Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
Water SA
versión On-line ISSN 1816-7950
versión impresa ISSN 0378-4738
Water SA vol.36 no.4 Pretoria jul. 2010
The effect of conditioning with NaCl, KCl and HCl on the performance of natural clinoptilolite's removal efficiency of Cu2+ and Co2+ from Co/Cu synthetic solutions
BB MambaI,*; DW NyembeI; AF Mulaba-BafubiandiII
IDepartment of Chemical Technology, University of Johannesburg, PO Box 17011, Doornfontein 2028, South Africa
IIMinerals Processing and Technology Research Group, Department of Extraction Metallurgy, University of Johannesburg, PO Box 526, Wits 2050, South Africa
ABSTRACT
Southern African clinoptilolite's capability as an ion-exchanger with respect to Cu2+ and Co2+ was investigated in order to consider its viability in the removal of metal cations from aqueous solutions. The effect of chemical conditioning was investigated using sodium chloride (NaCl), hydrochloric acid (HCl) and potassium chloride (KCl). The most efficient activating or conditioning reagent was found to be HCl at 0.02 M concentration, followed by KCl at 0.04 M and then NaCl at 0.04 M. The worst performing clinoptilolite was the original form under the conditions described in this study and it thus served as a control. The HCl-conditioned clinoptilolite was the most efficient in metal removal (79% Co2+ and 73% Cu2+) followed by the NaCl-conditioned form (69% Co2+ and 54% Cu2+), while the KCl-conditioned form adsorbed 54% and 73% of Co2+ and Cu2+, respectively. The column method was used for the cation-exchange processes with synthetic solutions of 0.0020 M, 0.0698 M and 0.2000 M of Co2+ and Cu2+ concentrations which were measured using atomic absorption spectroscopy (AAS).
Keywords: clinoptilolite, chemical conditioning, ion-exchange, copper and cobalt
Introduction
There are several factors affecting heavy metal removal from aqueous solutions (Inglezakis and Grigoropoulou, 2004). Particle size, chemical conditioning, contact time, initial metal ion concentration, pH and the presence of the competing ions in aqueous solutions can be given as some examples of significant factors affecting removal of metals using clinoptilolite (Reed and Arunachalam, 1994).
Natural zeolites which have been chemically conditioned can be used for various industrial applications since chemical conditioning improves their ion exchange performance (Semmens and Seyfarth, 1996) compared to the native clinoptilolite. Such applications include the sequestration of toxic pollutants from industrial effluent and wastes. These natural zeolites are crystals that are naturally occurring hydrated alumino-silicates of alkali and alkaline earth cations. They contain variable Si/Al ratios and have exchangeable ions such as Na+, K+, and Mg2+ in their structures (Culfaz and Yagiz, 2004). The zeolites' framework consists of alumino-silicates with a 3-dimensional structure of SiO4 and AlO4 tetrahedral molecules linked by shared oxygen atoms. Clinoptilolite is one of the most abundant and cosmopolitan natural zeolites, which is widely used because of its ion-exchange properties (Kuronen et al., 2006). Natural zeolites, such as clinoptilolite, are able to lose and gain water in a reversible manner and to exchange their extra framework cations in solution without crystal structural changes (Kuronen et al., 2006).
The performance of natural zeolites can be improved by performing chemical conditioning on them. This conditioning removes certain cations from zeolites that may hinder ionexchange while exposing the easily exchangeable ones. It has previously been reported in literature that sodium-type natural clinoptilolite favours the exchange of Cu2+ over Co2+ (Zamzow and Murphy, 1992; Coruh, 2008). Cationic exchange of Cu on chabazite and clinoptilolite, Zn exchange on clinoptilolite, Co exchange on clinoptilolite and Cr exchange on phillipsite and chabazite have all been reported (Blanchard et al., 1984; Assenov et al., 1988; Trgo et al., 2006).
Chemical conditioning of a zeolite is a process applied with the aim of replacing certain cations in the zeolite with exchangeable ones (Semmens and Martin, 1988). In this study a natural zeolite, clinoptilolite, was investigated in its original form and was also chemically conditioned using HCl, NaCl and KCl (Kesraoui-Ouki et al., 1993; Papachristou et al, 1993; Kesraoui-Ouki et al., 1994), subsequently referred to as HCl-activated, NaCl-activated and KCl-activated throughout this paper. Chemical conditioning also causes exchangeable ions that are already within the zeolite to be more accessible, contributing to the efficient performance of the zeolite. The finally conditioned zeolite should have improved effective ionexchange capability (Bremner and Schultze, 1995; Gradev et al., 1988; Panayotova and Velikov, 2003).
The Na+ ion is the most weakly bound ion in clinoptilolite and thus it can be easily exchanged with the cations in solution. The effect of treatment of clinoptilolite samples was investigated for the removal of NH4+ ions from aqueous solution. Cuban clinoptilolite has been previously transformed into its Na+, K+, Ca2+ and Mg2+ forms after treatment (Rozic et al., 2002; Zamzow et al., 1990; Milan et al., 1997; Rahmani et al., 2009; Langwaldt, 2008). These studies revealed an improved performance when Na+- clinoptilolite was used. Similarly, Inglezakis et al. (1999) determined the effect of batch mode NaCl treatment on Pb2+ removal by Greek clinoptilolite and found that the effective capacity was increased. The effect of Na+ conditioning with different sodium-based chemicals was also examined by several researchers who studied the ability of Na+ modified form Bulgarian clinoptilolites, prepared with NaOH and NaCl, for Cu2+ removal from solution and it was reported that NaCl treatment resulted in a greater improvement in Cu2+ uptake compared to NaOH treatment (Panayotova, 2001). The author attributed the difference to the limited ability of NaOH to displace K+, Ca2+ and Mg2+.
Among the reported conditioning agents, HCl is claimed to have a destructive effect on the clinoptilolite structure (Sprynskyy et al., 2005). Kurama and co-workers (2002) treated Bigadic clinoptilolite with 1 M HCl solution. Hydrochloric acid is reported to cause leaching of Al3+ from zeolites, which compromises the exchange capability of the zeolite (Bailey et al., 1999). Therefore it is important to study the parameters impacting proper zeolite conditioning, especially using HCl. Although NaCl is the most frequently used conditioning agent in chemical conditioning of zeolites, acid treatment offers advantages for cation exchange, as this study has revealed.
Natural clinoptilolite is abundant in South Africa and is therefore easily accessible which reduces the usually high cost of acquiring this natural mineral. In previous studies it was observed that the use of natural clinoptilolite in its original form was inefficient as a cation-exchanger and it was established that the performance of the zeolite could be improved by chemical conditioning (Mamba et al., 2009; Nyembe et al., 2010). In this study, the exploitation of the ion-exchange potential of Southern African clinoptilolite for the removal of Cu2+ and Co2+ from aqueous synthetic sulphate solutions in batch mode applications was pursued using 3 chemically-conditioned forms of clinoptilolite.
Experimental
Preparation of synthetic solutions
The synthetic solutions were prepared as described in Mamba et al. (2009). For AAS analyses respective solutions of copper and cobalt were prepared by dissolving CoSO4.7H2O (analytical grade) and CuSO4.5H2O (analytical grade) in deionised water. The effect of the presence of one cation on the other's removal efficiency was studied by varying the metal ion concentration in solution. Studies on the Co/Cu mixed synthetic solutions were based on solutions of copper and cobalt prepared at stoichiometric ratios of Co:Cu of 1:1, 1:5, 1:9, 5:1 and 9:1, which corresponded to the following concentration ratios of Co:Cu - 0.0020:0.0020 g/ℓ, 0.0020:0.0698 g/ℓ, 0.0020:0.2000 g/ℓ, 0.0698:0.0020 g/ℓ and 0.2000:0.0020 g/ℓ, respectively. These concentrations were arbitrarily chosen with the intention of generating weakly-, moderately- and highly-concentrated solutions. The aqueous synthetic solutions were stored at room temperature (approx. 25ºC). The samples were utilised within 48 h after preparation to minimise errors from precipitation and container-plating of the metal ions.
Preparation of clinoptilolite
In addition to original forms, conditioned forms of Southern African clinoptilolite were also used in this study. Conditioning of the samples was aimed at enhancing the ion exchange capacity of the material by replacing exchangeable cations on clinoptilolite structure with a single cation. Sodium chloride, potassium chloride and hydrochloric acid (NaCl, KCl and HCl) were selected as the conditioning agents since K+, H+ and Na+ ions are weakly bound ions on clinoptilolite, and thus once the clinoptilolite is converted to the homoionic form they can easily exchange with heavy metal cations (Inglezakis and Grigoropoulou, 2004). For this purpose, original clinoptilolite was conditioned and 2 different conditioned samples were prepared for each chemical reagent. The raw zeolite was crushed with jaw crushers and sieved through screens to a size range of 2.8 to 5.6 mm. A fraction of this average particle size was rinsed with distilled water and air-dried for 24 h. Chemical conditioning of clinoptilolite was performed using HCl, KCl and NaCl solutions at concentrations of 0.02 and 0.04 M, based on the findings of the authors' earlier work (Mamba et al., 2009). Fractions of clinoptilolite were soaked in the respective solutions for 8 h, rinsed 3 times in deionised water of pH 6.7 and dried in an oven at 50ºC for 24 h. Selection of the conditions used in the preparation of the clinoptilolite was based on preliminary experiments performed with HCl in the laboratory.
Scanning electron microscopy (SEM)
A scanning electron microscope (SEM) is used to study the surfaces of solids to give information about their morphology and topological presentations. Such presentations may provide possible explanations about the solid's behaviour. To study the morphology and topological patterns of the original HCl-activated, NaCl-activated and KCl-activated clinoptilolite, a SEM JEOL JSM-840 instrument was used. Grains of 2.8 to 5.6 mm size range were mounted on aluminium stubs with carbon tape on a graphite support unit. They were then coated with gold to improve visibility and also to make the surface conductive so as to prevent charging effects. The samples were then subjected to an electron beam under vacuum to obtain micrographs of the zeolites.
Adsorption experiments
The ion-exchange processes of Co2+ and Cu2+ on the clinoptilolite were performed at room temperature. Glass columns of 20 mm diameter and 300 mm of length were pre-loaded with 25 g of either original clinoptilolite or activated clinoptilolite and a volume of 25 mℓ of the prepared Co2+ and Cu2+ bearing solutions was passed through each form of zeolite (Kocasoy and Sahin, 2007). The contact time afforded for the experiments was 60 min. The effluent solutions were collected at 5, 10, 15, 30, 45 and 60 min intervals and analysed for final metal content using AAS. Figure 1 shows the experimental set-up used for the ion-exchange experiments.

Fourier Transform Infra Red (FTIR) spectroscopy characterisation
The FTIR technique was used to determine the functionalities present in the HCl-activated, NaCl-activated, KCl-activated and original forms of clinoptilolite, as reported in Mamba et al. (2009). This characterisation was done in order to ascertain whether chemical conditioning of original clinoptilolite exposes other latent functional groups. This would assist in offering interpretation pertaining to the ion-exchange capabilities of the clinoptilolite. Dry pellets were prepared by mixing finely milled clinoptilolite (approx. 75 µm) with a bromide binder in a ratio of 1:10 of sample to binder (0.05 g clinoptilolite: 0.5 g binder); mixing was done using a pestle and mortar until homogeneity of the mixture was achieved. The IR data and IR spectra were obtained for the different pellets after running samples using the Midac FTIR 5000 spectrophotometer on CaF2 plates with the various bands recorded in wavenumbers (cm-1).
Results and discussion
Fourier Transform Infra Red analyses
The IR-spectra for original and HCl-activated clinoptilolite are shown in Fig. 2.

At the range of 4 000 to 3 000 cm-1 the original and 0.04 M HCl-activated forms of clinoptilolite showed distinct stretching, which is typical of water absorption (Majedova, 2003). A small peak was observed for 0.02 M at this range. This shows that water adsorption and retention by clinoptilolite is increased by HCl activation at 0.02 M concentration. At the 1 750 to 1 500 cm-1 range, the 0.02 M HCl-activated clinoptilolite showed distinct peaks and again the original and the 0.04 M activated forms showed a small peak. This could be as a result of 0.02 M activation washing out the non-zeolitic impurities present in the original clinoptilolite, as confirmed by SEM. The small peaks at about 1 250 with the 0.04 M and 0.02 M HCl-activated form could be due to the strength of the acid which resulted in the leaching out of calcium. At this very wavelength, the original form of clinoptilolite showed a stretched band which is due to Ca-O. There were peaks observed for all the clinoptilolite forms at 1 558 cm-1 which may be due to the bending vibrations of adsorbed water. This is expected since, given its porous structure, desiccation of the zeolite at high temperatures (50ºC) will increase its hydrophilic (water absorption) properties (Ng and Mintova, 2008).
The stretching between 1 500 and 1 000 cm-1 observed in Fig. 2 indicates the presence of a high content of calcite in the sample as confirmed by SEM. The strong band at 1 341 cm-1 (due to Si-O stretching) is the main characteristic band for quartz (Al-Degs et al., 2003). The peaks observed between 1 000 and 600 cm-1 are present in all the forms of clinoptilolite; one characteristic band appears at 836 cm-1 for all the forms. This is the quartz band. Quartz is common with zeolites, especially those of the Heulandite family. The peak that appears at 753 cm-1 for the original clinoptilolite form appears at 759 cm-1 for the activated zeolite. There is a peak that appears at 686 cm-1 for the original form while for the acid-activated forms it appears at 635 cm-1. This shift could be attributed to the action of the acid.
In essence, the characteristic IR bands of the KCl- and NaCl-activated clinoptilolite forms were comparable to the HCl-activated forms. The differences were only in intensity and the slight apparent shifts in the positions of the peaks. These shifts were less pronounced in the KCl- and NaCl-activated clinoptilolite forms than in the HCl-activated forms as shown in Fig. 3.

There were peaks between 2 000 and 1 250 cm-1 which indicated the presence of a high content of calcite in the sample. The strong band at 850 cm-1 is the main characteristic band for quartz (Al-Degs et al., 2003). Other small characteristic quartz bands appear between 850 and 500 cm-1. The positions of the peaks appear to have shifted when compared to the HCl-activated forms. This could be attributed to the mild action of the KCl and NaCl. The 0.04 M KCl-activated and NaCl-activated clinoptilolites' FTIR spectra were identical to their 0.02 M activated counterparts. We have therefore only represented the spectra of the 0.04 M activated forms of clinoptilolite for both KCl and NaCl conditioning. It can be deduced that the 2 concentrations used did not markedly alter the zeolite's structure.
Copper and cobalt removal using original clinoptilolite
Figures 4 and 5 show that the 1:1 Co:Cu ratio showed the lowest removal of Cu2+ and the highest removal of Co2+. This is likely to have been due to the fact that the Cu2+ formed more bulky and stable complexes with the water molecules in the solution (Fernandez et al., 1994).
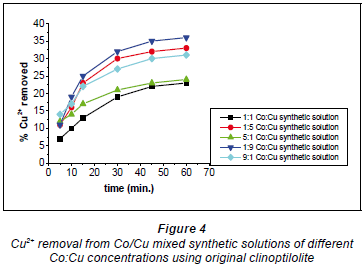

The bulky complex-formation by Cu2+ ions resulted in the availability of Co2+ ions for sorption. The highest Cu2+ removal recorded for this form of clinoptilolite (non-activated) was 35% and was recorded with a 1:9 Co:Cu ratio. The lowest removal efficiency for Cu2+ was at 23% with the 1:1 Co:Cu ratio. On the other hand, the highest removal efficiency recorded for Co2+ ions was 40% and was obtained with the 1:1 Co:Cu solution as shown in Fig. 5.
This was as expected since this solution recorded the lowest removal efficiency for Cu2+ ions. The lowest removal efficiency recorded for Co2+ was 26% and was recorded with the 9:1 Co:Cu synthetic solution where the Co2+ ion concentration was the highest. Another solution where Co2+ concentration was higher than that of Cu2+ (5:1) also recorded only 31% removal. It is evident that the higher the Co2+ ion concentration in solution, the higher the removal efficiency for Co2+ ions; the converse was also true.
The low metal removal efficiencies could be due to the original clinoptilolite's heterogeneous structure since there was no modification applied to it. It is also possible that the clinoptilolite surface and pore openings were partially covered by dust produced during crushing of the clinoptilolite, resulting in pore clogging which led to smaller ion-exchange capacity and slower ion-exchange rates (Inglezakis et al., 1999). Pore clogging by fine particles, which can be reduced by chemical conditioning of the zeolite, has also been reported as a possible cause of smaller ion exchange capacity and slower exchange rates (Mondale et al., 1995). According to one study, pore clogging can affect the ion-exchange capacity by up to 15% (Carland and Aplan, 1995). This could result in the locations of exchangeable ions not being evenly distributed within the zeolite, such that there would be delays in the ion exchange process due to exchangeable ions competing with other ions not involved in the ion-exchange process.
Copper and cobalt removal using NaCl-activated clinoptilolite
Figure 6 depicts Cu2+ removal with NaCl-activated clinoptilolite.
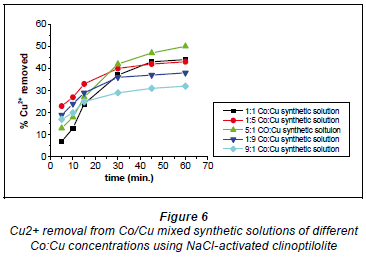
In synthetic solutions where there were more Cu2+ ions than Co2+ ions (1:5 and 1:9) the graphs displayed similar removal patterns. The removal efficiency started off at a higher rate and then slowed down almost abruptly before reaching a plateau. This must have been due to the nature of the zeolite's surface, i.e. surface charge which determines the ion-exchange mechanism in the uploading of Cu2+ onto the zeolite. It is possible that there were Cu2+affinitive sites immediately available on the zeolite's surface, such that the uploading started off very fast; these Cu2+ affinitive sites then became depleted with time as the Cu2+ adopted another uploading mechanism that proved to be slower than ion-exchange (Ederm et al., 2004).
The most likely mechanism to have occurred at first is the ion-exchange mechanism which is known to be quick (Ederm et al., 2004; Zachara et al., 1991; McBride, 1980). It is suggested that the latter part of the metal ion loading takes place inside the zeolite and is slow due to the percolation of the solution through the clinoptilolite channels. In light of the fact that there was more Cu2+ than Co2+ in solution there were more free and available Cu2+ ions in solution for exchange, such that the Cu2+ ions were uploaded first. As time went by the Cu2+ ions that had formed Co-Cu non-stable complexes in a dynamic fashion were later uploaded as they broke away from their Co2+ ion counterparts in a manner allowed by their complex formation dynamics. The removal patterns in the 5:1 and 1:1 Co:Cu aqueous solutions showed similar trends. Initially, there were low removal efficiencies which gradually picked up before reaching a plateau. It is possible in this case that both ion exchange and precipitation mechanisms happened simultaneously.
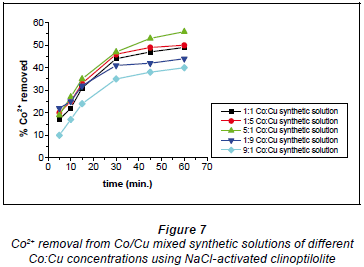
In the 5:1 Co:Cu aqueous solution shown in Fig. 7 there were more Co2+ ions than Cu2+ ions. This meant that most of the Cu2+ ions participated in the formation of Co-Cu non-stable complexes in solution such that the Cu2+ ions were not immediately available for uploading via ion-exchange mechanisms. Thus, the Cu2+ ions were uploaded as they broke away from the non-stable complexes as allowed by the chemical bonding dynamics. In the 1:1 Co:Cu solution that there were equal amounts of Cu2+ ions available to Co2+ ions for the formation of non-stable complexes. This results in the non-immediate availability of Cu2+ ions for uploading, causing the ion-exchange process to be slowed down. On the whole, the removal efficiency for both Cu2+ and Co2+ was highest with the 5:1 Co:Cu aqueous solutions and the lowest efficiencies were recorded with the 9:1 Co:Cu aqueous solutions. What was expected was that the ratio of removal that showed the highest removal for Co2+ would show the least removal for Cu2+ and vice versa. The highest removal efficiencies recorded for NaCl-activated clinoptilolite were 56% for Co2+ and 47% for Cu2+. The lowest efficiencies were 31% and 40% for Cu2+ and Co2+, respectively. These results were obtained with the 0.04 M NaCl-activated form of clinoptilolite.
Copper and cobalt removal using KCl-activated clinoptilolite
The highest percentage removal recorded for Cu2+ ion was 50% which was obtained with 5:1 Co:Cu solution (Fig. 8)

The 9:1 Co:Cu solution was expected to record a high percentage removal since it also contained a high concentration of Co2+ ions, however it recorded the lowest (38%). This discrepancy can be attributed to the non-homogeneous nature of the clinoptilolite. The 1:1, 1:5 and 1:9 Co:Cu solutions behaved in such a manner that the fewer Cu2+ ions that were present in the solutions the more efficiently the Cu2+ was adsorbed (48%, 47% and 42%, respectively).
The 1:1, 9:1 and 1:5 Co:Cu solutions recorded 70%, 68% and 66%, respectively (Fig. 9). The 5:1 and 1:9 Co:Cu solutions recorded 52% and 55%, respectively. It should be noted that when there was a high concentration of Cu2+ ions in the solution, Co2+ removal was limited. The optimal ratio resulting in the most efficient removal was 1:1 Co:Cu in solution.
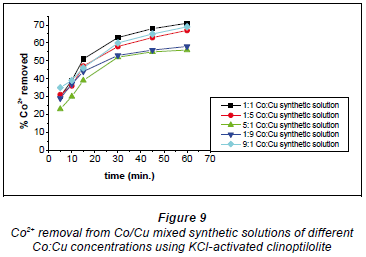
On the whole, the KCl-activated clinoptilolite showed the best performance over the original and NaCl-activated forms.
Copper and cobalt removal using HCl-activated clinoptilolite
It has been documented that acid treatment of natural clinoptilolite improves its adsorbent properties (Kuronen et al., 2006; Inglezakis and Grigoropoulou, 2004). This is due to de-catination, de-alumination and the dissolution of amorphous silica fragments blocking the channels. A study by Korkuna and coworkers also revealed that there is a change in the clinoptilolite structure after acid treatment with dilute acid activations accounting for improved removal capability of the HCl-clinoptilolite (Korkuna et al., 2006). Studies by Vasylechko and co-workers confirm the findings of this study with respect to the effect of acid-activation of natural clinoptilolite (Vasylechko et al., 1999; Vasylechko et al., 2000). From the kinetics graphs in Figs. 10 and 11 it was observed that a ratio of Co:Cu 1:1 favours removal efficiency for Co2+ more than it favours Cu2+ removal.
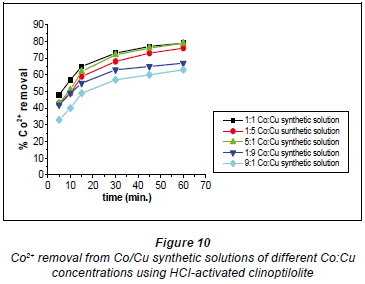
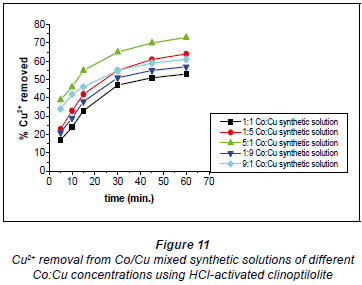
It is suggested that in this solution (Co:Cu 1:1) some of the Cu2+ ions form stable hydrated complexes with water and some of the Co2+ ions in the solutions form complexes which are too bulky to move into the zeolite pores. In the 1:5 Co:Cu solution, removal of Cu2+ ions is very high (73%). This could be as a result of there now being more 'free' available Cu2+ ions than in the 1:1 Co:Cu solution. However, a sudden change was observed for the 1:9 and 9:1 Co:Cu solutions. It was expected that the removal of Cu2+ would be more feasible if more of the Cu2+ ions were present in the matrix.
It apparently became difficult for the Cu2+ to upload, in comparison to the uploading of Co2+ ions (79% in the 1:1 Co:Cu solution), as shown in Fig. 11. This might be due to cationcation interactions of Co-Cu which could have resulted in the formation of stable complexes thus lowering metal uptake rates.
It is possible that an increase of Cu2+ ions in the solution is favoured only to a certain limited extent with respect to Cu2+ ions uptake. The ion-exchange in this case was expected to be faster than was apparent in the first 10 min, since the number of active sites in the zeolite were the same as those provided for the single-cation solutions. However there seemed to be a "struggle" in the quest to occupy the active sites. This could again be attributed to cation-cation interactions as well as to the water of hydration in the solutions and the non-homogeneous nature of the clinoptilolite. These active sites eventually become saturated with metal ions and unable to further accommodate more ions at higher concentrations.
The selectivity of Co2+ over Cu2+ in these solutions can be explained in the following manner: For larger anhydrite ions, the charge is more dispersed and the water of hydration is held less strongly by the metal cation. This leads to a situation where the larger the ion, the less hydrated it is and the smaller the relative size of the hydrated ion. For example, anhydrous Cu2+ is larger than the anhydrous Co2+ ion, but hydrated Cu2+ ion is smaller than the hydrated Co2+. The Cu2+ ion has a valence of +2 and can hold its hydration shell very strongly, while the Co2+ ion, a larger divalent cation, holds its hydration shell relatively less strongly. As a result, the Co2+ ion can lose its hydration shell readily when it is approaching the clinoptilolite surface and establish a stronger bond with the clinoptilolite than Cu2+, whose approach in the proximity of the clinoptilolite surface is prevented by the bulky surrounding water molecules.
On the whole, the low removal efficiencies exhibited by original clinoptilolite provide evidence that the clinoptilolite surface and pore openings are partially covered by dust, resulting in pore clogging which may lead to smaller ionexchange capacity and slower ion-exchange rates of the cations (Athanasiadis and Helmreich, 2005). The Na-activated clinoptilolite form did not show any marked improvement or decline in metal removal efficiency when the activating concentration was increased to 0.04 M. The Na-activated clinoptilolite form exhibited fair metal removal efficiency. HCl-activation of the clinoptilolite samples was found to improve their effective sorption capacity with the 0.02 M HCl concentration. HCl-activation of the clinoptilolite samples was found to improve the effective sorption capacity with the 0.02 M HCl concentration.
Scanning electron microscopy analyses
The SEM analysis was performed in order to determine the morphological changes brought about by the conditioning chemicals. The original clinoptilolite was observed to have crystalline calcium on its surface as shown in Fig. 12.

It is on these sites that most of the ion-exchange and adsorption takes place, in the case of original clinoptilolite. The abundance of the calcium must have limited metal upload in the inner parts of the clinoptilolite since the highest recorded result for cation removal using this form of clinoptilolite was 40% for Co and 35% for Cu.
The SEM images in Fig. 13a show a more open structure of the HCl-activated clinoptilolite's surface compared to the inactivated zeolite shown in Fig. 13b.
This explains why the acid-activated clinoptilolite performed better than all of the other forms of clinoptilolite. There were some morphological changes where the clinoptilolite was observed to exhibit a more open and somewhat softened structure brought about by HCl-activation, when comparing the 2 SEM images (Misaelides et al., 1995). Such was not the case with the clinoptilolite forms conditioned with NaCl and KCl. It is this modification that is thought to be responsible for the ease of cation removal observed with the HCl-activated clinoptilolite. It was observed that conditioning with NaCl and and KCl did not alter the morphology of the clinoptilolite, as shown in Figs. 14a and 14b.
The retention of structural integrity of the clinoptilolite is due to the non-corrosive nature of the 2 chemicals (NaCl and KCl) compared to HCl. The corrosive nature of HCl is able to eliminate the particles that clog the pores of the zeolite thus improving its adsorption and ion-exchange properties.
Conclusions
The HCl-conditioned clinoptilolite was the most efficient form in the removal (79% Co2+ and 73% Cu2+) followed by the NaCl-conditioned form (69% Co2+ and 54% Cu2+) while the KCl-conditioned form adsorbed 54% and 73% of Co2+ and Cu2+, respectively. The results obtained with the HCl-activated and KCl-activated clinoptilolite using synthetic Co/Cu solutions show that chemically activated Southern African clinoptilolite can be used effectively for the removal of metal cations from aqueous solutions, especially leached solutions obtained during mineral processing.
Acknowledgements
Funding obtained from the National Research Foundation (NRF), Mintek/DST, Nanotechnology Innovation Centre (NIC) and the University of Johannesburg is gratefully appreciated.
References
AL-DEGS Y, TUTUNJI M and BAKER H (2003) Isothermal and kinetic adsorption behaviour of Pb2+ ions on natural silicate minerals. Clay Miner. 38 501-509. [ Links ]
ASSENOV A, VASSILEV C and KOSTOVA M (1988) Simultaneous sorption of metal ions on natural zeolite clinoptilolite. In: Kallo D and Sherry HS (eds.) Occurrence, properties and utilization of Natural zeolites. Akadémiai Kiado, Budapest. 471-480. [ Links ]
ATHANASIADIS K and HELMREICH B (2005) Influence of chemical conditioning on the ion exchange capacity and on kinetics of zinc uptake by clinoptilolite. Water Res. 39 1527-1532. [ Links ]
BAILEY SE, OLIN T J, BRICKA RM and ADRIAN DD (1999) A review of potentially low cost sorbents for heavy metals. Water Res. 33 2469-2479. [ Links ]
BLANCHARD G, MAUNAYE M and MARTIN G (1984) Removal of heavy metals from water by means of natural zeolites. Water Res. 18 1501-1507. [ Links ]
BREMNER PR and SCHULTZE LE (1995) Ability of clinoptilolite-rich tuffs to remove metal cations commonly found in acidic drainage. In: Ming DW and Mumpton FA (eds.) Natural zeolites '93: Occurrence, properties, use. International Committee on Natural Zeolites, Brockport, New York. 397-403. [ Links ]
CORUH S (2008) Treatment of copper industry waste and production of sintered glass-ceramic. Waste Manage. Res. 24 234-241. [ Links ]
CULFAZ M and YAGIZ M (2004) Ion exchange properties of natural clinoptilolite: lead-sodium and cadmium-sodium equilibria. Sep. purif. Technol. 37 (2) 93-105. [ Links ]
EDERM E, KARAPINAR N and DONAT R (2004). The removal of heavy metal cations by natural zeolites. J. Colloid Interface Sci. 280 309-314. [ Links ]
FERNANDEZ A, RENDUELES M, RODRIGUES A and DIAZ M (1994) Co-ion behavior at high concentration cationic ion exchange. Ind. Eng. Chem. Res. 33 2789-2794. [ Links ]
GRADEV G, AVRAMOVA A and STEFANOVA I (1988) Silver(I) sorption on clinoptilolite and vermiculite and their modifications: In: Kallo D and Sherry HS (eds.) Occurrence, properties and utilizations of Natural zeolites. Akadémiai Kiado, Budapest. 463-470. [ Links ]
INGLEZAKIS VJ and GRIGOROPOULOU H (2004) Effect of pore clogging on kinetics of lead uptake by clinoptilolite. J. Haz. Mater. B112 37-43. [ Links ]
INGLEZAKIS VJ, DIAMADIS NA, LOIZIDOU MD and GRIGORO-POULOU H P (1999) J. Colloid Interface Sci. 215 54-57. [ Links ]
KURAMA H, ZIMMER A and RESCHETILOWSKI W (2002) Chemical modification effect on the sorption capacity of natural clinoptilolite. Chem. Eng. and Technol. 25 (3) 301-305. [ Links ]
KURONEN M, WELLER M, TOWNSEND R and HARJULA R (2006) Ion exchange selectivity and structural changes in highly aluminous zeolites. React. Funct. polym. 66 1350-1361. [ Links ]
KESRAOUI-OUKI S, CHEESEMAN R and PERRY R (1993) Effects of conditioning and treatment of chabazite and clinoptilolite prior to lead and cadmium removal. Environ. Sci. Technol. 27 1108-1116. [ Links ]
KESRAOUI-OUKI S, CHEESEMAN CR and PERRY R (1994) Natural zeolite utilisation in pollution control: A review of applications to metals' effluents. J. Chem. Technol. Biotechnol. 59 121-126. [ Links ]
KOCASOY G AND SAHIN V (2007). Heavy metal removal from industrial wastewater by clinoptilolite. J. Environ. Sci. Health A 42 (14) 2139-2146. [ Links ]
KORKUNA O, LEBODA R, SKUBISZEWSKA-ZIEBA J, VRU-BLEVS'KA T, GUN'KO VM and RYCZKOWSKI J (2006) Structural and physicochemical properties of natural zeolites: clinoptilolite and mordenite. Micropor. Mesopor. Mater. 87 243-254. [ Links ]
LANGWALDT J (2008) Ammonium removal from water by eight natural zeolites: a comparative study. Sep. Sci. Technol. 43 (8) 2166-2182. [ Links ]
MAMBA BB, NYEMBE DW and MULABA-BAFUBIANDI A F (2009). Removalof copper and cobalt from aqueous solutions using natural clinoptilolite. Water SA 35 307-314. [ Links ]
MCBRIDE MB (1980) Chemisorption of Cd2+ on calcite surfaces. Soil Sci. Soc. Am. J. 44 26-28. [ Links ]
MILAN Z, SANCHEZ E, WEILAND P, DE LAS POZAS C, BORJA R, MAYARI R and ROVIROSA N (1997) Ammonia removal from anerobically treated piggery manure by ion exchange in columns packed with homoionic zeolite. J. Chem. Eng. 66 65-71. [ Links ]
MISAELIDES PA, GODELITSAS A, FILIPPIDIS A, CHARISTOS D and ANOUSIS I (1995) Thorium and uranium uptake by natural zeolitic materials. Sci. Total Environ. 173/174 237-246. [ Links ]
MONDALE KD, CARLAND RM and APLAN FF (1995) The comparative ion exchange capacities of natural sedimentary and synthetic zeolites. Miner. Eng. 8 (4) 535-548. [ Links ]
NYEMBE DW, MAMBA BB AND MULABA AF (2010). Adsorption mechanisms of Co2+ and Cu2+ from aqueous dolutions using natural clinoptilolite: equilibrium and kinetic dtudies. J. Applied Sci. 10 (8) 599-610. [ Links ]
PANAYOTOVA M and VELIKOV B (2003) Influence of zeolite transformation in a homoionic form on the removal of some heavy metal ions from wastewater. J. Environ. Sci. Health A 38 (3) 545-554. [ Links ]
PANAYOTOVA MI (2001) Kinetics and thermodynamics of copper ions removal from wastewater by use of zeolite. Waste Manage. 21 671-676. [ Links ]
PAPACHRISTOU P, HARALAMBOUS KJ, LOIZIDOU M and SPYRELLIS N (1993) Studies on the nickel removal from aqueous solution. J. Environ. Sci. Health A 28 135-142. [ Links ]
RAHMANI AR, SAMADI MT and EHSANI HR (2009) Investigation of clinoptilolite natural zeolite regeneration by air stri`pping followed by ion exchange for removal of ammonium from aqueous solutions. Iran. J. Environ. Health. Sci. Eng. 6 (3) 167-172. [ Links ]
REED BE and ARUNACHALAM S (1994) Removal of lead from aqueous solutions by condensed tannin gel adsorbent J. Environ. Eng. 120 (2) 416-436. [ Links ]
ROZIC M, STEFANOVIC SJ, KURAJICA S, MAEEFAT MR, MARGETA K and FARKAS A (2002) Ion exchange properties of natural clinoptilolite: lead-sodium and cadmium-sodium equilibria. J. Colloid Interface Sci. 284 48-56. [ Links ]
SEMMENS MJ and MARTIN WP (1988) The influence of pretreatment on the capacity and selectivity of clinoptilolite for metal ions. Water Research 22 (5) 537-542. [ Links ]
SEMMENS M, SEYFARTH IN, SAND LB and MUMPTON FA (1978) (eds.) (1996) Natural zeolites. Occurrence, properties and use. Pergamon Press. Elmsford, NY. 517 pp. [ Links ]
SPRYNSKYY M, LEBEDYNETS M, TERZYK AP, KOWALCZYK P, NAMIESNIK J and BUSZEWSKI B (2005) Ammonium sorption from aqueous solutions by the natural zeolite Transcarpathian clinoptilolite studied under dynamic conditions. J. Colloid Interface Sci. 284 408-415. [ Links ]
TRGO M, PERIC J and VUKOJEVIC MEDVIDOVIC N (2006) A comparative study of ion exchange kinetics in zinc/lead-modified zeolite-clinoptilolite systems. J. Haz. Mater. 136 (3) 938-945. [ Links ]
VASYLECHKO VO, GRYSHCHOUK GV, LEBEDYNETS LO, KUZ'MA YB, ZAKORDONSKIY VP, VASYLECHKO LO and KALYTOVS'KA MB (1999) Adsorption of cadmium on acidmodified Transcarpathian clinoptilolite. J. Chem. Anal. (Warsaw). 44 (6) 1013-1024. [ Links ]
VASYLECHKO VO, GRYSHCHOUK GV, KUZ'MA YU B, LEBEDYNETS LO and OLIYARNYK YA O (2000) Adsorption of cadmium on transcarpathian clinoptilolite. Adsorption Sci. Technol. 18 (7) 621-630. [ Links ]
ZACHARA JM, COWAN CE and RESCH CT (1991) Sorption of divalent metals on calcite. Geochim. Cosmochim. Acta 55 1549-1563. [ Links ]
ZAMZOW MJ, EICHBAUM BR, SANDGREN KR and SHANKS DE (1990) Heavy metal removal from motorway storm water using zeolites. Sep. Sci. Technol. 25 13-15. [ Links ]
ZAMZOW MJ and MURPHY JE (1992) Natural zeolites as cation exchangers for environmental protection. Sep. Sci. Technol. 1969-1984. [ Links ]
Received 20 November 2009; accepted in revised form 31 May 2010.
* To whom all correspondence should be addressed. +27(11) 559 6516; fax: +27(11) 559 6425; e-mail: bmamba@uj.ac.za














