even completely mineralises the compounds. However, the time-scale and the dissipated power necessary to obtain complete mineralisation of the pollutants in the case of ultrasound treatment are not economically acceptable. Hence ultrasound is found more effective when used in combination with other conventional treatment processes than as a stand-alone process (Torres et al., 2009).
It is well known that biological treatment is perhaps less costly and more environmentally friendly than any other destructive treatment. Therefore, several papers have reported that sonochemical degradation could be successfully used as a pre-treatment to enhance the biodegradability of wastewater, but not for the complete mineralisation of the pollutants (Klavarioti et al., 2009; Torres et al., 2009; Sangave and Pandit, 2004).
Cephalexin, as one of the most popular antibiotics, is produced in great quantities. Nowadays this drug is ubiquitously present in the aquatic environment due to its resistance to biodegradation. Considered from the perspective of economy and technology, ultrasound irradiation processes should be used as a pre-treatment technique to enhance the biodegradability of the solution; once biodegradability has been achieved, the effluent is transferred to a cheaper biological treatment (Arslan- Alaton and Akmehmet, 2002).
The purposes of this study were:
• To investigate the decomposition of cephalexin under ultrasound irradiation
• To evaluate the enhanced biodegradability of the solution
• To study the effects of various operating conditions on removal efficiency
According to our knowledge, this is the first time that ultrasound has been used to improving the biodegradability of wastewater containing cephalexin.
Experimental
Cephalexin hydrate (CAS No. 15686-71-2) was provided by Sigma. Its structural formula is shown in Fig. 1. All other chemicals were of analytical grade and used as received. The water used in all experiments was purified by a Milli-Q system.

Sonication was carried out with an ultrasonic generator (JY92, Ningbo Xinzhi Instrument Co., China), equipped with a 24 kHz transducer and a titanium horn tip of 2.0 cm diameter.
The ultrasonic generator was operated at 200 W (calorimetric power: 17.3 W). The tip of the probe was placed 2 cm into the liquid layer. The sonication was administered in a pulse mode of 30 s on and 15 s off.
All tests were carried out in a 100 m cylindrical glass reaction vessel. A constant temperature of 25±2C was maintained during the sonication by circulating water through a jacket around the sonication cell. In all cases, 50 m
cylindrical glass reaction vessel. A constant temperature of 25±2C was maintained during the sonication by circulating water through a jacket around the sonication cell. In all cases, 50 m of cephalexin solution with a typical concentration of 20 mg/
of cephalexin solution with a typical concentration of 20 mg/ was added into the jacketed reactor. The pH value of each reaction solution was adjusted to the desired level using appropriate concentrations of sulphuric acid or sodium hydroxide solutions. At different time intervals, samples were withdrawn from the reactor by syringe and filtered through 0.45 μm membranes.
was added into the jacketed reactor. The pH value of each reaction solution was adjusted to the desired level using appropriate concentrations of sulphuric acid or sodium hydroxide solutions. At different time intervals, samples were withdrawn from the reactor by syringe and filtered through 0.45 μm membranes.
The analytical methods used for determination of COD and BOD5 were 5220D and 5210D, respectively (APHA-AWWA-WEF 1998). The closed reflux colorimetric method was used to determine COD values. Test solution (2 ml) was pipetted into the dichromate reagent and digested at 150ºC for 2 h in an Aqualytic AL 32 COD reactor. COD concentration was measured colorimetrically at λ=430 nm using an Aqualytic PCcompact COD vario photometer. BOD5 was measured using an automatic BOD measurement apparatus (Hach BODTrak, Hach Co., USA). A measured sample of wastewater and nutrient buffer was poured into each of 6 BODTrak bottles. The bottles were then sealed and incubated on the BODTrak instrument, which automatically monitored the BOD continually over 11 days. The samples were continually stirred at 20ºC using magnetic stir bars.
Results and discussion
Sonolysis of cephalexin in aqueous solution
The degradation of cephalexin in aqueous solution under ultrasound irradiation was studied (Fig. 2). Sonolysis was performed under the following conditions: ultrasonic frequency set at 24 kHz, applied power set at 200 W, temperature maintained at 25ºC, and pH value of 7.5 under air atmosphere. As can be seen in Fig. 2, cephalexin could be destructed by ultrasound and the COD value of the solution decreased gradually along with reaction time. At the end of the experiment (150 min) the COD was reduced to about 70% of its initial value.

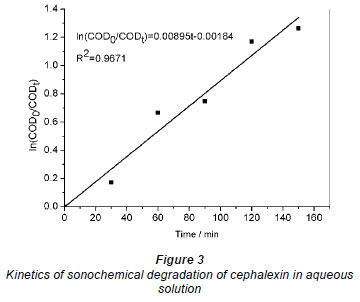
The degradation kinetics of cephalexin in aqueous solution by sonication is shown in Fig. 3. It was found that the degradation of cephalexin in aqueous solution follows a pseudo-first order kinetics. The kinetic rate constant for the degradation of cephalexin and the correlation coefficient (R2 values) are 0.00895 min-1 and 0.9671, respectively. The R2 value indicates that the degradation of cephalexin under ultrasound irradiation indeed follows pseudo-first order kinetics.
Effect of ultrasonic irradiation on biodegradability
As we all know, ultrasonic treatment alone may not be suitable for decontaminating completely organic pollutants. The process efficiency may be improved by coupling with biological treatment, which will carry on mineralisation as far as possible. Therefore, a sonication process, as a previous step of an ensuing biological treatment, was applied in order to improve the biodegradability of wastewater (Sangave and Pandit, 2004).
In the present study, the solution of cephalexin was sonicated and then the changes of the BOD5/COD ratio were measured to evaluate the biodegradability of the solution (Fig. 4). The BOD5 of 20 mg/ cephalexin solution was about zero, indicating that cephalexin is not readily biodegradable. Contaminants with a ratio of BOD5/COD>0.3 are generally accepted as biodegradable, while those with ratios of between 0.2 and 0.3 units are partially biodegradable. The results presented in Fig. 4 show that the ultrasonic action significantly enhanced the biodegradability of the solution. The BOD5/COD ratio was improved from 0 to 0.36 after 60 min of sonolysis. These results provide evidence that ultrasound transforms cephalexin, in a rapid and quite selective way, into more biodegradable intermediates that will be degraded in a posterior biological step. This is an interesting advantage because, in fact, the most cost-efficient way for applying sonolysis is not to aim for the mineralisation of the organic pollutant but to transform its chemical structure in order to make it less toxic and more biodegradable. It should be emphasized that the sonication time and power should be kept as low as possible in order to reduce the wastewater treatment costs and make the sonolysis suitable for industrial application.
cephalexin solution was about zero, indicating that cephalexin is not readily biodegradable. Contaminants with a ratio of BOD5/COD>0.3 are generally accepted as biodegradable, while those with ratios of between 0.2 and 0.3 units are partially biodegradable. The results presented in Fig. 4 show that the ultrasonic action significantly enhanced the biodegradability of the solution. The BOD5/COD ratio was improved from 0 to 0.36 after 60 min of sonolysis. These results provide evidence that ultrasound transforms cephalexin, in a rapid and quite selective way, into more biodegradable intermediates that will be degraded in a posterior biological step. This is an interesting advantage because, in fact, the most cost-efficient way for applying sonolysis is not to aim for the mineralisation of the organic pollutant but to transform its chemical structure in order to make it less toxic and more biodegradable. It should be emphasized that the sonication time and power should be kept as low as possible in order to reduce the wastewater treatment costs and make the sonolysis suitable for industrial application.

As can be seen in Fig. 4, the biodegradability was initially enhanced by sonication, but this was followed by a decrease in biodegradability. The reason for this may be that the biodegradable intermediates are easily removed under ultrasound irradiation. Similar results have also been reported in previous studies (Cokgor et al., 2004).
Effect of ultrasound power on the degradation of cephalexin
The effect of ultrasonic power on the sonolysis of cephalexin was investigated. As shown in Fig. 5, the sono-degradation rate of cephalexin in aqueous solution is strongly dependent on ultrasound power. The degradation rate of cephalexin increases greatly with an increase in power, reaches a maximum at about 200 W, and then decreases rapidly with increasing power. This indicates clearly that the output power of 200 W will be the optimum to obtain a maximum degradation rate of cephalexin. Sivakumar and Pandit (2001) have also obtained similar results with decomposition of a dye solution.

With increasing the output power of ultrasound, there will be an increase in the number of cavities generated and hence the cumulative pressure pulse (number of cavities multiplied by the collapse pressure due to a single cavity) will also increase at higher levels of power (Sivakumar and Pandit, 2001). This results in a corresponding increase in the extent of degradation of cephalexin. At higher output power, however, a large number of gas bubbles exist in the solution, which scatter the sound waves to the walls of the vessel or back to the transducer. Thus less energy is dissipated in the liquid due to cavitational activity, although the vessel is exposed to higher and higher power. Again, bubble cloud formation may occur at the surface of the horn if the power is too high, resulting in attenuation of the sound wave. Also, it may happen that, due to the very high number of cavities per unit volume of liquid, there is a coalescence of the cavities resulting in the formation of a larger cavity (the collapse pressure is inversely proportional to the size of the cavity).
Therefore, the enhancement of the sonochemical effect with increasing power dissipation in the system is only obtained until an optimum power value, beyond which the rates of degradation decrease with increased output power.
Effect of pH on the degradation of cephalexin by sonochemistry
The initial pH of the medium is an important parameter for the degradation of chemical pollutants under ultrasound irradiation. The influence of pH on the degradation rate of cephalexin was investigated at pH range 4.5-10.0 using 20 mg/ cephalexin aqueous solution; the results are shown in Fig. 6. It was observed that the degradation rate of cephalexin in aqueous solution is strongly dependent on pH value. The degradation rate of cephalexin is enhanced with increasing pH value from 4.5 to 6.5, and there is almost no change in the pH range of 6.5-8.5. Further increase of pH to about 10.0 leads to a great reduction of degradation rate. The removal rate of COD is only 11.47% at pH 10.0.
cephalexin aqueous solution; the results are shown in Fig. 6. It was observed that the degradation rate of cephalexin in aqueous solution is strongly dependent on pH value. The degradation rate of cephalexin is enhanced with increasing pH value from 4.5 to 6.5, and there is almost no change in the pH range of 6.5-8.5. Further increase of pH to about 10.0 leads to a great reduction of degradation rate. The removal rate of COD is only 11.47% at pH 10.0.
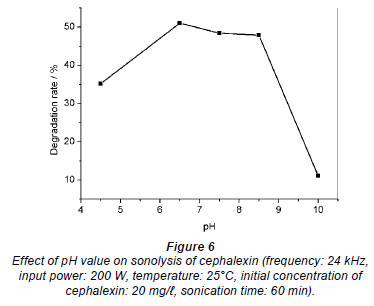
The effect of pH on the rate of sono-degradation is dependent on the state of the pollutant molecule, i.e. whether the pollutant is present as ionic species or as a molecule (Ghodbane and Hamdaoui, 2009). Cephalexin is a non-volatile compound and the region of sono-decomposition would be at the cavitation bubble interface where the hydroxyl radical concentration is at a maximum. The change in the solution pH results in a change in the hydrophobic property of cephalexin, because cephalexin is a zwitterion, i.e. the molecule contains both a basic and an acidic group. In the pH range 4.5-6.5, cephalexin mainly exists in zwitterionic form in solution. Therefore, cephalexin exists in higher concentrations at the bubble surface and thus is more readily subjected to the hydroxyl radical attack. However, at lower and higher pH conditions, cephalexin mainly exists in cationic or anionic form in solution. Therefore, the hydrophilicity and solubility of cephalexin are enhanced, and thus the decomposition is carried out in the bulk of the solution where there is a lower concentration of hydroxyl radicals, because only about 10% of the hydroxyl radical generated in the bubble can diffuse into the bulk solution (Goel et al., 2004).
Additionally, a high pH value may create more free radical scavengers (i.e., CO3 2-, HCO3 -) and results in the decrease in the concentration of hydroxyl radical (•OH) (Wang et al., 2009). This may be another reason for the decrease of removal rate of cephalexin at higher pH value.
Conclusions
The ultrasound irradiation process was found to be a successful treatment for both the removal of, and improvement of the biodegradability of, cephalexin in wastewater. Ultrasound can effectively decompose cephalexin in aqueous solution and the extent of degradation depends strongly on the operating conditions, such as ultrasound power and pH value of the medium. Sonolysis of cephalexin follows a pseudo-first order kinetics and ultrasound noticeably increases the biodegradability of the cephalexin solution.
These conclusions lead us to suggest integrating the technology of ultrasound and biological treatment as an optimal strategy for antibiotic removal from industrial wastewater.
Acknowledgments
The authors greatly acknowledge The Foundation for Excellent Young Scientists of Shandong Province for financial support (2008BS09019).
References
AMIN MM, ZILLES JL, GREINER J, CHARBONNEAU S, RASKIN L and MORGENROTH E (2006) Influence of the antibiotic erythromycin on anaerobic treatment of a pharmaceutical wastewater. Environ. Sci. Technol. 40 (12) 3971-3977. [ Links ]
APHA-AWWA-WEF (1998) Standard Methods for the Examination of Water and Wastewater (20th edn.). American Public Health Association, Washington DC. [ Links ]
ARSLAN-ALATON I and AKMEHMET BI (2002) Biodegradability Assessment of ozonated raw and biotreated pharmaceutical wastewater. Arch. Environ. Con. Tox. 43 (4) 425-431. [ Links ]
CHOI K, KIM S and KIM S (2008) Removal of tetracycline and sulfonamide classes of antibiotic compound by powdered activated carbon. Environ. Technol. 29 (3) 333-342. [ Links ]
COKGOR EU, ALATON IA, KARAHAN O, DOGUEL S and ORHON D (2004) Biological treatability of raw and ozonated penicillin formulation effluent. J. Hazard. Mater. 116 (1-2) 159-166. [ Links ]
GHODBANE H and HAMDAOUI O (2009) Intensification of sonochemical decolorization of anthraquinonic dye Acid Blue 25 using carbon tetrachloride. Ultrason. Sonochem. 16 (4) 455-461. [ Links ]
GOEL M, HONGQIANG H, MUJUMDAR AS and RAY MB (2004) Sonochemical decomposition of volatile and non-volatile organic compounds - a comparative study. Water Res. 38 (19) 4247-4261. [ Links ]
ISARIEBEL QP and CARINE J (2009) Sonolysis of levodopa and paracetamol in aqueous solutions. Ultrason. Sonochem. 16 (5) 610-615. [ Links ]
KLAVARIOTI M, MANTZAVINOS D and KASSINOS D (2009) Removal of residual pharmaceuticals from aqueous systems by advanced oxidation processes. Environ. Int. 35 (2) 402-417. [ Links ]
SANGAVE PC and PANDIT AB (2004) Ultrasound pre-treatment for enhanced biodegradability of the distillery wastewater. Ultrason. Sonochem. 11 (3-4) 197-203. [ Links ]
SIVAKUMAR M and PANDIT AB (2001) Ultrasound enhanced degradation of Rhodamine B: optimization with power density. Ultrason. Sonochem. 8 (3) 233-240. [ Links ]
TORRES RA, MOSTEO R, PETRIER C and PULGARIN C (2009) Experimental design approach to the optimization of ultrasonic degradation of alachlor and enhancement of treated water biodegradability. Ultrason. Sonochem. 16 (3) 425-430. [ Links ]
WANG X, WANG J, GUO P. GUO W and WANG C (2009) Degradation of rhodamine B in aqueous solution by using swirling jet-induced cavitation combined with H2O2. J. Hazard. Mater. 169 (1-3) 486-491. [ Links ]
Received 4 February 2010; accepted in revised form 3 September 2010.
* To whom all correspondence should be addressed. +86 531 82765719; fax: +86 531 82765719; e-mail: chm_guowl@ujn.edu.cn














