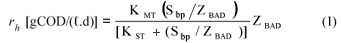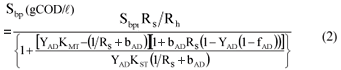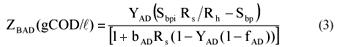Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Water SA
On-line version ISSN 1816-7950
Print version ISSN 0378-4738
Water SA vol.36 n.3 Pretoria Apr. 2010
Biological sulphate reduction with primary sewage sludge in an upflow anaerobic sludge bed reactor – Part 5: Steady-state model
J Poinapen; GA Ekama1
Water Research Group, Department of Civil Engineering, University of Cape Town, Rondebosch 7701, South Africa
ABSTRACT
This paper describes the development of a steady-state anaerobic digestion model for biological sulphate reduction using primary sewage sludge (PSS) as substrate. The model comprises: a chemical oxygen demand (COD) based hydrolysis kinetics part in which the PSS biodegradable COD and sulphate removals are calculated for given hydraulic and sludge retention times; a C, H, O, N, P, S, COD and charge mass balance stoichiometry part in which the alkalinity generated (from both the HCO3- and HS-) is determined from the COD and sulphate removals; and an inorganic carbon (CO2) and sulphide mixed weak acid/base chemistry part in which the digester pH is calculated from the HCO3- and HS- species formed. From the stoichiometry, it was found that the PSS is carbon limited in that it does not generate sufficient HCO3- alkalinity for the sulphate reduction, i.e., its COD/C ratio is too high which accounts for the observed zero gas (CO2) generation. The H2S/HS- system provides the alkalinity shortfall and establishes the system pH. Once developed and calibrated, the model results were compared with experimental data from 2 laboratory-scale upflow anaerobic sludge bed reactors (operated at 35ºC and 20°C respectively) fed PSS and sulphate. The predicted COD and sulphate removals, alkalinity and digester pH correspond very well to the measured data. The model assists in identifying design and operation parameters sensitive to the system and provides a basis for developing an integrated biological, chemical and physical process dynamic model.
Keywords: biological sulphate reduction, primary sewage sludge, upflow anaerobic sludge bed reactor, steady state model, kinetics, stoichiometry, mixed weak acid/base chemistry
Nomenclature
| a | molar nitrogen composition of organics in CxHyOzNaPb |
| AD | anaerobic digestion |
| Alk H2S | alkalinity with respect to the H2S reference species excluding the water species |
| AMD | acid mine drainage |
| b | molar phosphorus composition of organics in CxHyOzNaPb |
| bAD | endogenous respiration rate of acidogens |
| BPO | biodegradable particulate organics |
| BRT | bed retention time |
| BSO | biodegradable soluble organics |
| BSR | biological sulphate reduction |
| COD | chemical oxygen demand |
| E | flux of acidogen and endogenous mass wasted per day as a fraction of the flux of hydrolysable biodegradable organics utilised per day |
| EDC | electron donating capacity |
| ƒ | proportion H2PO4- in phosphate (H2PO4- + HPO42-) weak acid base species |
| ƒAD | unbiodegradable fraction of acidogen biomass |
| FBR | fluidised bed reactor |
| ƒc | mass carbon to mass (VSS) ratio |
| ƒcv | mass COD to mass (VSS) ratio |
| ƒh | mass hydrogen to mass (VSS) ratio |
| ƒn | mass OrgN to mass VSS ratio |
| ƒo | mass oxygen to mass (VSS) ratio |
| ƒp | mass OrgP to mass VSS ratio |
| ƒPS'up | unbiodegradable fraction of PSS with respect to total COD (Sti) |
| FRBCOD | fermentable readily biodegradable (soluble) COD |
| FRBO | fermentable readily biodegradable (soluble) organics |
| FSA | free and saline ammonia |
| H2CO3* alk | Alkalinity with respect to the H2CO3 reference species including the water species |
| HAc | acetic acid |
| HRT | hydraulic retention time |
| ISS | inorganic suspended solids |
| k | molar carbon composition of acidogen biomass in CkHlOmNnPp |
| KI | sulphide inhibition kinetic constant |
| KM | Monod maximum specific hydrolysis rate for saturation kinetics |
| KMT | Monod maximum specific hydrolysis rate for saturation kinetics at TºC |
| KM20 | Monod maximum specific hydrolysis rate for saturation kinetics at 20ºC |
| KS | Monod half saturation coefficient for hydrolysis for saturation kinetics |
| KST | Monod half saturation coefficient for hydrolysis for saturation kinetics at TºC |
| KS20 | Monod half saturation coefficient for hydrolysis for saturation kinetics at 20ºC |
| l | molar hydrogen composition of acidogen biomass in CkHlOmNnPp |
| m | molar oxygen composition of acidogen biomass in CkHlOmNnPp |
| M | experimentally measured |
| MB | molar mass of acidogen biomass |
| MS | molar mass of biodegradable organics |
| n | molar nitrogen composition of acidogen biomass in CkHlOmNnPp |
| OLR | organic loading rate |
| OP | ortho-phosphate |
| P | theoretically predicted |
| p | molar phosphorus composition of acidogen biomass in CkHlOmNnPp |
| PBR | packed bed reactor |
| pK'S1 | 1st dissociation constant for the sulphide weak acid base system corrected for ionic strength effects |
| PSS | primary sewage sludge |
| Qe | effluent flow |
| Qi | influent flow |
| Qr | recycle flow |
| Qw | waste flow |
| R1 | UASB Reactor 1 |
| R2 | UASB Reactor 2 |
| RBCOD | readily biodegradable COD (Sbsi) |
| rh | volumetric hydrolysis rate – gCOD/(ℓ.d) |
| Rh | hydraulic retention time |
| Rs | sludge age |
| Sbp | biodegradable particulate COD concentration |
| Sbpi | biodegradable particulate COD concentration in influent |
| Sbsi | biodegradable soluble COD concentration in influent |
| Sbsai | VFA (all assumed acetic acid) COD concentration in influent |
| Sbsfi | fermentable biodegradable soluble COD concentration in influent |
| SBR | sequencing batch reactor |
| SLR | sludge loading rate |
| SRB | sulphate reducing bacteria |
| SRT | solids retention time |
| SS | steady state |
| SSD | sample standard deviation |
| ST | total sulphide species concentration |
| Sti | total COD concentration in influent |
| Sup | unbiodegradable particulate COD concentration |
| Supi | unbiodegradable particulate COD concentration in influent |
| Susi | unbiodegradable soluble COD concentration in influent |
| Sbpi | biodegradable particulate COD concentration in influent |
| T | temperature in ºC |
| TKN | total Kjeldahl nitrogen |
| TOC | total organic carbon |
| Total alk | sum of weak acid/base subsystem alkalinities |
| TSS | total suspended solids (VSS+ISS) |
| UASB | upflow anaerobic sludge bed reactor |
| UCTADM1 | University of Cape Town Anaerobic Digester Model No. 1 |
| UCTADM1-BSR | University of Cape Town Anaerobic Digester Model No. 1 including biological sulphate reduction |
| UPO | unbiodegradable particulate organics |
| USCOD | unbiodegradable soluble COD |
| USO | unbiodegradable soluble organics |
| Vd | volume of digester (equivalent to bed volume, Vb) |
| VFA | volatile fatty acids |
| VSS | volatile suspended solids |
| Vup | hydraulic upflow velocity in UASB reactor |
| WS | waste sludge |
| WWTP | wastewater treatment plant |
| x | molar carbon composition of organics in CxHyOzNaPb |
| y | molar hydrogen composition of organics in CxHyOzNaPb |
| YAD | specific yield coefficient of acidogens |
| z | molar oxygen composition of organics in CxHyOzNaPb |
| ZBAD | acidogen biomass concentration mgCOD/ℓ |
| ZEAD | acidogen endogenous mass concentration mgCOD/ℓ |
| γS | electron donating capacity of acidogen biomass |
| γS | electron donating capacity of biodegradable organics |
| θ | Arhenius temperature sensitivity coefficient for KM |
Introduction
Biological sulphate reduction (BSR) is an attractive treatment process in the remediation of sulphate-rich waters such as acid mine drainage (AMD). Conventionally, organic substrates such as molasses, ethanol, acetate or lactate have been used as electron donor and organic carbon source for BSR. However, these organics are relatively expensive, making AMD remediation via BSR costly. Since the economics of BSR are governed by the cost of the carbon source, the BioSURE® technology was developed, in which BSR is achieved using primary sewage sludge (PSS) as carbon source and electron donor (Rose et al., 2002). The core unit process in the BioSURE® system is BSR with PSS. To assist in, and optimise, the design, operation of, and research into this unit process, mathematical models (both steady-state and dynamic) represent very useful process evaluation tools. Mathematical models provide quantitative descriptions of the treatment system of interest that allow predictions of the system response and performance to be made. Based on the predictions, design and operation criteria can be identified to optimise the system performance. The model predictions can be evaluated and as such make it possible to test hypotheses of the system behaviour, such as biological processes and their response to system constraints, in a consistent and integrated fashion. In this paper, a steady-state (SS) anaerobic digestion model for BSR using PSS as energy source is developed and calibrated. The SS BSR model results are compared with experimental data from the 2 laboratory-scale UASB reactors, R1 at 35°C (when fed 1 500 and 1 800 mgSO42-/ℓ) and R2 at 20°C (fed 1 500 mgSO42-/ℓ) (Poinapen et al., 2009).
Importance of steady-state models
Steady-state models are comparatively simple kinetic and stoichiometric models based on constant flows and loads as inputs to determine the system design parameters. They are based on the slowest process kinetic rate governing the overall behaviour of the system and relate this process to the system design and operating parameters. These design and operating parameters, such as reactor volume, recycle ratios and retention time, can be estimated in a relatively simple and quick way with explicit equations from the system performance criteria, for instance, effluent quality. Usually, steady-state models constitute the initial step to estimate the design and operating parameters of a system. These parameters then serve as input to the more complex kinetic dynamic simulation models to explore the time-varying behaviour of the system and refine the design and operating parameters. A dynamic model for BSR in a UASB reactor fed PSS is developed in the last paper of this 6-part series (Poinapen and Ekama, 2010).
Characterisation of PSS
In conformity with mass balance and continuity principles, the effluent parameters (COD (all constituents), TKN, FSA, VFA, H2CO3* alk, Alk H2S, H2S/HS- and pH) are defined by the influent PSS and SO42- constituents transformed in the system. Thus, in the development of the steady-state (SS) model for BSR, the PSS is fully characterised based on the COD (total and unbiodegradable particulate fraction, fPS'up), short chain (volatile) fatty acids (VFA) COD, TKN and FSA and the PSS CHON composition of the particulate solids, i.e. x, y, z and a in CxHyOzNa. This approach is similar to that used by Sötemann et al. (2005a) and characterises the PSS in terms of the measurable parameters used in calculating the COD, C, H, O, N, S and charge mass balances.
Anaerobic digestion steady-state model for methanogenesis
Sötemann et al. (2005a) developed a SS model for anaerobic digestion (AD) of PSS under methanogenic conditions. This model consists of 3 sequential parts, namely:
• A COD-based kinetic part in which the influent COD concentration hydrolysed, VFA COD utilised, methane gas COD generated, biomass COD produced and COD concentrations of the effluent are determined for a given sludge age
• A C,H,O,N, charge and COD mass balance based stoichiometry part in which the gas composition (or partial pressure of CO2), ammonia released and alkalinity generated are calculated from the VFA and PSS COD concentration hydrolysed (and utilised) and its x, y, z and a composition in CxHyOzNa of the biodegradable organics
• An inorganic carbon system weak/acid-base chemistry part in which the pH of the digester is obtained from the partial pressure of CO2 and HCO3- (or H2CO3*alkalinity) generated
Based on the above, a steady-state model for BSR of sulphate-rich waters using a generic biodegradable organic CxHyOzNaPb as carbon source was developed. This SS BSR model will be useful to:
• Estimate product generation from influent organic C, H, O, N and P composition and establish whether or not a particular organic type is C-deficient, i.e. generates insufficient inorganic C to supply the alkalinity (HCO3-) required for the SO42- reduction
• Estimate reactor volume and retention time for a required substrate COD loading and sulphate removal rate
• Estimate product concentrations (such as hydrogen sulphide) and their sensitivity to system performance
• Provide a basis for cross-checking BSR kinetic dynamic simulation model results
In the development of the SS model using PSS as organic, it is assumed that the slowest biological process, i.e. hydrolysis/acidogenesis, generates directly the BSR end-products, which are H2S, HS-, HCO3-, CO2, NH4+ and biomass. Thus, the SS BSR model includes the same 3 parts as the methanogenesis SS AD model of Sötemann et al. (2005a), namely:
COD-based kinetics of the hydrolysis/acidogenesis process (as for Sötemann et al. (2005a) because Ristow et al. (2005) found that this also applies to BSR)
C,H,O,N,P,S, charge and COD mass balance based stoichiometric conversion of the reactants from the 1st part and utilisation of VFA to BSR end-products
Effect of the end-products on the digester pH by applying mixed weak acid/base chemistry of the inorganic carbon (CO2) and sulphide systems. For PSS, the ortho-phosphate and, under normal operating conditions, the VFA (acetic acid) weak acid-base systems are low enough to have a negligible effect on digester pH.
Steady-state AD model for BSR
With the modified UASB configuration operated in this research, the sludge recycle line from the top to the bottom of the reactor bed ensured that the biomass was fairly evenly distributed along the bed axis. This biomass recycle line offered 2 advantages – it initiated BSR at the bottom of the bed thus maximising the system performance, and it allowed the UASB reactor bed to be modelled as a completely mixed digester. This avoids the necessity of evaluating uncertain and complex granular sludge dynamics, along the reactor bed height in UASB reactors, caused by dispersion, sedimentation and convection.
Hydrolysis of primary sewage sludge
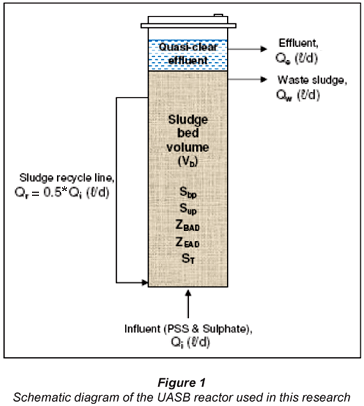
Consider a UASB reactor of bed volume Vb (ℓ) and influent flow rate Qi (ℓ/d). The UASB reactor configuration has the benefit of uncoupling the solid and liquid (hydraulic) retention times compared with a flow-through digester. For this reason, the fundamental design parameter, sludge age, (Rs in days) is considered in this case (Fig. 1).
The influent PSS COD is characterised in terms of measurable parameters (Fig. 2). The influent parameters are as follows:
• Total influent PSS COD, Sti (mgCOD/ℓ)
• Total soluble COD (membrane filtered), Sbsi + Susi (mgCOD/ℓ)
• Volatile fatty acids (VFA), Sbsai (mgHAc/ℓ, then converted to mgCOD/ℓ), with the 5 point titration method of Moosbrugger et al. (1992)
With a known (or assumed) value of the unbiodegradable particulate fraction (fPS'up) of the influent total PSS COD (Sti), the biodegradable particulate COD (Sbpi) concentration in the influent is defined. The unbiodegradable soluble COD (Susi) concentration forms part of the total soluble COD. Since the Susi concentration is very low in relation to the Sbsi, it can be given an approximate value based on previous research. Usually Susi is about 50 to 75 mgCOD/ℓ in PSS. With the above, the influent PSS COD can be fully characterised (Fig. 2). Knowing Susi and Sbsai, the fermentable readily biodegradable soluble COD (FRBCOD, Sbsfi) concentration can be quantified. The Sbsfi also undergoes the same hydrolysis/acidogenesis processes as the Sbpi and both are converted to VFA and H2 which then get utilised in BSR to generate hydrogen sulphide (H2S/HS-), bicarbonate (HCO3-), NH4+ and biomass.
In contrast, the influent VFA (Sbsai) is not included in the hydrolysis process but it does generate H2S/HS- and HCO3- with negligible (assumed zero) biomass production. So, Sbsai is included in the stoichiometry part of the steady-state model. The zero sludge production for the utilisation of influent VFA is accepted in the SS model because the yield of acetoclastic sulphidogens is very low compared with that of the acidogens.
Ristow et al. (2005) concluded that the rate of PSS hydrolysis is the same under both methanogenic and sulphidogenic conditions. Since BSR does not affect the rate of PSS hydrolysis, the same hydrolysis kinetics (rate formulations and rate constants) for methanogenic AD can be applied to sulphidogenic AD conditions. As outlined by Sötemann et al. (2005a), the acidogens have the highest yield coefficient (YAD = 0.089 gCOD biomass/gCOD organics hydrolysed) and constitute more than 77% of the total biomass formed in the AD of hydrolysable organics. By increasing the YAD value from 0.089 to 0.113, the biomass formation of the other organism groups is taken into account. This adjustment in the YAD value resulted in similar percentage COD removal predictions and so was also accepted for BSR. The SS model for BSR derived here also uses the COD to quantify the organics and biomass concentrations and the saturation equation for the hydrolysis/acidogenesis rate.
The steady-state anaerobic digester equations for the hydrolysis part of the SS BSR model applied to the UASB system (Fig. 1) were derived and are listed below.
• Hydrolysis rate equation – saturation (Contois) kinetics:
• Residual biodegradable organics concentration in reactor and waste flow:
• Acidogen biomass concentration in reactor and waste flow:
• Unbiodegradable organics concentration in reactor and waste flow,
• The acidogen endogenous residue concentration:
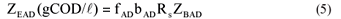
where
| KMT | = the saturation maximum specific hydrolysis rate constant at TºC |
| = 5.27 gCOD organics/(gCOD biomass·d) at 35ºC | |
| KST | = the half saturation coefficient at TºC |
| = 7.98 gCOD organics/gCOD biomass at 35ºC | |
| YAD | = pseudo acidogen yield coefficient = 0.113 gCOD biomass/gCOD organics hydrolysed |
| Sbpi, Sbp | = Influent and waste flow (bed) COD concentration to and from digester (gCOD/ℓ) |
| bAD | = acidogen endogenous respiration rate |
| = 0.041 (/d) | |
| Rs | = bed solids retention time or sludge age (d) |
| Rh | = hydraulic retention time in the bed volume (d) |
| ƒAD | = endogenous residue of acidogens (assumed zero) |
Equations (2) to (4) are the same as for flow-through AD systems except that the influent particulate COD concentrations (Supi and Sbpi) are multiplied by Rs/Rh to take account of the bed solids retention effect.
From the hydrolysis kinetics, the COD concentration of the biodegradable particulate organics utilised in the BSR AD (Sbpi Rs/Rh – Sbp) is known. Following the hydrolysis process, the stoichiometry of BSR needs to be established taking into account the utilisation of the influent volatile fatty acids (VFA, undissociated and dissociated, and assumed to be all acetate) which also affect alkalinity generation and hence digester pH.
Stoichiometry of BSR
By following the generalised procedure of McCarty (1975), the general stoichiometry of BSR with a biodegradable organic compound of composition CxHyOzNaPb and generating sludge mass of composition CkHlOmNnPp and CO2 gas (i.e. C sufficiency) is given by:
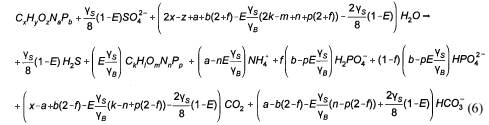
where

where
| ZBAD, ZEAD | = COD concentration of the anaerobic biomass and endogenous residue respectively (gCOD/ℓ). |
| Vd | = volume of the digester (ℓ) |
| = UASB Sludge bed volume (ℓ) | |
| Qi | = influent flow to digester (ℓ/d) |
| Qw | = bed waste flow from the digester (ℓ/d) |
From γS and γS, the COD of the biodegradable organics and sludge mass (accepting the live biomass and endogenous residue have the same composition) is 8γS and 8γB gCOD/mol respectively. Also, from Ekama (2009), with known values of the COD/VSS (ƒcv), TOC/VSS (ƒc), OrgN/VSS (ƒn) and OrgP/VSS (ƒp) ratios, the elemental composition of the biodegradable organics (x, y, z, a and b) can be calculated from Eq. (9), which also applies to the biomass (k, l, m, n and p). Accepting y = 7, then:
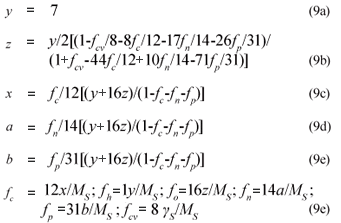
where ƒc, ƒh, ƒo, ƒn, ƒp and ƒcv are the mass fractions of C, H, O, N, P and COD of the organics respectively (9)
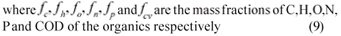
Alternatively, if the composition of the biodegradable influent organics (x, y, z, a, b) and biomass (k, l, m, n, p) are known, the COD/VSS (ƒcv), TOC/VSS (ƒc), OrgN/VSS (ƒn) and OrgP/VSS (ƒp) ratios of the influent organics and biomass can be calculated from Eq. (10).
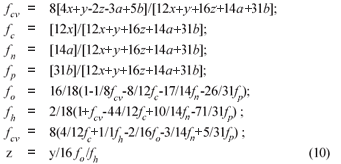
The influent VFA is assumed to be acetate. The split between the undissociated and dissociated acetate (HAc and Ac- respectively) species is governed by the influent pH and, since the influent pH was always greater than 5.9, almost all the influent VFA was in the dissociated (Ac-) form.
Equation (6) holds also for acetate, both associated (HAc) and undissociated (Ac-) provided the correct composition x(=2), y(=4 for HAc, =3 Ac-), z(=2) and charge (=0 for HAc, =-1 for Ac-) are inserted. Because the yield of sulphidogens is very low, E = 0 when applying Eq. (6) to acetate, which yields:

From Eqs (11a) and (11b) it can be seen that the influent VFA (both undissociated and dissociated) concentration is important for establishing the digester pH because its utilisation makes a significant contribution to the alkalinity generated.
Equation (6) is valid for organics that generate sufficient CO2 for the required alkalinity increase. This will be the case if the CO2 term in Eq. (6) is positive, i.e.
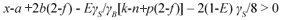
Accepting zero biomass production (E=0) and negligible organic P content (b=0), substituting 4x+y-2z-3a+5b for γS yields 2z>y+a. So organics with a composition that conforms to 2z>y+a are carbon sufficient. Most organics do not conform to this, e.g. the amino acids, alcohols, all of the fatty acids except formic and acetic acids, and PSS. The mono-, di- and polysaccharides and acetic acid conform exactly, i.e. 2z=y for a=0, but with biomass growth (E>0) they also become carbon deficient. The COD/C ratio of these organics is 2.67, so organics with a COD/C ratio>2.67 are C deficient for BSR in the sense that they can donate more electrons for BSR than supply CO2 for the alkalinity increase.
So for most organics, the gaseous CO2 term in Eq. (6) is negative. In this event the sulphide system produces the alkalinity shortfall in the form of HS-. Re-arranging Eq. (6) for C deficiency (HS- and zero gaseous CO2 production) yields:
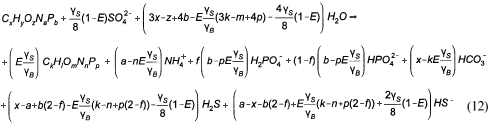
The PSS (which contained negligible P, ƒp<0.015 gP/gVSS) with its determined composition from this investigation (see below) of C3.35H7O1.45N0.45 is C deficient, so Eq. (12) instead of Eq. (6) applies in this SS BSR model development.
Mixed weak acid/base chemistry
Once the BSR products are known from the stoichiometry above, the digester pH is predicted using the mixed weak acid/base chemistry. For C deficient systems and low P content PSS, in effect the H2S/HS- system with a pK'S1 value near 7 establishes the reactor pH because a gaseous CO2 phase is absent, namely:
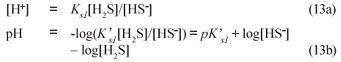
where

The validity of Eq. (13b) to approximate the reactor pH can be shown from the principles of mixed weak acid-base systems (Loewenthal et al., 1989; 1991). For a mixed weak acid/ base system comprising the inorganic carbon, acetic acid, ammonia, phosphate and sulphide systems in water, the total alkalinity with respect to the most protonated species is defined as:
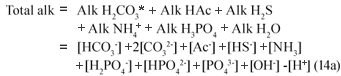
In Eq. (14a), the nomenclature of Loewenthal et al.(1989) is adopted, i.e. Alk as a prefix refers to the alkalinity with respect to the named reference species without the water (+[OH-] -[H+]) terms, i.e. the alkalinity of only the named weak acid-base system by itself. Alk as a suffix refers to the alkalinity with respect to the named reference species including the water terms. For example, H2CO3* alk = Alk H2CO3* + [OH-] - [H+].
Assuming the acetic acid is completely utilised so its concentration is too low to affect pH and noting that in the pH range 6.5 to 8, [CO32-], [NH3], [PO43-], [OH-] and [H+] are negligible compared with [HCO3-], [HS-], [H2PO4-] and [HPO42-] , the Total alkalinity reduces to:
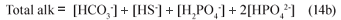
With 6 weak acid-base systems (inorganic carbon, acetic acid, ammonia, phosphate, sulphide and water), 6 parameters need to be known to define all the system species including the pH. From the stoichiometry (Eq. (12)), these 6 knowns are the [HCO3-], the Total alk (Eq. (14b)), the total sulphide and OP species concentrations (ST = [HS-] + [H2S], PT =[H2PO4-] + [HPO42-]), the acetic acid concentration (assumed zero) and the ammonia concentration (not required in Eq. (14b) – completely protonated between pH 6.5 and 8). Accepting that for PSS the organic P content is very low (b≈0), so that the alkalinity generated by the phosphate system is very low in relation to the inorganic carbon alkalinity, the Total alk reduces to:

For the sulphide system, from equilibrium chemistry it can be shown that:
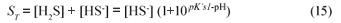
Hence Total alk = [HCO3-] + [HS-] = [HCO3-] + ([H2S]+[HS-]) /(1+10pK's1-pH) from which Eq. (13b) can be obtained.
For the experimental system fed carbon deficient organics, the 6 parameters which are required to be known are the same 6 parameters as mentioned above, except that the [HCO3-] concentration is exchanged for the pH. The reactor in situ pH, total sulphide (ST), OP (PT) and ammonia (NT) concentrations are direct measurements; the sulphide is measured with the COD test (Poinapen et al., 2009b). The H2CO3* Alk and VFA concentrations are measured with the 5-point titration method of Moosbrugger et al. (1992) using the 4 direct measurements as input.
Interestingly, the Total alk generated is governed only by the composition of the biodegradable organics utilised and the type of bioprocess, e.g. methanogenesis or sulphidogenesis. With methanogenesis, the Total alk generated is a consequence of the difference between the protons taken up or released in the breakdown of the biodegradable organics and the production of biomass. When organic N is present in the biodegradable organics in significant concentrations, effectively in the non-ionised NH3 form, the released NH3 takes up a proton from the aqueous phase to form NH4+. The H+ is supplied by the dissolved CO2 (H2CO3*) to form HCO3-, viz. H2CO3*+NH3 → NH4+ + HCO3-. The Total alk increases because the released NH3 is not reference species for the ammonia system. The CO2 produced by the breakdown of the biodegradable organics that cannot be 'held' in the digester in this way escapes as gas with the methane (which is governed by the COD of the biodegradable organics) and sets the partial pressure of the gas phase. Similarly, when dissociated acetic acid (Ac-) is utilised, a H+ is taken up, viz. H2CO3*+Ac- → HAc + HCO3-. So the N content of the biodegradable organics and the influent acetic acid concentration and pH, fix the Total alkalinity (≈[HCO3-]) generated in the digester and partial pressure of the gas phase and hence the pH in the digester. When organic P is present in the biodegradable organics in significant concentrations, effectively in the non-ionised H3PO4 form, the released H3PO4 releases protons to the aqueous phase to form H2PO4- and HPO42-. The released protons (H+) react with HCO3- to form dissolved CO2 and water, viz. HCO3- + H+ → H2CO3* → H2O + CO2. This decreases the dissolved CO2 that can be 'held' in the digester with the result that more CO2 escapes as gas. This decreases the Alk H2CO3* (HCO3-) and increases the CO2 partial pressure of the gas phase, but the Total alk (= [HCO3-] + [H2PO4-] + 2[HPO42-]) remains constant. The reason the Total alk remains constant is because the phosphate species released by the organics are reference species (H3PO4) for the phosphate system. Although the Total alk remains constant, the species making up the Total alk are not the same and now include phosphate system species, so the pH in the digester is now governed by both the inorganic carbon and phosphate systems.
The above also applies to sulphidogenic systems but, additionally, protons are taken up by the sulphate reduction, i.e. in effect H2SO4 is utilised. This increases the Total alkalinity by so much that not even all of the CO2 produced by the breakdown of the biodegradable (carbon deficient) organics and 'held' in the aqueous phase, is sufficient to supply it. The alkalinity deficit therefore has to be supplied by the other weak acid/base systems to meet the Total alkalinity required. The phosphate system in effect makes more of the CO2 available to form HCO3- and the difference between the Total alkalinity required and the sum of the Alk H2CO3* (= [HCO3-]) and Alk H3PO4 (=[H2PO4-] + 2[HPO42-]) produced has to be supplied by the sulphide system as Alk H2S (= [HS-]) from H2S → H+ + HS-. So the Total alkalinity generated is governed only by the composition of the biodegradable organics utilised and the type of bioprocess.
Steady-state BSR model validation
Figure 3 illustrates the characterisation of the different components of the influent (PSS) and bed waste sludge (WS) and the determination of their respective elemental compositions from the mass fraction ratios (ƒcv, ƒc, ƒn; ƒp was accepted as zero). The circled notes 1 to 4 marked in Fig. 3 are described below.
Characterisation of primary sewage sludge (PSS)
(Unbiodegradable particulate COD fraction (Fig. 3; Note 1):
In this study, the unbiodegradable particulate fraction (ƒPS'up) of the PSS was set at 0.36. Ristow et al. (2005) and Sötemann et al. (2005a) conducted studies on methanogenic and sulphidogenic anaerobic digestion of PSS at different retention times (sludge ages) between 5 and 60 d and so were able to determine the ƒPS'up value of PSS. They found that the ƒPS'up of different batches of PSS collected from the same wastewater treatment plant (WWTP, Athlone, Cape, South Africa) varied from 0.34 to 0.36. The PSS used in this study was obtained from the same WWTP. Because running UASB systems at different sludge ages to determine the ƒPS'up was beyond the scope of this investigation, the fPS'up value from previous studies was accepted. Accordingly, an ƒPS'up value of 0.36 was used in the steady-state (SS) BSR model developed in this study.
Dissolved organic compounds (USO, FRBO, VFA) (Fig. 3; Note 2):
The concentration of the unbiodegradable soluble organics (USO) of the PSS was assumed to be 75 mgCOD/ℓ, which is very low with respect to the total PSS COD (~ 50 000 mgCOD/ℓ). The volatile fatty acids (VFA) (and H2CO3* Alk) concentration was measured using the 5-pH point titration method (Moosbrugger et al., 1992) and the unit mgHAc/ℓ converted to mgCOD/ℓ by multiplying by 64/60. The concentration of the total soluble organic comprising the USO, VFA and fermentable readily-biodegradable organics (FRBO) was measured in the COD test. Thus, by difference the FRBO concentration was calculated. The compositions of the USO and FRBO were determined using ƒcv, ƒc and ƒn ratios of 1.42, 0.487, 0.049 and 1.42, 0.470, 0.022, respectively, taken from Brink and Ekama (2008), who obtained these ƒc and ƒn from wastewater characterisation tests using an assumed ƒcv = 1.42 for both the USO and FRBO fractions. The actual VFA composition (C2H4O2 for HAc and C2H3O2- for Ac-) was used.
Biodegradable particulate organics (BPO) composition (Fig. 3; Note 3):
To determine the composition of the biodegradable particulate organics CxHyOzNa of the PSS, 4 measurements are required, COD, TKN, VSS and total organic carbon (TOC), because there are 4 unknowns, namely x, y, z and a. The COD, TKN and VSS (and TSS) of the PSS particulate organics (comprising both unbiodegradable (UPO) and biodegradable (BPO) particulate organics were determined with the COD, TKN and VSS/TSS tests, whereas the TOC was obtained from elemental analysis of dried PSS (TSS). The TOC of the influent PSS was found to be ~43% of the total suspended (dried) solids (TSS). From this, the TOC/VSS ratio (ƒc) of the PSS particulate organics (PO=BPO+UPO) was calculated.
With the accepted ƒPS'up= 0.36, the UPO concentration was calculated and its composition, i.e. x, y, z and a in CxHyOzNa, was determined from ƒcv, ƒc and ƒn values of 1.480, 0.515 and 0.0597, respectively, taken from Wentzel et al. (2006), which conforms to the composition of these organics used in Activated Sludge Model No.1 (ASM1, Henze et al., 1987). A composition of C4.26H7O2.2N0.42 was obtained for UPO. By fractionating the particulate COD concentrations of the PSS using the UPO ƒcv, ƒc and ƒn values of 1.480, 0.515 and 0.0597, respectively, the biodegradable particulate organics (BPO) of the PSS was found by mass difference between the PO (UPO + BPO) and unbiodegradable (UPO) and fcv, fc and ƒn values of 1.682, 0.524, 0.083, respectively, were obtained for the BPO. Accordingly, from these values, the composition of the BPO calculated from Eq. (9) was found to be C3.35H7O1.45N0.45.
Characterisation of waste sludge (WS)
WS concentration and composition (Fig. 3; Note 4)
To characterise the waste sludge (WS), the same principle as above was applied except that the WS comprised: Supi (or UPO) with known concentration (Eq. (4)) and the same composition as the influent UPO; residual biodegradable organics (BPO or Sbp) with concentration calculated from the hydrolysis kinetic model (Eq. (2)) and with the same composition as the influent BPO (Sbpi); and biomass with concentration also calculated from the hydrolysis kinetic model (Eq. (3) but with an unknown composition (endogenous residue concentration was assumed zero, ƒAD = 0). The kinetic model saturation rate values were taken from Sötemann et al. (2005a) with KM (the maximum specific hydrolysis rate constant) = 5.27 gCOD organics/(gCOD biomass.d) and KS (the half saturation coefficient) = 7.98 gCOD organics/gCOD biomass, both at T = 35ºC. The FRBO and VFA concentrations combined were very low (< 0.8%) with respect to that of the total WS and were thus considered to have all been utilised and therefore zero in the waste sludge.
To determine the biomass composition, 4 measurements are again required (COD, TKN, VSS and TOC). However, in using the TOC value determined from elemental analysis of the WS, it was found that the biomass composition was far out of the normal range obtained from previous studies, in that the oxygen composition (m) in the biomass formulation of CkHlOmNn was < 1.2. This value was considered too low and so the results from the elemental analysis of the waste sludge were not used to determine the biomass composition. Instead, the composition of the biomass was obtained from the measured WS COD/VSS and TKN/VSS ratios and an assumed value for m = 2 in the biomass composition. This assumption makes a small difference to the overall WS composition because the biomass is only a small proportion (<8%) of the total. Adding the concentrations of all the three above waste sludge constituents (residual Sbp, Supi and biomass) gives the total particulate COD concentration of the waste sludge (and reactor sludge bed). The calculated waste total particulate COD concentration was found to be very close to the measured value; this was expected because the biomass concentration is a very small part (<10%) and indicates the selected hydrolysis kinetic constants and unbiodegradable particulate COD fraction apply to the UASB systems.
Knowing the COD concentrations of the Supi and residual Sbp in the WS, their VSS concentrations were calculated from their fƒcv values of 1.48 and 1.682, respectively, (as determined for the influent). The only ƒcv ratio still missing and required to obtain the overall (combined) ƒcv of the total particulate organics in the WS is that of the biomass. For instance, in the case of R1 at 1 500 mgSO42-/ℓ, the measured waste total particulate COD/VSS (ƒcv) and TKN/VSS (ƒn) ratios were 1.512 and 0.065 respectively. To match these measured values, the ƒcv of the biomass was found by iteration so that the combined ƒcv and ƒn equalled to the 1.52 and 0.065 measured. This yielded a biomass ƒcv of 1.599. Now, from this ƒcv of 1.599, the k and n values for l=7 and m=2 in the CkHlOmNn biomass composition were also determined by iteration and were found to be 5 and 0.55, respectively, giving a biomass composition of C5H7O2N0.55, identical to the C5H7O2N1 accepted by Sötemann et al. (2005b) in the UCTADM1 model, except for the N content. This biomass composition gives a TKN/VSS (fn) ratio of 0.072 mgN/mgVSS, compared with 0.124 mgN/mgVSS for C5H7O2N1. A different biomass N content is expected because Sötemann et al. (2005b) assumed the C5H7O2N1 composition from the commonly accepted value for activated sludge. From the residual Sbp, Supi and biomass individual COD concentrations in the WS, and their respective fn ratios, the combined ƒn ratio of the WS was calculated and found to be 0.063 mgN/mgVSS, very close to the measured value of 0.065 mgN/mgVSS as expected and therefore the C5H7O2N0.55 was accepted here. The same principle was applied for UASB R1 fed 1 800 mgSO42-/ℓ and UASB R2 (20ºC) fed 1 500 mgSO42-/ℓ. It was found that using the biomass composition of C5H7O2N0.55 and biomass ƒcv ratio of 1.599, the calculated waste sludge ƒcv and ƒn closely matched the measured waste sludge ƒcv and ƒn values of both systems.
As stated above, the saturation (Contois) hydrolysis rate equation and its associated kinetic constants were used in the hydrolysis kinetic part of the steady-state BSR model (and in the dynamic simulation model, UCTADM1-BSR, Poinapen and Ekama, 2010). At 35ºC, the saturation maximum specific hydrolysis rate KM = 5.27 gCOD organics/(gCOD biomass·d) and the half saturation coefficient KS = 7.98 gCOD organics/gCOD biomass. For UASB reactor R2 operated at 20ºC, both KM and KS were adjusted for temperature dependency in the steady-state model application. The temperature function 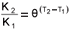 was used where K1 and K2 are here the maximum specific hydrolysis rate constants at T1 = 35°C and T2 = 20°C, respectively, and θ = 1.133. KM20 was found to be 0.808 gCOD organics/(gCOD biomass·d) and KS= 1.223 gCOD organics/gCOD biomass.
was used where K1 and K2 are here the maximum specific hydrolysis rate constants at T1 = 35°C and T2 = 20°C, respectively, and θ = 1.133. KM20 was found to be 0.808 gCOD organics/(gCOD biomass·d) and KS= 1.223 gCOD organics/gCOD biomass.
SS BSR model application
Once developed and calibrated, the SS BSR model was validated by applying it to the UASB systems operated in this study. Table 1 compares the SS BSR predicted results with the experimental data from the 2 laboratory-scale UASB reactors. Overall the SS model predictions correspond well with the measured data for all 3 systems. The COD removal is lower for the measured data because the UASB effluent contains some particulate COD (since the effluent is not 100% soluble COD) while in the steady-state model this is assumed to be zero. The predicted digester pH values for R1 (1 800 mgSO42-/ℓ) and R2 correspond very well to the measured values, while for R1 (at 1 500 mgSO42-/ℓ) it is 0.1 pH unit lower. Though not significant, this pH difference may be ascribed to either minor experimental error in pH measurements or to the composition of the primary sludge. The composition of the influent biodegradable particulate organics (BPO) CxHyOzNa is calculated from the influent PSS characterisation using the stoichiometric equations and is found to be C3.35H7O1.45N0.45 which differs slightly from that found by Sötemann et al. (2005a) for the primary and humus sludge mixture of Izzett and Ekama (1992), i.e. C3.5H7O2N0.196 and their own tests on pure PSS, i.e. C3.65H7O1.97N0.190. In fact, with C-deficient substrates for BSR, as PSS is, it is possible to calculate the C released from the utilised biodegradable organics from the C content of the HCO3- concentration, which is known from the H2CO3* alkalinity, because no CO2 gas is generated by the system (Eq. (12) and the C in the biomass generated is small. For R1 (1 500 mgSO42-/ℓ), R1 (1 800 mgSO42-/ℓ) and R2, the C released in the utilisation of BPO, VFA and FRBO was 283, 349 and 300 mgC/ℓ, respectively, and the C in the H2CO3 Alk measured was 279, 326 and 275 mgC/ℓ respectively. This validates the C content of the biodegradable organics and established the C balance over the 3 systems, i.e. 98.5%, 94.0% and 91.6%. In addition, the good correlation between the measured and steady-state results suggests that the assumption of a completely mixed digester for the UASB reactor bed is valid and reasonable. This was made possible because of the introduction of the sludge recycle line from the top to the bottom of the reactor.
Conclusions
A steady-state AD model for BSR using PSS as carbon source and electron donor has been developed. The model comprises 3 sequential parts: a COD-based hydrolysis kinetics part, a C,H,O,N,P,S, COD and charge mass balanced stoichiometry part and a mixed weak acid/base chemistry part. The hydrolysis kinetics of PSS were taken from Ristow et al. (2005) and Sötemann et al. (2005a) since they concluded that this is the same for both sulphidogenic and methanogenic systems. From the stoichiometry, it was found that the PSS is carbon limited in that it does not generate sufficient HCO3- alkalinity for the sulphate reduction, i.e. its COD/C ratio is too high (>2.67), which accounts for the observed zero gas (CO2) generation. As a result, the H2S/HS- system provides the alkalinity shortfall, establishes the system pH and allows the C released in the utilisation of the biodegradable organics to be accounted for in the C of the H2CO3* alkalinity (HCO3-) generated. Once developed and calibrated, the model results were compared with experimental data from 2 laboratory-scale UASB reactors (operated at 35°C and 20°C, respectively) fed PSS and sulphate. The model-predicted results, including pH, correlate very well with the experimental results. This provides support for:
• The PSS hydrolysis rate determined by Ristow et al. (2005) and Sötemann et al. (2005a)
• The developed BSR stoichiometry which gives considerable insight into the inter-relationships between the biological processes and weak acid/base chemistry systems
• The method of characterising the organics via the ƒcv, ƒc, ƒn and ƒp ratios for sulphidogenic and methanogenic AD systems.
The SS BSR model also provides a basis for crosschecking the results of an integrated 2-phase (aqueous-gas) mixed weak acid/base chemistry and biological processes simulation model for BSR which is presented in the last paper of this series (Poinapen and Ekama, 2010).
Acknowledgements
This research was supported financially by the Water Research Commission, National Research Foundation and University of Cape Town and is published with their permission.
References
BRINK IC and EKAMA GA (2008) Measurement of composition of organic constituents of municipal wastewater for plant wide modelling. Research Report No. W131, Department of Civil Engineering, University of Cape Town. [ Links ]
EKAMA GA (2009) Using bioprocess stoichiometry to build a plant wide mass balanced based steady state WWTP model. Water Res. 43 (8) 2010-2120. [ Links ]
HENZE M, GRADY CPL (Jnr), GUJER W, MARAIS GvR and MATSUO T (1987) Activated Sludge Model No. 1. IWA Scientific and Technical Report No. 1. IWA, London. [ Links ]
IZZETT HB and EKAMA GA (1992) The effect of thermophilic heat treatment on the anaerobic digestibility of primary sludge. Research Report No. W76, Department of Civil Engineering, University of Cape Town. LIDE D (2001) CRC Handbook of Chemistry and Physics (82nd edn.). CRC Press, Boca Raton, FL. [ Links ]
LOEWENTHAL RE, EKAMA GA and MARAIS GVR (1989) Mixed weak acid/base systems – Part 1: Mixture characterisation. Water SA 15 (1) 3-24. [ Links ]
LOEWENTHAL RE, WENTZEL MC, EKAMA GA and MARAIS GVR (1991) Mixed weak acid/base systems – Part II: Dosing estimation, aqueous phase. Water SA 17 (2) 107-122. [ Links ]
McCARTY PL (1975) Stoichiometry of biological reactions. Prog. Water Tech. 7 (1) 157-172. [ Links ]
MOOSBRUGGER RE, WENTZEL MC, EKAMA GA and MARAIS GvR (1992) Simple Titration Procedures to Determine H2CO3* Alkalinity and Short Chain Fatty Acid Concentrations in Aqueous Solutions Containing Known Concentrations of Ammonium, Phosphate and Sulphide Weak Acids/Bases. WRC Report No. TT 57/92. Water Research Commission, Pretoria, South Africa. [ Links ]
POINAPEN J, EKAMA GA and WENTZEL MC (2009) Biological sulphate reduction with primary sewage sludge in an upflow anaerobic sludge bed reactor – Part 3: Performance at 20ºC and 35ºC. Water SA 35 (5) 543-552. [ Links ]
POINAPEN J and EKAMA GA (2010) Biological sulphate reduction with primary sewage sludge in an upflow anaerobic sludge bed reactor – Part 6: Dynamic Model. Water SA 36 (3) 203-214. [ Links ]
RISTOW NE, SÖTEMANN SW, LOEWENTHAL RE, WENTZEL MC and EKAMA GA (2005) Hydrolysis of Primary Sewage Sludge under Methanogenic, Acidogenic and Sulphate Reducing Conditions. WRC Report No. 1216/1/05. Water Research Commission, Pretoria, South Africa. [ Links ]
ROSE PD, CORBETT CJ, WHITTINGTON-JONES K and HART OO (2002) The Rhodes BioSURE® Process Part 1: Biodesalination of Mine Drainage Wastewaters, WRC Report No. TT 195/02. Water Research Commission, Pretoria, South Africa. [ Links ].
SÖTEMANN SW, RISTOW NE, WENTZEL MC and EKAMA GA (2005a) A steady state model for anaerobic digestion of sewage sludges. Water SA 31 (4) 511-527. [ Links ]
SÖTEMANN SW, VAN RENSBURG P, RISTOW NE, WENTZEL MC, LOEWENTHAL RE and EKAMA GA (2005b) Integrated chemical, physical and biological processes modelling – Part 2: anaerobic digestion of sewage sludges. Water SA 31 (4) 545-568. [ Links ]
WENTZEL MC, EKAMA GA and SÖTEMANN SW (2006) Mass balance-based plant-wide wastewater treatment plant models – Part 1: Biodegradability of wastewater organics under anaerobic conditions. Water SA 32 (3) 269-276. [ Links ]
Received 10 August 2009; accepted 15 February 2010.
1 To whom all correspondence should be addressed.
+2721 650 2585; fax: +2721 689 7471;
e-mail: George.Ekama@uct.ac.za