Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Water SA
On-line version ISSN 1816-7950
Print version ISSN 0378-4738
Water SA vol.36 n.4 Pretoria Jul. 2010
The effect of silica concentration on the biosorption of Cu2+ and Co2+ from aqueous solutions mediated by strains of Bacillus
NP DlaminiI; BB MambaI,*; AF Mulaba-BafubiandiII
IDepartment of Chemical Technology, University of Johannesburg, PO Box 17011, Doornfontein, 2028, South Africa
IIDepartment of Extraction Metallurgy, University of Johannesburg, PO Box 526, Wits 2050, South Africa
ABSTRACT
Bacillus strains were isolated from a mine tailings dump in Nigel town, south-east of Johannesburg. These were then grown at 37(±0.5)ºC in a trace element-agitated liquid media. The effects of pH, contact time, initial ion concentration and the presence of co-cations were studied to ascertain the optimal conditions for biosorption to take place. Test solutions contained 0.002 M, 0.07 M and 0.2 M of either copper or cobalt ions. The Bacillus strains removed the copper and cobalt more efficiently from solutions of low concentration (0.002 M and 0.07 M) than from solutions of high concentration (0.2 M) over a 48 h period. Maximum biosorption was obtained at pH 6.5 and 5.5 for copper and cobalt solutions, respectively. The presence of silica led to an initial increase in both copper and cobalt biosorption, though higher concentrations of silica resulted in a decrease in metal uptake by Bacillus strains.
Keywords: biosorption, Bacillus, silica, copper, cobalt
Introduction
Pollution of the environment with heavy metals, mainly as a result of industrialisation, has become a challenge for scientists and environmental health practitioners. Reported methods of metal removal from an aqueous solution include adsorption (biosorption, chemisorption or specific adsorption) and precipitation in the form of metal hydroxides (Fe3+, Cr3+ and Al3+), metal carbonates (Fe2+, Mn2+) or metal sulphides (Pb2+, Co2+, Cd2+, Cu2+, Ni2+, Fe2+, Zn2+) (Bojinova and Velkovar, 2001) ion exchange, oxidation, reduction and reverse osmosis (Bueno et al., 2008).
Metal uptake by biosorption is complex and may involve the contribution of diffusion, adsorption, chelation, complexation, coordination and microprecipitation mechanisms, depending on the specific biomass or substrate (Veglio and Beolchini, 1997). Therefore, this biological process can be affected by other chemical and physical variables like pH, ionic strength, biomass concentration and the presence of other heavy metals in solution. All of these factors have to be taken into account in order to understand the mechanism of biosorption and to optimise the operating conditions.
In this study, copper and cobalt were chosen for biosorption experiments due to their wide use in industry and their potential water pollution impact. Hence, the main objective of this research was to evaluate the impact of silica on the adsorption of Co and Cu from an aqueous solution by selected micro-organisms. The aqueous solutions used in this study simulate the natural composition of an oxidised Co-Cu mixed ore with a siliceous gangue, after leaching the mineral ore using an acid such as sulphuric acid. Thus, the possible effect of the remaining silica on the Cu and Co solution during the biosorption process is explored, with the aim of proposing the use of micro-organisms to metallurgical processing divisions of mining companies, as a viable and less expensive means for extracting metals from leached solutions. While chemical technologies pertaining to adsorption are associated with sludge generation and large capital demands, the biosorption process has the advantages of low operating costs, a high probability of metal recovery and potential biosorbent regeneration (Wase and Foster, 1997).
Methods
Microorganisms and growth conditions
Bacillus strains were isolated from soil and water sampled from mine dumps in Nigel, a small mining town founded in 1909 and located south-east of the city of Johannesburg, South Africa. The bacterial strains were then grown at 37(±0.5)ºC (carefully controlled) in agitated liquid media containing (NH4)2SO4 (1.0 g -1), KCl (0.1 g
-1), KCl (0.1 g -1), K2HPO4 (0.5 g
-1), K2HPO4 (0.5 g -1), MgSO4 (0.5 g
-1), MgSO4 (0.5 g -1), elemental sulphur (8.5 g
-1), elemental sulphur (8.5 g -1) and FeSO4.7H2O (7.5 g
-1) and FeSO4.7H2O (7.5 g -1). The medium was sterilised by autoclaving, using the HUXLEY HL 341 speedy autoclave at 120(±0.05)ºC for 15 min. The pH was adjusted to 6.5 with diluted KOH solutions. Bacterial growth was allowed to proceed for 72 h with continuous stirring and thereafter the cultures were concentrated by centrifugation. From this solution a glycerol stock culture was prepared for further experiments.
-1). The medium was sterilised by autoclaving, using the HUXLEY HL 341 speedy autoclave at 120(±0.05)ºC for 15 min. The pH was adjusted to 6.5 with diluted KOH solutions. Bacterial growth was allowed to proceed for 72 h with continuous stirring and thereafter the cultures were concentrated by centrifugation. From this solution a glycerol stock culture was prepared for further experiments.
Phylogenetic analysis
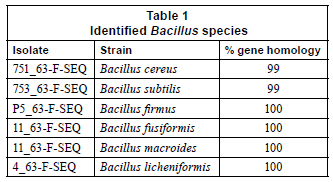
The strains used were Bacillus cereus, Bacillus subtilis, Bacillus fusiformis, Bacillus firmus Bacillus macroides and Bacillus licheniformis, as confirmed by PCR analysis.
Test solutions
The simulated laboratory experiments involved the use of 3 test solutions. These were prepared from Cu and Co salts with the addition of the impurity component (silica) in some cases. The test solutions were divided into: single metal system (Co or Cu solution), binary metal solution (Co and Cu mixed system) and multi-metal system (Co and/or Cu plus an impurity in various ratios). The solution concentrations of Cu2+ and Co2+ in the solutions were chosen arbitrarily to cover a broader spectrum; concentrations were 0.002 M, 0.07 M and 0.2 M for both Cu2+ and Co2+. The ratios of these ions were varied depending on the category in testing.
Batch biosorption experiments
The factors that affect the adsorption rate were examined in a batch system. All experiments were carried out with the microorganism suspension in Erlenmeyer flasks in an incubator at 37(±0.5)ºC with constant shaking to elucidate optimum conditions for adsorption. The contents of the flasks were filtered and the filtrates were diluted (10 m was pipetted into a 100 m
was pipetted into a 100 m volumetric flask and filled up to the mark with distilled water.) This was done in order to obtain lower concentrations which could be analysed by flame absorption spectroscopy to determine residual metal concentrations. The percentage of metal adsorbed by biomass was calculated as follows:
volumetric flask and filled up to the mark with distilled water.) This was done in order to obtain lower concentrations which could be analysed by flame absorption spectroscopy to determine residual metal concentrations. The percentage of metal adsorbed by biomass was calculated as follows:
% metal adsorbed by biomass = 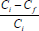 x 100 - MAS
x 100 - MAS
where:
Ci is the initial metal concentration, Cf is the final metal concentration and MAS is the total amount of metal adsorbed by impurities, if any, which in this study was silica.
Control experiments
Control experiments were conducted to monitor other possible factors which may affect metal concentrations in solution, for example, precipitation. This was done by setting up a similar experiment but with no bacterial strains added to the solutions, i.e. solutions with the3 different concentrations charged into Erlenmeyer flasks in an incubator at 37(±0.5)ºC, with constant shaking in order to elucidate optimum conditions for adsorption (contact time, pH, initial metal concentration). Contact times between 6 and 72 h were examined and the results obtained were compared with results from relevant literature. The pH range of pH 3.5 to 9 was also based on standard literature procedures.
Bacterial interaction with metal species
Interaction of bacteria with the different categories of metal species was studied using scanning electron microscopy (SEM) (model SEM Joel JSM 5600). Imaging of bacteria required fixing. Fixing was achieved by first adding a drop of the sample onto a microscope slide and then covering it with a few drops of 2.5% gluta-aldehyde in phosphate buffer and then drying the sample at 60ºC. All samples were carbon-coated before SEM imaging. Transmission electron microscopy (TEM) was also performed, using a Joel 100S TEM model. This was done to monitor differences in the manner in which the bacteria interacted with the metal species in the different treatment protocols, to confirm the presence of metal species within the microorganisms, and to investigate the manner in which metal uptake occurred.
Sorption isotherms
The Langmuir equation represents one of the first theoretical treatments of non-linear sorption and suggests that uptake occurs on a homogenous surface by monolayer sorption without any interaction between adsorbed molecules. Langmuir isotherms were plotted for the single element category. The Langmuir equation is expressed as follows:
x/m = (ab/Cr)/ (1+bCr)
where:
Cr is the equilibrium concentration of the metal in solution remaining after adsorption (mg/), x/m is the number of metal ions adsorbed onto the bacteria, a and b are Langmuir constants related to adsorption capacity and adsorption energy, respectively. Constant a is the maximum sorption capacity and represents single layer coverage of the adsorbent (bacteria) in contact with the adsorbate (metal solution). The variable b represents enthalpy of adsorption and changes with temperature.
To draw the Langmuir plots, the equation was first linearised as follows:
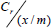 = (1 / ab) + (1/a)Cr
= (1 / ab) + (1/a)Cr
1/(x/m) is then plotted against 1/Cr. to obtain a straight line.
Another mathematical equation used to fit the data obtained in this study was the Freundlich equation. This model is known for its good fit of data over a wide range of concentrations (Ho and Mckay, 1998). It uses an equation that incorporates the heterogeneity of the surface of the ion exchanger and the exponential distribution of active sites and their energies. The Freundlich equation is given by:
LogCads = LogK + 1/ nLogCeq
where:
Ce is the equilibrium concentration in µg·g-1 and Cads is the number of metal ions adsorbed onto the bacteria. K is related to temperature and n is a characteristic constant for any system under study.
Results
Isolation of the bacteria
The bacteria showed positive results when grown in a trace-element medium. This was observed by the change in colour of the medium from dark green to reddish-orange after about 36 h, confirming that oxidation had taken place.
Bacterial species characterisation
A preliminary classification of the prokaryotes which was based on their cellular and physiological characteristics was later confirmed by phylogenetic analysis (16S rRNA gene sequence analysis) of the 6 representative isolates selected. Four of the isolates exhibited 100% homology and only isolates 753_63-F-SEQ and 751_63-F -SEQ exhibited a 99% gene homology as shown in Table 1.
Bacterial interaction with metal species
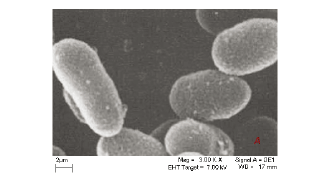
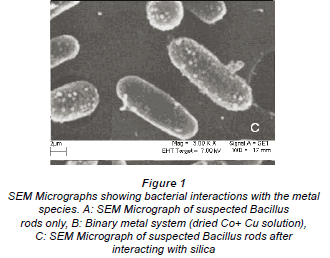
The interactions of the micro-organisms with the metal species were noted in the different categories of metal combinations i.e. single element, binary metal and multi-element matrices. This is graphically illustrated by the SEM micrographs in Fig. 1a-c.
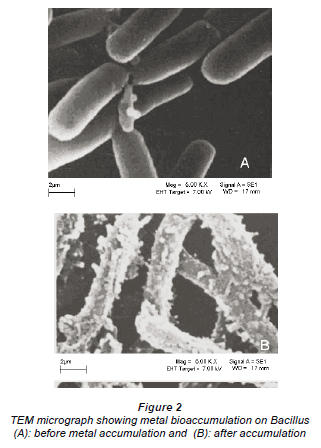
Figure 1a is a micrograph showing the Bacillus rods only, Fig. 1b shows dried Cu/Co solutions and Fig. 1c shows an image from a slide of washed cells of Bacillus after interacting with the mixed element species with the bacteria acting on it. In Fig. 1c, the intact morphology is diminished and this suggests the presence of bacteria in the matrix. The cavities observed in Fig. 1c may be as a result of ion exchange processes in the abundant active sites which were created by the presence of the silica impurity. The holes and cracks thus increase the surface area for bacterial interaction with the metallic species. Figure 2 shows metal bioaccumulation on the periplasmic wall of the Bacillus rods. It is worth noting that accumulation is higher on the longer dimension than on the shorter one.
The effect of solution pH
It is well accepted that pH has a significant effect on the solubility, speciation and biosorption capacity of heavy metals (Al-Rub et al., 2006) The increase in metal removal from solution with increase in pH can be explained using the analogy between the reaction of metal hydrolysis and the reaction between binding sites of the sorbent and the metal, where in both interactions hydrogen ions are released and substituted by the metal (Pagnanelli et al., 2003). Figure 3 shows metal uptake as a function of pH. It is observed that the best results occurred at pH 6.5 for Cu (II) and 5.5 for Co (II), where 94% and 88% were taken up by the biomass, respectively.
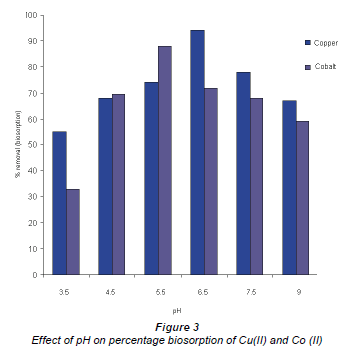
A similar trend has been reported by other researchers who have investigated the effect of pH on biosorption using different biomaterials (Kiran et al., 2005; Fiol et al., 2006; Uslu and Tanyol, 2006). The different pH binding values of these components could be as a consequence of the nature of the chemical interactions of each component with the microbial cells. At highly acidic pH values, metal ions compete for binding sites on the cell wall, which results in a lower metal uptake (Bueno et al., 2008).
The effect of contact time
The effect of contact time in the biosorption of Cu and Co by Bacillus strains was investigated by varying the contact time between the bacteria and the test solutions. The choice of the contact time range (6 h to 72 h) was based on previous literature reports. The results of these tests are shown in Fig. 4. The maximum removal of all metallic species occurred within a 48 h time period where uptakes of 94% for Cu and 83% for Co were recorded. After the 48 h contact period, an equilibrium was reached. Similar results were reported in other studies using different biosorbents for the uptake of various heavy metals, where equilibrium was established after a certain period of contact time (Kumar et al., 2006; Uslu and Tanyol, 2006; Pan et al., 2006). This was attributed to complexation of metal ions in solution which introduces competition between surface and soluble active sites for the binding of metal ions, thus resulting in a reduction in metal removal capacity.
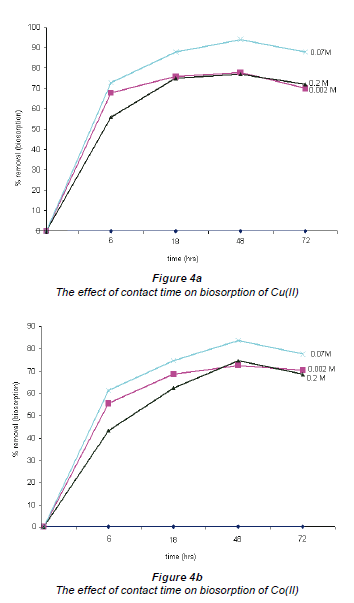
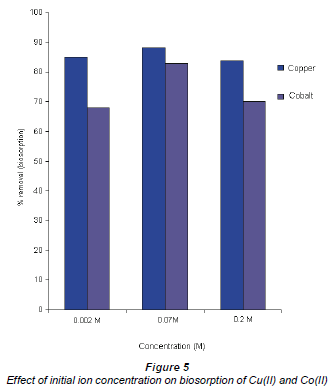
The effect of the initial concentration of Co(II) and Cu(II)
The effect of the initial concentration of Co(II) and Cu(II) ions is illustrated in Fig. 5, i.e. metal uptake as a function of the initial concentration. It is evident that an increase in equilibrium concentration leads to an increase in metal uptake. This observation is linked to the metallic concentration gradient with respect to the active and available sites of the Bacillus spp., which suggests that metal uptake increased with an increase in initial ion concentration. However, at very high metal concentrations the adsorption rate decreases. For example, cobalt shows very good removal efficiency at 0.07 M relative to 0.2 M. The suggestion is that adsorption in dilute solutions is mainly through ion exchange processes whereas in concentrated solutions adsorption occurs mainly through precipitation which is much slower in comparison to ion exchange (Zachara and Cowan, 1991;Papadopoulos and Rowell, 1988).
Sorption isotherms
The results plotted in the Langmuir and Freundlich equations are presented in Figs. 6 and 7, which depict the surface properties and affinity for metal ions of the Bacillus strains (Ho and McKay, 1998).
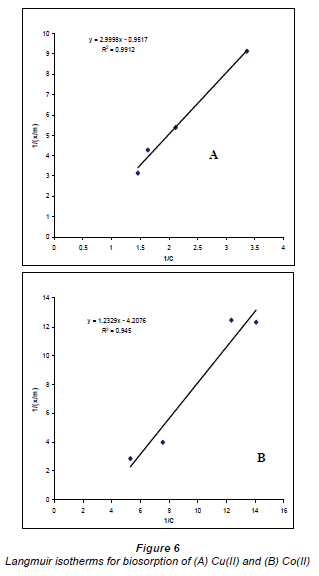
The data of the single element solutions fit the Langmuir equation very well, with R2 values > 0.9 for the dilute sulphate solutions of both Cu and Co. According to the graphs based on the Langmuir equation, Cu2+ adsorption is more favoured than Co2+ adsorption.
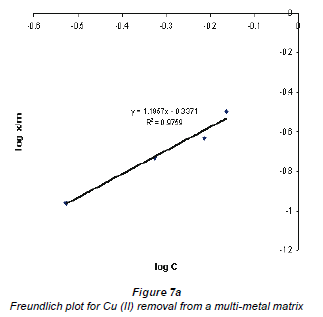
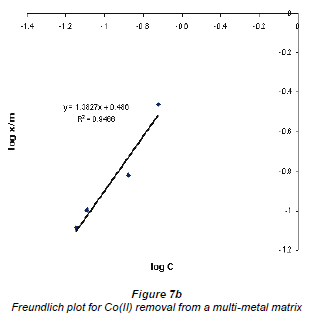
When log Cads was plotted against log Ceq , the data plotted conformed to the Freundlich equation, which had values of R2 higher than 0.9. Figures 7a and b show graphs emanating from plotting this data using the Freundlich equation for the adsorption of Cu2+ and Co2+ in a multi-element matrix, i.e. mixed Cu2+ and Co2+ in the presence of the silica impurity.
The Freundlich plots still show a better adsorption of Cu2+ compared to Co2+. This may be attributed to the nature of the 2 metal ions; although the charges on both Cu2+ and Co2+ ions are the same, their hydrated radii differ - Cu2+ has a lower hydrated radius than Co2+. Adsorption of an ion by an ion exchanger largely depends on the hydrated radius. The larger the diameter the slower its mobility and the less likely its exchange will occur (Ederm et al., 2004). It appears that a smaller amount of water molecules surrounding the cation allows for ease of adsorption.
Effect of silica impurity
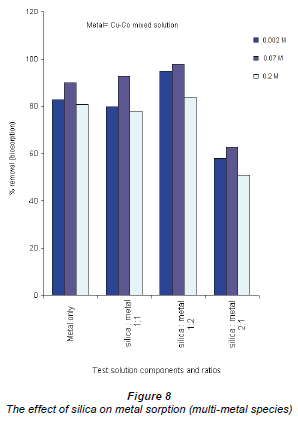
The effect of silica contamination was investigated by monitoring the uptake of Cu and Co by the Bacillus strains, using 3 test solutions: a solution containing equal amounts of the mixed Cu-Co and silica,and and 2 solutions with either of the Cu-Co/silica concentrations in varying ratios. The control experiments had only silica (not shown in the graph since it did notadd any value to the findings) or onlythe Cu-Co mixed solutions.
The presence of the silica in the solutions in all categories increased the adsorption efficiencies of both Co and Cu ions. These observations contradict conclusions drawn by Stumm and Morgan, who suggested that this increase in metal adsorption is due to the silica adsorbing the metal ions (Stumm and Morgan, 1996). In this study, it was found that silica only adsorbed negligible amounts of the metal ions. Silica has 4 silicon-oxygen bonds and may dissociate in solution by ion exchange, leaving free-floating oxygen which can be used by the bacteria for respiration thus increasing their activity.
When the silica impurity was present at a high concentration there was a significant reduction in sorption rate. This is depicted in Fig. 8 where the concentration, for example, of Cu2+ adsorbed at 0.002 Mdecreased from 96% to 64% at 0.2 M Cu2+ concentration.
Conclusions
This study has demonstrated that microorganisms can offer a viable and less costly alternative technology for the uptake of metal species from metallurgical process solutions and polluted water sources. Since biosorption is pH dependent, this study is now being extended to adjacent water sources which are being polluted as a result of acid mine drainage. Therefore, from the cloning studies that are currently underway in our laboratories, it is anticipated that novel and robust bacterial strains will be obtained and used for the sequestration of metals from aqueous solutions.
Acknowledgements
The authors gratefully acknowledge the National Research Foundation (NRF) and the University of Johannesburg for funding this project. Assistance from Mr E Fosso-Kankeu with bacterial isolation is also appreciated.
References
AL-RUB AFA, EL-NAAS MH, ASHOUR I and AL-MARZOUQI M (2006) Process Biochem. 41 457-464. [ Links ]
BOJINOVA DY and VELKOVAR RG (2001) Bioleaching of metals from mineral waste product. Acta Biotechnol. 21 275-282. [ Links ]
BUENO BY, TOREM ML, MOLINA F and DE MESQUITA LM (2008) Biosorption of lead(II), chromium(III) and copper(II) by R. opacus. Miner. Eng. 21 65-75. [ Links ]
EDERM E, KARAPINAR N and DONAT R (2004) The removal of heavy cations by natural zeolites. J. Colloid Interface Sci. 280 (2) 309-314. [ Links ]
FIOL N, VILLAESCUSA I and MARTINEZ M (2006) Sorption of Pb(II), Ni(II), Cu(II) and Cd(II) from aqueous solution by olive stone waste. Sep. Purif. Technol. 50 132-140. [ Links ]
HO YS and MCKAY G (1998) Kinetic model for lead (II) sorption on to peat. Adsorp. Sci. Technol. 16 243-255. [ Links ]
KIRAN I, SIBEL T and TAMER A (2005) Chromium (VI) biosorption characteristics of Neurospora crassa fungal biomass. Miner. Eng. 18 (7) 681-689. [ Links ]
KUMAR YP, KING P and PRASAD VS (2006) Equilibrium and kinetic studies for the biosorption system of copper(II) ion from aqueous solution using Tectona grandis L.f. leaves powder. J. Hazardous Mater. B137 1211-1217. [ Links ]
PAN J, GE X, LIU R and TANG GH (2006) Characteristic features of Bacillus cereus cell surfaces with biosorption of Pb (II) ions by AFM and FT-IR. Colloids Surfaces B: Biointerfaces 52 89-90. [ Links ]
PAGNANELLI F, ESPOSITO A, TORO L and VEGLIO F (2003) Metal speciation and pH effect on Pb, Cu, Zn and Cd biosorption onto Sphaerotilus natans: Langmuir type empirical model. Water Res. 37 627-633. [ Links ]
PAPADOPOULOS P and ROWELL D (1988) The reactions of cadmium with calcium carbonate surfaces. J. Soil Sci. 39 23-35. [ Links ]
USLU G and TANYOL M (2006) Equilibrium and thermodynamic parameters of single and binary mixture biosorption of lead (II) and copper (II) ions onto Pseudomonas putida: Effect of temperature. J. Hazardous Mater. 135 (1-3) 87-93. [ Links ]
STUMM W and MORGAN JJ (1996) Aquatic Chemistry (3rd edn.) John Willey, New York. [ Links ]
VEGLIO F and BEOLCHINI F (1997) Removal of metals by biosorption: A review. Hydromet. 44 301-316. [ Links ]
WASE DA and FORSTER CF (1997) Biosorption for Metal Ions. Taylor and Francis Ltd. UK. [ Links ]
ZACHARA JM and COWAN CT (1991) Sorption of divalent metals on calcite. Geochim. Cosmochim. Acta 55 (6) 1549-1562. [ Links ]
Received 26 October 2009; accepted in revised form 31 May 2010.
* To whom all correspondence should be addressed. +27(11) 559 6516; fax: +27(11) 559 6425; e-mail: bmamba@uj.ac.za