Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Water SA
On-line version ISSN 1816-7950
Print version ISSN 0378-4738
Water SA vol.35 n.5 Pretoria Oct. 2009
The influence of land use on water quality and diatom community structures in urban and agriculturally stressed rivers
G Walsh*; V Wepener
Centre for Aquatic Research, Department of Zoology, University of Johannesburg, PO Box 524, Auckland Park 2006, Johannesburg, South Africa
ABSTRACT
Epilithic diatom communities offer a holistic and integrated approach for assessing water quality as they remain in one place for a number of months and reflect an ecological memory of water quality over a period of time. The objective of this study is to use diatom assemblages to distinguish between particular land types and associated water quality impacts that are linked to these land-;use patterns. Water quality and diatom community data were collected from sites in the Crocodile and Magalies Rivers (Gauteng and North West Province, South Africa) associated with agricultural, urban and natural (reference) adjacent land use respectively. The data collected were subjected to multivariate statistical techniques to analyse spatial and temporal patterns in water quality (principal component analysis) and diatom community structures (non-;metric multidimensional scaling) to elucidate hypothesised differences in community structure per land-;use type. Five diatom response indices (Generic Diatom Index, Specific Pollution Sensitivity Index, Biological Diatom Index, Eutrophication/Pollution Index and Percentage Pollution Tolerant Valves) incorporated in the OMNIDIA software were implemented to assess the integrity of diatom communities per land-;use type. Principle component ordination of water quality describes 56.6% of the variation in data observed, and indicates the separation of reference sites from test sites for low and high flow conditions combined. It was, however, not possible to distinguish between the agricultural and urban land-;use sites using PCA based on water quality data. One-;way ANOSIM showed a significant difference (p < 0.05) between reference groups, agricultural groups and urban groups, with no significant differences noted (p > 0.05) between groups made up of sites exhibiting the same land-;use patterns. Diatom indices showed that agricultural sites were in a slightly more modified ecological state than urban sites overall. Based on the species similarity (SIMPER analyses), reference sites showed strong associations with Achnanthes minutissima, Gomphonema venusta and Cocconeis placentula var. euglypta, whilst urban sites were associated with Diatoma vulgaris, Navicula tripunctata and Amphora pediculus. Agriculture could be separated into high-; and low-;intensity practices based on species composition. Sites where high-;intensity agriculture took place were dominated by motile species of the genus Nitzschia, and low-;intensity agriculture was indicated by motile species of the genus Navicula. Urban sites contained a combination of species that were tolerant of spikes in water quality.
Keywords: diatoms, agriculture, urban, land use, water quality, community structure, aut-;ecological indices
Introduction
The use and relevance of diatoms as biomonitoring tools in riverine environments in South Africa have recently been investigated (De la Rey et al., 2004; Harding et al., 2005; Taylor et al., 2005b; Taylor et al., 2007a). Diatoms have recently been used in the State of the Rivers Report for the Crocodile (West) Marico Water Management Area as an indicator of water quality and have been useful in indicating specific water quality problems such as organic pollution, eutrophication and heavy metal pollution (RHP, 2005; Taylor et al., 2005b). The motivation for using diatoms for biomonitoring is that they are cosmopolitan, their cell cycle is rapid and they can provide a relatively rapid indicator of disturbance. Unlike other aquatic biota, diatoms do not have specialised habitat niches and are not predominantly governed by streamflow.
Because diatoms comprise a large proportion of epilithic algal communities they provide a representative group of species that are indicative of the effects of specific water quality problems. Changes in water chemistry will inhibit the multiplication of some species, while supporting that of others, thus the percentage composition of certain species within a community will be changed (Cholnoky, 1960 cited in Harding et al., 2005). Changes in species composition can thus be used to reflect changes in water quality in a more integrated manner than traditional monitoring of water chemistry.
Modern agriculture is responsible for chemical and physical impacts due to increased contaminant and nutrient runoff, increases in suspended solids and changes in discharge and channel morphology (Skinner et al., 1997). Thus, potentially there could be high water pollution at sites where the upstream and adjacent land use is agricultural. A multi-;spatial scale assessment of land-;use and diatom assemblages using partial canonical correspondence analysis (CCA) showed that the percentage of agricultural land use at varying spatial scales explained between 3.7% to 6.3% of variability in the diatom species dataset, suggesting that linkage between agricultural land use and diatom community structure was weak (Pan et al., 2004). This study did, however, show that 72% of diatom taxa sampled from agriculturally impacted streams were salt-;tolerant taxa which suggest that sampled streams are affected by salinisation due to irrigation. It is possible to use diatom community structures to differentiate between impacts of urban wastewaters and farmland nutrient pollution, with subtle differences between eutrophication and biodegradable organic pollution discernible through the presence of certain indicator species (Rott et al., 1998).
The objective of this study is to compare and relate changes in diatom species assemblages to land use. This was undertaken by elucidating how water chemistry is changed by land-;use patterns (agricultural, urban and relative reference) and, subsequently, how community structures of diatoms are modified due to agricultural impacts on the aquatic system. The relative importance of the effects of agricultural land use and other environmental variables such as water chemistry on diatom assemblages will be explored. Two seasons of water quality and diatom data were collected at 7 sites to elucidate the effects of land use on stream water chemistry and diatom assemblages between seasons.
Materials end methods
Study sites
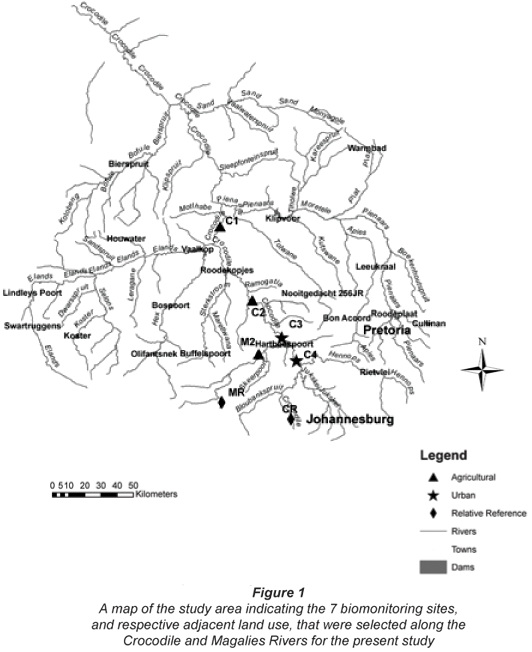
Two different types of study sites were selected, namely 'reference' sites that were unimpacted sites relative to the impacts being assessed in this study; and monitoring sites, which have site-;specific impacts relating to land use (Eekhout et al., 1996).
A total of 7 sites, 2 on the Magalies River and 5 on the Crocodile River, were selected. These study sites, as well as their main adjacent land uses, are shown in Fig.1. They range from perceived (based on land-;use GIS-;based shape files and aerial imagery) least-;impacted to highly impacted in terms of land-;use practices. Field sampling took place during a high-; (April 2006) and a low-;flow (August 2006) period.
Water quality
Physicochemical variables were measured in situ in flowing sections of each river at each site before biotic sampling was carried out and included pH, temperature (°C), dissolved oxygen (mg/ℓ and % saturation) and electrical conductivity (µS/cm). Handheld water quality meters were used for in situ analysis (Eutech pH 110 RS232C; Eutech DO6 dissolved oxygen meter and a Eutech CON 110 RS232C conductivity, TDS and temperature meter).
Single subsurface water samples were collected at each site. Spectrophotometric analysis was carried out on water samples using a Merck Photometer SQ 118. Variables measured in the laboratory using the spectrophotometric method were turbidity (NTU), nitrite (mg/ℓ NO2-;N), nitrate (mg/ℓ NO3-;N), orthophosphate (mg/ℓ PO4-;P), total phosphate (mg/ℓ TP), calcium (mg/ℓ Ca), soluble chloride (mg/ℓ Cl), sulphate (mg/ℓ SO4), ammonium (mg/ℓ NH4-;N), ammonia (mg/ℓ NH3-;N) and chemical oxygen demand (COD expressed as mg/ℓ O2). Water quality results were compared to the Target Water Quality Requirement (TWQR) figures for aquatic ecosystems as set out by DWAF (1996).
Total suspended solids (TSS), total suspended organic matter (TSOM) and total suspended inorganic matter (TSIM) were determined by filtering 1 ℓ of water collected from each site, for each sampling season, through a pre-;weighed, pre-;dried filter membrane (47 mm/0.45 µm pore size), using a Millipore glass vacuum filtration system. Once the samples had been filtered, the filter papers were dried at 60ºC for 48 h, after which they were weighed again. The dried filter paper was placed inside a pre-;dried and pre-;weighed crucible and incinerated at 600ºC for 8 h to determine the proportions of TSOM to TSIM. Total suspended solids concentrations were estimated using the weight of residuals on the membrane filters and was expressed as g/ℓ.
Diatoms
Epilithic diatom field sampling and laboratory procedures were carried out according to the methodology described by Taylor et al. (2005b). Diatoms were collected from 5 cobbles in a 10 m reach of the flowing sections of the rivers. Diatom samples were prepared for microscopy by using the hot hydrochloric acid (HCl) and potassium permanganate (KMnO4) method (Hasle, 1978). The taxonomic guide by Taylor et al. (2007b) was consulted for identification purposes in this study. Where necessary, Krammer and Lange-;Bertalot (1986; 1988; 1991a; b) were used for identification and for confirmation of species identification. Multiple endpoints were used (species assemblages, aut-;ecological metrics and diatom indices) to relate diatom assemblages to land-;use impacts. The diatom indices used in this study, as well as reasons for their selection are listed in Table 1.
For the purposes of this study 300 to 600 diatom frustules were counted for ecological analysis (Prygiel et al., 2002). Suggested rules for counting diatoms according to CEN (2004) were followed. A Zeiss Photomicroscope I with differential interference contrast optics (DIC) was used for identification and enumeration at a magnification of 100 x 1.3 NA (oil immersion objective). The microscope was attached to a JVC video camera with a frame grabber and the images were captured using Automontage software.
Diatom community data were entered into OMNIDIA (Lecointe et al., 1993) software which incorporated the above indices, with the calculation of the index scores. In all cases (excluding the % PTV) the diatom indices were calculated using the weighted average formula of Zelinka and Marvan (1961) (cited in Taylor et al., 2007a):
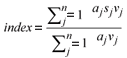
where:
aj = abundance of species j in sample
vj = indicator value
sj = pollution sensitivity of species j
For all of the above indices (except % PTV which has a maximum value of 100) the maximum value is 20, where a score tending to zero indicates an increasing level of pollution or eutrophication. Class values for diatom index scores for SPI, GDI, BDI and EPI indicating varying levels of pollution were awarded according to Eloranta and Soininen (2002). These values were used in this study for the interpretation of the scores yielded by the various indices.
Statistical data analyses
Primer Version 6 was used for the statistical analysis of water quality data. Temperature data were excluded after initial analyses because of the contribution of the variation in temperature between seasons to skewness in the data. Selected water quality data variables (DO, conductivity, pH, NO2-;N, NO3-;N, COD, NH3-;N and NH4-;N) were log-;transformed due to the skewness in data indicated by the scatter plot for variable pairs (Draftsman Plot). Principal component analysis (PCA) was carried out on normalised data. A lower triangular resemblance matrix was created based on the Euclidean distance between samples. The resemblance matrix was subjected to two-;dimensional non-;metric multidimensional scaling (NMDS). Finally, the BIOENV procedure using biota and/or environment (BEST) matching based on Spearman's correlation was used to identify variables that best explained species ordination.
Diatom community statistics
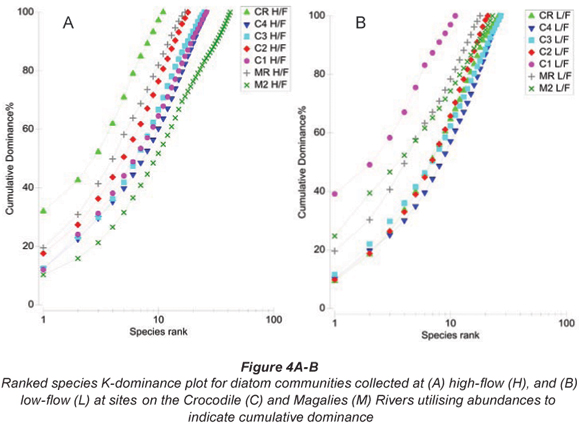
Primer Version 6 was used to construct Bray-;Curtis similarity matrices from square-;root transformed diatom species abundance data recorded for each site on high-; and low-;flow occasions. Similarity matrices were subjected to group-;averaged hierarchical clustering (CLUSTER) and ordination by NMDS to summarise patterns in species composition. Factors were assigned to Bray-;Curtis resemblance matrices based on groupings from the CLUSTER analysis and NMDS ordination. Permutation-;based hypothesis testing using one-;way Analysis of Similarities (ANOSIM) was undertaken to determine the extent of the differences between diatom community structures for the different samples at different flow periods. Pair-;wise comparisons from ANOSIM were used to identify significant differences (p < 0.05) between groupings of diatom community compositions. One-;way Analysis of Similarity Percentages (SIMPER) based on species contribution was used to identify the species of diatoms that primarily provided discrimination between sample clusters. K-;dominance plots were included to indicate sites that have an increased dominance of species relative to the other samples and flow periods.
Results
Water quality
Water chemistry was characterised by spatial and temporal variability in water variables among the 7 sites during both high-; and low-;flow periods (Table 2). The data indicate that the measured variables were generally within the target water quality range for aquatic ecosystems (DWAF, 1996). Exceptions were increased NO3-;N levels for relative reference sites CRH, agricultural Site C2L and urban influenced Sites C4H and C4L; increased PO4-;P levels at urban impacted Sites C4H, C4L and C3H; increased conductivity, SO4 and Cl levels at the agriculturally influenced Sites C1 and C2 for both seasons and a sharp increase in COD levels from high to low flow for Site C3 (2 to 17 mg O2/ℓ), and Site C4 (0.5 to 5 mg O2/ℓ).
The TSS increased for all sites except for CR from high flow to low flow (Table 2). The TSIM fraction of the TSS increased from high to low flow at agricultural Sites C1, C2 and M2, as well as C3 which has an urban influence. The remainder of the sites, which include the relative reference sites and Site C4 showed an increase in the organic fraction between seasons.
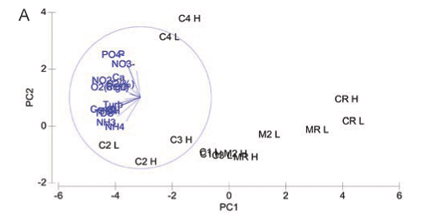
Relationships between sites and water quality variables are displayed using PCA bi-;plots showing samples and water quality parameters (Figs. 2A to C). Measure of fit is indicated by the length of the arrow in relation to the placement of the variable, and the distance between sampling sites approximates the dissimilarity of water chemistry as measured by Euclidean distance (Clarke and Warwick, 2001).
Temporal analysis
Principal component analysis for water quality variables at both high and low flow showing (dis)similarity amongst study sites on the Crocodile and Magalies Rivers is represented in Fig. 2A. The two-;dimensional PCA bi-;plot describes 56.6% of the variation in data, where 41.1% is displayed on the 1st axis and 15.5% is displayed on the 2nd axis.
The reference Sites CRH, CRL, MRL, MRH and Magalies River agricultural test Sites M2H and M2L were separated from the remainder of the sites along the PC1 axis, indicating their dissimilarity to test sites. Urban Site C4 was more influenced by TP levels at both high and low flow than the other sites, and thus is separated from the other sites on the PC2 axis. No apparent trends in water quality based on the specific land-;use practices are indicated in the PCA for the remainder of the urban and agricultural test sites on the Crocodile River; however, there was a degree of spatial variation for the remainder of the test sites. The subset of water quality variables that best described the existing classification and placement of sites at high flow according to BIO-;ENV matching are DO (%), TP, PO4-;P , Cl and NH3-;N.
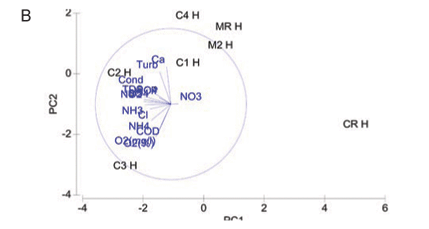
A PCA ordination of water quality variables for study sites at high flow is indicated in Fig. 2B. The two-;dimensional bi-;plot describes 67% of the variation in data, where 46.9% is displayed on the 1st axis and 20.1% displayed on the 2nd axis. Study sites on the Magalies River are similar to each other in terms of water quality, indicating spatial variation. Referring to specific variables agricultural Sites C1H and C2H varied in water quality due to the higher nutrient levels at C2H in comparison to C1H, but were similar in terms of higher COD, conductivity and turbidity levels overall. Urban test Sites C3H and C4H at high flow show dissimilarity on the PC2 axis that was contributed to by the difference in values for Ca, NH3-;N and NH4-;N. Urban sites were, however, very similar in terms their TP and PO4-;P levels, which were higher than those of the agricultural sites. The subset of water quality variables that best describes the existing classification and placement of sites at high flow according to BIO-;ENV matching remained DO (%), TP, PO4-;P , Cl and NH3-;N.
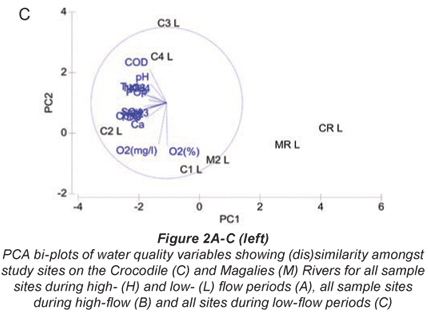
A PCA bi-;plot of water quality variables for all sample sites at low flow is shown in Fig. 2C. The two-;dimensional bi-;plot describes 74.0% of the variation in data, where 53.8% displayed on the first axis and 20.2% is displayed on the second axis. Variables from a 2D configuration plot, overlaid on the PCA plot showed that relative reference sites CRL and MRL, as well as M2L were similar in terms of water quality at low flow. Water quality variables contributing to this similarity were relatively low levels of turbidity, conductivity, nutrients and COD. Urban sites were separated from agricultural sites on the PC2 axis for low flow indicating trends in water quality that may be related to land use. Urban sites were placed due to the higher COD, PO4-;P, TP and NO3-;N levels relative to the agricultural sites. Agricultural sites differed from the urban sites in terms of higher conductivity and salts (Cl and SO4) levels. Relative reference sites are shown to be less influenced by water quality drivers according to the NMDS ordination. A trend was noted between water quality and land use in Fig. 3, where sites that have agriculture as their main land use have strong positive correlations to DO, and where urban impacted sites show negative correlations to DO. According to BIO-;ENV matching, the subset of water quality variables that best described the placement of sites at low flow was conductivity, pH and NH3-;N.
Diatom community composition
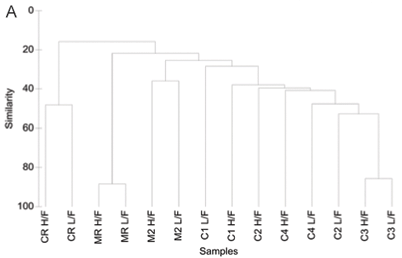
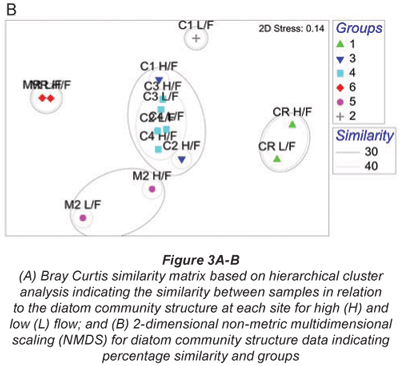
A total of 99 diatom species were identified from the 7 study sites. Species richness varied from 11 to 42 with an average of 23. The grouping of sample sites according to CLUSTER analysis and NMDS ordination based on diatom assemblages for high and low flow are shown in Figs. 3A and B. The NMDS ordination indicated that sites group mostly according to land use, with the exception of Site C1L which was an outlier, and Site C2L which is an agricultural site that grouped with sites exhibiting urban impacts.
One-;way ANOSIM showed that there was a significant difference (p < 0.05) between relative reference groups, agricultural groups and urban groups. There were no significant differences (p > 0.05) between groups that were made up of sites exhibiting the same land-;use patterns.
The diatom species for high-; and low-;flow periods that contributed to the groupings of sample sites and similarity within the diatom groupings are shown in Table 3. The reference sites were contained in Group 1 (Crocodile River relative reference group) and Group 6 (Magalies River relative reference group), and these 2 groups showed different species contributions in relation to each other. SIMPER analysis for the Magalies River reference site showed that Achnanthes minutissima (Syn. Achnanthidium minutissimum) and Gomphonema venusta were dominant species, whereas Cocconeis placentula var. euglypta and Navicula gregaria were dominant for the Crocodile River reference group.
The agriculturally influenced sites (with the exception of Site C2L) were contained within 3 different groups, separated seasonally and spatially, with varying dominant taxa; Group 2 (dominated by Diatoma vulgaris), Group 3 (Nitzschia frustulum and N. palea) and Group 5 (Navicula tripunctata and N. cryptotonella). Urban sites were placed together in Group 4, along with agricultural Site C2L. These sites were dominated by 3 species of relatively equal contributions, namely D. vulgaris, N. tripunctata and Amphora pediculus.
Ranked species k-;dominance plots for diatom communities indicated by Figs. 4A and B show relative species abundance as a percentage of the total abundance, plotted for each site. At high flow the site where there was a dominance of a single species is CRH, whereas at low flow the sites that are dominated by a particular species are Sites C1L and M2L.
Diatom index scores
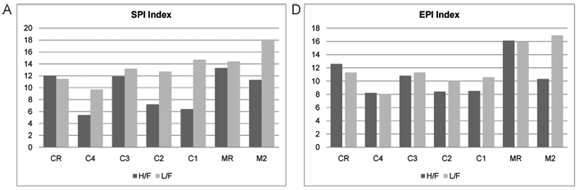
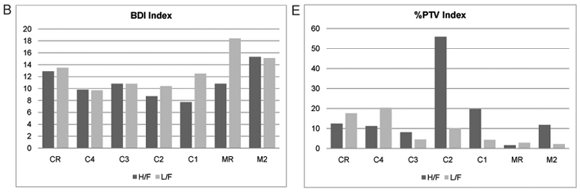
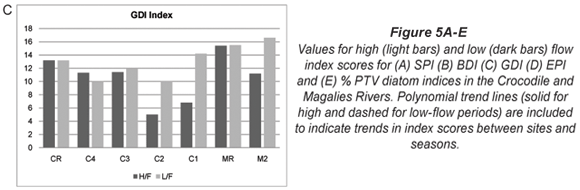
The index scores calculated by OMNIDIA for the selected diatom indices as well as their classes for each site on the Crocodile and Magalies Rivers at high-; and low-;flow periods are shown in Table 4 and Figs. 5A to E. Diatom index scores are presented as values from 0 to 20, where a decreasing score indicates an increasing level of pollution or eutrophication.
The diatom index scores indicated that the Magalies River was in an overall better class of ecological health than the Crocodile River. The agricultural Sites (C1 and C2) were in a slightly more modified ecological state than urban impacted Sites (C3 and C4) referring to Fig. 5. The exception is urban Site C4, which has the lowest scores for the SPI and EPI diatom indices (Figs. 5A and D), where Site C4 was the more impacted site of the two urban land-;use sites according to the diatom indices. Agricultural Site C2 showed the lowest overall scores for GDI and an increased tolerance to organic pollution (Figs. 5C and E).
The overall diatom index scores for the Magalies River ranged from moderate to high integrity, and the percentage of diatom species that are tolerant to organic pollution (% PTV) were low (Table 4). It was noted that M2 (agricultural test site) was classified as having a higher integrity than CR (reference site for Crocodile River).
During high flow, the overall integrity classes of the agricultural sites were 'poor' according to diatom indices, whereas integrity classes increased in the low-;flow season. Urban sites were generally classed from 'moderate/poor' to 'moderate' overall. There was an overall increase in the integrity from high flow to low flow noted for the diatom index classes.
Sites on the Magalies River were in a state of oligotrophy (with the exception of M2H which at high flow was classed as eutrophic) and diatom communities present were made up of species that had a continuously high DO requirement (Table 5). There was a low DO requirement at high flow for M2, indicating impairment of the diatom community due to changes in water quality.
The agricultural sites were in a state of eutrophy, were β-;mesosaprobous and had a low to moderate DO requirement (Table 5). Diatom communities at Site C1 at high flow showed that the salinity was tending towards brackish-;fresh indicating an increase in halophilous species at this site. Site C2 was classed as nitrogen heterotrophic tolerant, indicating an increased number of diatom species that are dependent on periodically elevated concentrations of nitrogen. It is important to note that CR at low flow shows α-;mesosaprobity, which indicates a site that is strongly polluted with restricted fauna due to an increase in organic pollution.
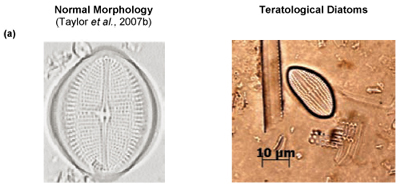
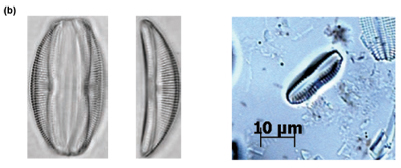
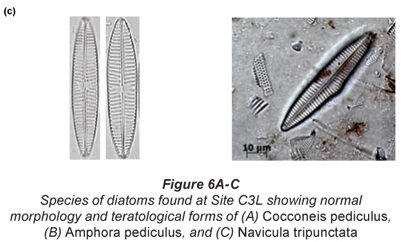
Of particular interest was Site C3 at low flow, where individuals of Cocconeis pediculus (Fig. 6A), A. pediculus (Fig. 6B) and N. tripunctata (Fig. 6C) having reasonably severe deformities were observed.
Discussion
Water quality
Referring to water quality data and results collected at high-; and low-;flow seasons for relative reference, agricultural and urban test sites during 2006, it is evident that, overall, agricultural sites displayed increased conductivity, TSIM, SO4 and Cl levels, whereas urban sites were impacted by elevated levels of nutrients (in particular TP) and COD. Water quality in relation to site land uses are discussed below (Table 2).
Agricultural sites
As one would expect from a site dominated by agricultural activities, conductivity values were relatively high at Site C1 (Schofield and Ruprecht, 1989; Williams, 1987; Williams, 2001). Values for conductivity (612 µS/cm to 739 µS/cm) increased from high to low flow, as did salt concentrations with values increasing from 286 mg/ℓ to 412 mg/ℓ for Cl and 63 mg/ℓ to 124 mg/ℓ for SO4 respectively. An increase in TSS from 0.0108 g/ℓ to 0.04 g/ℓ from high to low flow, most of which was in the inorganic fraction, was noted.
The seasonal trend in the pollution gradient (i.e. an increase in variable concentrations from high to low flow) for C1 may be explained by a combination of decreased flows in the winter months and abstraction of water for irrigation that leads to concentration of these variables. Data from this study agrees with studies done by Qader (1998) who found that reduced flows were correlated with an increase in sedimentation and conductivity concentrations downstream of abstraction points and reduced the dilution capacity of the river.
An increase in TSS and TSIM has been shown to be directly correlated with stripping of land and riparian zones for crop planting (Allan et al., 1997). Centre-;pivot farming occurs extensively along the length of the Crocodile River at, and preceding this site.
Nitrogen enrichment in the form of elevated levels of NO3-;N also occurred from high to low flow for C1 (0.30 mg/ℓ to 3.50 mg/ℓ). When considering that historic data for this section of river collected by DWAF (2008) from 1993 to 2006 showed that the 90th percentile for NO3-;N was 1.011 mg/ℓ, and the median value 0.129 mg/ℓ, this value has increased exponentially. Inorganic nitrogen content may increase in rivers that are downstream from dams that are not efficiently regulated (Deksissa et al., 2003). Releases from Roodekopjes Dam, upstream of Site C1 as well as fertiliser utilisation in the area may explain the increase in NO3-;N levels. Flows from Vaalkop Dam on the Elands River may also have impacts in terms of intermediate levels of nutrients which are speculated to come from surrounding platinum mines (RHP, 2005).
Water quality impacts for Site C2 were much the same as for Site C1, with a downward trend in water quality from high flow to low flow. When compared to historical data collected from the DWAF weir at Brits, these water quality variables have increased from previously recorded values (DWAF, 2008).
Overall, nutrient levels at Site C2 were higher than at Site C1, with an increase in COD levels. The town of Brits and the sewage works are situated upstream of this site and in conjunction with the intensive cultivation and orchards leading up to this site this may contribute to the increase in nutrients and COD. According to DWAF (1996), oxidisable organic matter originating from waste discharges causes spikes in COD. Increases in inorganic nitrogen concentrations are usually met with increases in the COD and pH values; this agrees with data collected at this site.
Site C2 at low flow particularly, showed elevated nitrogen and TP levels, which would explain its grouping with urban sites which are more likely to be affected by increases in nutrient values due to point source discharges of domestic and sewage effluents (DWAF, 1996).
One would expect the cumulative effect of the intensive agricultural practices prior to C1 to show a decrease in the water quality from Sites C2 to C1. However, this is not the case and water quality at the downstream agricultural Site C1 appears to be of a better quality than that of Site C2. An explanation is that the Elands River joins the Crocodile River just before Site C1, and water from the Elands River is known to be in a 'fair' state, while that of the Crocodile River is 'poor' (RHP, 2005). This confluence may have a dilution effect on pollutants in the middle Crocodile River. The combination of cumulative agricultural and urban water quality impacts at Site C2 appears to have an additive and interactive effect on water quality at this site.
Impacts in terms of water quality at Site M2 were nominal in comparison to agricultural test Sites C1 and C2. The relatively low water quality impact at agricultural test Site M2 is of interest, as there is intensive cultivation and livestock farming occurring along the length of the Magalies River upstream of this site. A study on land use and instream integrity in the Crocodile (West) Marico WMA, looking at the relative extent of different land uses at different spatial scales (ranging from 100m to catchments) showed that cultivated land was not a significant predictor of instream integrity at any scale (Amis et al., 2007).
This section of the Upper Crocodile sub-;management area is less populous and exponentially less urbanised than quaternary catchments where the other agricultural test sites have been selected (DWAF, 2004). The site borders on the Magaliesberg Nature Area and this Reserve may have remediation effects on water quality. Amis et al. (2007) did find that the total area under natural vegetation was the best predictor of instream health, as it inferred good riparian integrity and hence good water quality control.
Urban sites
Site C4 – above Hartbeespoort Dam
Site C4 is on the Crocodile River above the Hartbeespoort Dam wall, and below the confluence of the Hennops River. Referring to Table 2 nutrient values were very high overall and similar on both sampled occasions.
A value exceeding 10 mg/ℓ for inorganic nitrogen is considered to be indicative of hypertrophy, in which there is very low species diversity, but in which algae flourishes (DWAF, 1996). Levels of NO3-;N were above 25 mg/ℓ, a level that is toxic to human beings (Dodds and Welch, 2000). The immediate surroundings are natural, but there is an urban influence in terms of water quality from upstream land use, and this part of the river receives effluent from the Northern Sewage works which services the extensive urban settlements at Alexandra and Diepsloot. This would explain the consistently high values noted with regard to nutrients, as nutrients are being received from a point source.
Historical water quality data collected from 1972 to 2006 show that the 90th percentile and median values for NO3-;N recorded from water received from the Hennops was 7.9 mg/ℓ and 4.6 mg/ℓ respectively, with the highest recorded value measuring 27.6 mg/ℓ (DWAF, 2008). Water quality is known to be 'poor' at this point of the Crocodile River, because of high levels of nutrients received from the Hennops and Jukskei Rivers. The high nutrient levels are caused by sewage spillages and industry discharges into the sewer system. Increased development in Soweto and inadequate infrastructure is the likely cause for these increases in NO3-;N levels (RHP, 2005).
Sewage effluent regularly contains NO3 concentrations that exceed the TWQR's. Industrial effluent received from the urban area increases the oxygen demand. This severe nutrient and chemical loading is accompanied by increases in COD values (DWAF, 2008). As was noted, there was a rise in the COD level from 1 mg/ℓ in summer to 5 mg/ℓ in winter.
Site C3 – below Hartbeespoort Dam wall
At this site the main impairments in water quality were changes from summer to winter of NO3-;N levels (0.80 mg/ℓ to 9.30 mg/ℓ), a sharp decrease in DO (10.91 mg/ℓ to 3.79 mg/ℓ) in a manner consistent with COD increase (2 mg/ℓ to 17 mg/ℓ).
The shift in COD levels indicates that the oxygen deficit has increased from <15% to <75% between seasons. These values also indicate a shift from a 'slightly' polluted oligosaprobic to a 'strongly' polluted ά-;mesosaprobic river reach (Taylor et al., 2007b). The water quality impacts are associated with the development and maintenance of the residential areas surrounding the dam and river, and impacts due to urban runoff and sewage effluent. It is well known that these activities are responsible for nutrient and COD increases (Morrison et al., 2001).
A tenfold increase in TSOM was noted for this site from summer to winter. This increase could be related to sewage inputs and algal blooms in the Hartbeespoort Dam. Algal blooms in this dam are common and well documented and will augment the organic fraction of the suspended sediments during spillage. The ongoing intensive construction prior to this site would contribute to the TSS values.
Reference sites
With the exception of high NO3-;N levels in the summer months (19.60 mg/ℓ), water quality parameter values for CR were within the TWQRs for aquatic ecosystems, and were generally quite low (DWAF, 1996). The adjacent land use is recreational; however the upstream land use is urban. A source of the high NO3-;N levels is urban run-;off as this is known to cause increases in nutrients (Carpenter et al., 1998).
As was expected for Site MR, there were no major water quality impacts with exception of somewhat higher conductivity (431 µS/cm and 256 µS/cm) values, and an increase in the organic fraction of the TSS from 0.016 g/ℓ to 0.0236 g/ℓ for this reach of the river.
Upstream of this site is a small aquaculture operation. Organic matter fractions in suspended solids have been shown to increase downstream from fish farms (Brown, 1996 cited in Dallas and Day, 2004). An increase in the organic fraction of the TSS in winter could also be due to leaf litter from the riparian canopy that covers the river. Illegal water abstraction of ground water from the aquifer supplying Maloney's Eye is more than likely a contributing factor to the increase in conductivity values from reference conditions due to lowered flows (Qader, 1998; Magalies River Forum, 2007)
All of the other water quality parameters fell within the TWQRs as set out by DWAF (1996). The dolomitic source of the Magalies River (Maloney's Eye), is a few kilometres upstream of this site, hence water variable concentrations were expected to be low as the upper reaches of rivers are generally oligotrophic (Davies and Day, 1998). Contributing to this good water quality is the land adjacent to MR which is in a relatively un-;impacted condition. As discussed earlier, natural land use is the best predictor of good stream integrity (Amis et al., 2007).
Relationships between water quality and land use
High and low flow combined
The 2 Magalies River sites (MR and M2) and the reference site on the Crocodile River are distinctively separate from the other sampling sites on the first ordination axis (PC1 axis -; Fig. 2). This is an indication that the water quality at the reference sites and Site M2 clearly differs in water quality parameter composition from the test sites. The reason for this separation would be that the water quality variables at these sites are lower, indicative of oligotrophic to mesotrophic conditions that tend to move away from pollution gradients that are shown in Fig. 2A.
A distinct separation between Site C4 and the remainder of the Crocodile River test sites (C1, C2, and C3) was noted along the PC2 axis (Fig. 2A). The placement of urban site C4 was due to consistently high nutrient levels that distinguished it from the other sites, in particular phosphorus levels. This is most likely from the influence of the sewage works that are associated with this site.
Interestingly enough, agricultural Site C2 and urban Site C3 showed more similarity in terms of placement than Sites C2 and C1 did. Referring to Table 2, Sites C2 and C3 were more similar in terms of nutrient levels (nitrogen levels in particular) than the agricultural sites were. Site C2 is impacted by sewage inputs from the town of Brits upstream, and this could be a possible factor influencing its similarity with the urban Site C3.
Agricultural Site C1 is more similar to the sites with better water quality than to the other more impacted sites. This shows that a recovery takes place downstream from agricultural Site C2 to Site C1. As discussed earlier, this is thought to be from the influence of the water of the Elands River that joins the Crocodile before Site C1 (RHP, 2005). Another plausible reason could be attributed to the Roodekopjes Dam that is upstream from Site C1. Dams may have the ability to settle out sediments and nutrients before water re-;enters the river.
Nearly half of the variation in the water quality for high and low flow combined is not explained in Fig. 1. This variation is likely to be related to the presence of unknown water quality constituents entering the system from the surrounding catchment. Ansara-;Ross et al. (2008) indicated that the agricultural sites below the Hartbeespoort Dam are at risk of being affected by large scale pesticide use. Further, in this paper we also ascribe gross diatom cell-;wall deformities to metal exposure. It is therefore likely that contaminants such as pesticides and heavy metals could offer more explanation in terms of the interpretation of Fig. 2A.
High flow
At high flow a separation of Sites CR, MR and M2 along the PC1 axis is again noted, indicating dissimilarity in variable concentrations in comparison to higher values noted for test sites (Fig. 2B). The close placement of both of the Magalies River sites on the PCA bi-;plot signifies a spatial dissimilarity to sites on the Crocodile River.
The reason that there are no distinct patterns that link water quality to land use in the high-; flow PCA is because each of the remaining test sites (C1, C2, C3 and C4) have variable combinations (combinations of concentrations) that are unique to each site. This is thought to be contributed to by a combination of water quality impacts from runoff from land after summer rains, and releases from respective dams that are upstream of Sites C1, C2 and C3. Land runoff would contain pollutants that would be associated with the practices of each specific adjacent land use, while dam water would contain a mixture of pollutants from a larger and more diverse, catchment area.
Urban Site C3 had the highest DO concentration noted for any of the sites, and these notably higher values contribute to its dissimilarity in relation to other test sites. The higher DO is probably due to the high cobble contribution to the instream habitat at this site which contributes to mechanical mixing.
Low flow
Figure 2C shows that a pollution gradient exists between CR, MR and M2, and the remainder of the test sites along the PC1 axis. As mentioned previously, the water quality values for these sites were low. Low flow data shows a separation of the agricultural sites (Sites C1 and C2) and urban sites (Sites C3 and C4). The placement of the agricultural sites is driven by variables such as salts and conductivity, whereas urban sites' placements were more driven by nutrient and COD. This indicates a more specific pollution gradient were distinct impacts are seen for each land-;use type. The variables contributing to placement at low flow are examples of well known specific pollution problems associated to each land use. Irrigation in the dry season is known to cause salinity gradients in agricultural regions due to abstraction and concentration of salts around the roots of crops (Moore et al., 1990 cited by Lemly, 1994; Williams, 2001).
Diatom community composition in relation to land use
Site groupings
Reference groups
Group 1 (Table 3 & Figs. 3A and 3B) contains the reference site for the Crocodile River (CR) at high and low flow. Cocconeis placentula var. euglypta, N. gregaria and Gomphonema pumilum contributed to 50.9% of the make-;up of this group. The species in Group 1 are all tolerant of eutrophic to hyper-;eutrophic environments and N. gregaria and G. pumilum can tolerate critically to strongly polluted waters (Taylor et al., 2007b). Figure 6a indicating K-;dominance curves for sample at high flow also showed disturbance at this site to be the greatest in terms of the dominance of C. placentula var. euglypta. Considering the adjacent river land use, this result was not expected.
The immediate surroundings of this site are recreational/ natural with a relatively intact riparian zone; however, on a wider spatial scale the upstream activities are of a high-;intensity, i.e. the dominance of high density residential areas on the runoff to the Crocodile River, which would explain the presence of these particular diatom species. This indicates that scale and continuum play an extremely important role and that instream health was not predicted by natural land cover at this scale. The extent to which land use influences the instream integrity is scale-; dependent and generally is more predictable on a catchment scale rather than a localised scale as in this study (Allan et al., 1997; Amis et al., 2007).
The Magalies reference site (MR) for both seasons is contained in Group 6 (Table 3 & Figs. 3A and B). Achnanthes minutissima (21.80%) and G. venusta (11.49%) accounted cumulatively for 33.3% of the total diatom species contribution by abundance. The diatom A. minutissima is numerically dominant in upland streams and is typically found in rivers with low salinities and phosphorus concentrations, and dominates when turbidity values are low (Eloranta and Soininen, 2002; Blinn and Bailey, 2001). Taylor et al. (2007b) also noted that A. minutissima is generally found in clean, freshwaters that are well oxygenated. The findings of this study are in agreement with the findings of Lavoie et al. (2004), who found A. minutissima to be prevalent at reference/natural sites. In a study on organic and agricultural pollution, this species was found only in the uppermost river sites where conditions were oligotrophic (Rott et al., 1998).
Because G. venusta is thought to be an endemic species to South Africa, environmental preferences have not been established in European indices used for calculating integrity (Taylor et al., 2007b; Taylor et al., 2007c). In a study on the relevance of diatom-;based pollution indices to South Africa (Taylor et al., 2007c), G. venusta was shown to be associated with good water quality and with low levels of water quality variables at sites in the Crocodile (West) Marico WMA.
The presence of Cocconeis placentula and C. pediculus in the remainder of the similarity percentage make up suggests that possible underlying nutrient and salinity problems exist. Cocconeis placentula can tolerate mesotrophic to eutrophic conditions, whereas C. pediculus may tolerate moderate to high salinities (Van Dam et al., 1994; Hill et al., 2001; Taylor et al., 2007b). Higher than expected conductivity values, and an increase in NO3-;N from the high-; to low-;flow season confirms the indicator values of these species for this headwater site.
Agricultural groups
Agricultural sites were separated into Group 2, Group 3 and Group 5 (Table 3 and Figs. 3A and B). An unexpected positioning of Site C1L was noted when referring to NMDS and CLUSTER ordination based on diatom community structure for samples at high-; and low-; flow periods. It was expected that agricultural Site C1L would group with other agricultural sites on the Crocodile River at low flow (i.e. C2L). However, C1L was dissimilar to any of the other sites in terms of diatom community composition. This outlier group (Group 2) was placed due to the strong domination of D. vulgaris (77%) at this site (Table 5). This dominance of D. vulgaris is also noted in the K-;dominance plots for low flow, indicating disturbance at this site (Fig. 4B).
Environmental preferences for D. vulgaris include conductivity of 100 µS/cm to 500 µS/cm and mesotrophic to eutrophic conditions (Taylor et al., 2005a; Hill et al., 2001). According to Table 2 Site C1L exhibited an increase in Cl and SO4 levels for the low-;flow period, almost doubling from high-;flow figures, and showed an increase of close to 400% in TSS concentrations.
These findings contradict evidence of D. vulgaris being one of the most sensitive species in relation to increases in sediment loads and embeddedness in the USA (Blinn and Herbst, 2003). According to indicator values from the Netherlands, Diatoma spp. have relatively high indicator values for pH, organic nitrogen, oxygen, saprobity and trophic state (Van Dam et al., 1994). However, South African studies have linked D. vulgaris specifically to freshwaters with elevated levels of phosphate-;phosphorus (Taylor et al., 2007b). According to Taylor (2007) this diatom species is routinely found in high abundances below Bloemhof Dam, which occurs in an irrigated agricultural region. This somewhat contrasting information suggests that cosmopolitan indicators, such as D. vulgaris, which are used in European and American diatom indices of pollution, may not have the same value in a South African context. Apprehension has been expressed as to the viability of using data concerning the ecological preferences of diatoms between the Northern and Southern Hemispheres (Kelly et al., 1998).
When considering the remainder of the diatom community composition at C1L, impacts point in the direction of nutrient problems, more than salinity and conductivity problems as indicated by the water quality variable figures (Table 2). Amphora pediculus, Cocconeis pediculus, C. placentula var. euglypta and N. gregaria make up the majority of the remainder of the community. The combination of these sub-;dominant species with dominant D. vulgaris suggests that there were major problems related to eutrophication directly preceding sampling. At the low-;flow site visit, it was noted that there was a bloom of filamentous algae on cobbles which is indicative of over-;fertilisation of adjacent land and subsequent runoff into freshwater ecosystems. Increases in algal biomass were recorded in artificial channels of stream reaches that were artificially fertilised (Hart and Robinson, 1990).
As discussed above, NO3-;N levels rose from high to low flow far exceeding historic levels for this section of river. Preparation of land with fertiliser, and subsequent irrigation and runoff may be the cause of the increase in NO3-;N levels (Dallas and Day, 2004). Problems with interpretation of such results were shown by Biggs (1996). Over time many large-;scale indirect factors such as climate and geology, limit or obscure the expression of direct small-;scale factors such as nutrients and flow. This ultimately makes data interpretation difficult and ambiguous.
Crocodile River agricultural Sites C1H and C2H were placed in Group 3. The groups comprised N. frustulum, N. palea, A. pediculus and Aulacoseira granulata which are associated with increased salts, caused most probably from runoff from these highly cultivated areas. The composition of pollution-;tolerant taxa at this agricultural site points directly to salinity problems, which were noted in the water quality data.
Nitzschia frustulum and N. palea were linked to high conductivity gradients (5 000 to 7 500 µS/cm) in lowland streams with moderate to heavy agricultural practices. The above species and A. granulata were also found to be important indicators for high phosphorus environments in streams in Australia (Blinn and Bailey, 2001). Slightly elevated TP levels were noted at C2H.
When observing the individual composition of the diatom assemblages at C1H and C2H, Navicula recens and Nitzschia liebertruthii were found to be dominant at the respective sites. The presence of these species reiterates the presence of a salinity gradient at these agricultural sites, as Taylor et al. (2007b) describes these 2 diatoms as occurring in 'very' electrolyte rich to brackish waters. The significant contribution of the genus Nitzschia to this grouping also suggests siltation problems, as this genus is known to have many species that are motile and may escape the effects of siltation (Hill et al., 2001).
Group 5 was composed of agricultural Site M2 at high and low flow. As noted, water quality impacts for this agricultural site were less severe than sites on the Crocodile River. There was an increase in NO3-;N between the summer and winter months; however, the remainder of the water quality values were relatively constant. The combination of the present dominant species points strongly to a eutraphentic trophic state, bordering on hyper-;eutraphentic. The most dominant diatom of this group's assemblage is Navicula tripunctata, which is considered to be 'a good indicator of eutrophic waters that can tolerate critical levels of pollution' (Taylor et al., 2007b).
Two species belonging to the genus Navicula (N. tripunctata and N. cryptotonella) cumulatively make up the initial 27.37% of the assemblage of Group 5 (Table 3). Navicula spp. have been used in siltation indices as they are motile and can escape habitats that have silt issues (Kutka and Richards, 1996; Hill et al., 2001). Bahls (1993) reported that catchments that generate higher levels of silt that end up in receiving streams have a higher percentage of motile diatoms present, even if there are no other pollution gradients of disturbance present. Gomphonema parvulum, which made up 6.78% of the composition of Group 5, is also a sediment increaser taxa that is tolerant of 'extremely polluted waters' (Telpy and Bahls, 2006; Taylor et al., 2007b). Rott et al. (1998) showed N. tripunctata and Navicula cryptotonella were excellent indicator species in relation to agricultural disturbances. The frequency of occurrence for above taxa at sites associated with agriculture was 80%, and these species occurred at their highest abundances at these sites.
The presence of the diatom species that are moderately tolerant of pollution are counteracted by 2 diatoms that are associated with moderate to good quality waters (Taylor et al., 2007b); namely A. minutissima and Achnanthidium pyrenaicum (Syn. Achnanthes biasolettianum). According to Kahlert et al. (2009), an A. minutissima complex occurs which hosts 3 different varieties of the species that vary in water quality preferences according to their respective morphology, in particular the valve width. Although the valve width of A. minutissima individuals was not measured in the current study, we speculate that the A. minutissima noted in association with the Navicula species at Site M2 (Group 5) was a more tolerant variety of the complex. Achnanthes minutissima is a pioneer coloniser in systems where the water velocities are high, as they are capable of strong attachment to substrate and have a rapid growth rate (Kelly, 2002; Martinez de Fabricius et al., 2003), and is also associated with calcareous waters of dolomitic springs which are associated with the area (Sabater and Roca, 1992; Taylor, 2009). The combination of species noted at Site M2 infers that a combination of recent physical disturbance, geo-;hydrological contributions and the underlying geology are factors contributing to the assemblage.
Site M2L shows a large degree of dominance by a single species (A. minutissima) in K-;dominance plots for low flow (Fig. 6B). This species is a common pioneer species in head waters, and often dominates surfaces that have previously been impacted by physical abrasion or by pollution. The dominance of this species for low flow would indicate the recovery of this site from an unknown impact.
Urban group
Species making up the composition of Group 4, which contained mostly urban-;impacted sites, were not entirely unique to this group. One diatom species that does stand out however, is Eolimna subminuscula. This species is a very good indicator of industrial organic pollution and of strongly polluted waters (Gevrey et al., 2006; Taylor et al., 2007b). Urban sites did exhibit high nitrogen and phosphorus levels overall, and it is well known that compounds of nitrogen and phosphorus are often present in high concentrations in organic discharges.
Examining more closely the species composition of Sites C3 and C4 at high and low flow, one notices the contribution of Fistulifera saprophila, Mayamaea atomus and Eolimna minima to the urban sites. These species are considered to be some of the most pollution-;tolerant diatoms and are generally indicative of organic pollution (sewage), or are associated with organic detritus (Taylor et al., 2007b). The increases in COD from summer to winter at these sites would select for the above species.
The grouping of Site C2L with urban sites in Group 4 was interesting. This site suffers from impacts of agriculture and urban practices with sewage discharges contributing to an increase in the organic pollution at this site. Rott et al. (1998) found that sites that were impacted by both agricultural and urban practices were highly affected in terms of water quality. It appears that in the low-;flow season water quality, and hence diatom assemblage, is more affected by organic inputs rather than inorganic nutrients from farming practices than in the low-;flow season. Author to check
Diatom community integrity
A range of diatom indices that were tested by Taylor (2004) and Taylor et al. (2007a) were selected for representation of the extent of aquatic pollution at each of the study sites for the Magalies and Crocodile Rivers. Figures 5A to 5E and Table 4 indicate graphs and display trends in water quality as represented by the selected diatom pollution-;based indices. These data indicate that the Magalies River was more ecologically stable than the Crocodile River according to the diatom indices. The relative reference site MR remained in a 'Good' class overall, whereas the agricultural site showed recovery from high to low flow moving from a 'Moderate' integrity to a 'Good/High'. The water quality integrity for M2L as indicated overall by diatom indices superseded that of MR. These data are in agreement with the water quality data collected for the Magalies River in this study.
Huizenga (2004) used historical data to show that the Magalies and Skeerpoort Rivers did not show any sign of water quality pollution or change from the 1970's to present and had good to excellent water quality index scores. According to the causal principle (Schonfelder, 2000) this would provide an ambient environment that would favour the colonisation of diatoms that have ecological preferences for oligotrophic to mesotrophic water. The confluence of the Skeerpoort and the Magalies Rivers before Site M2 may be a contributing factor the good water quality noted at this site for low flow, as the Skeerpoort is known to have a good water quality and a high ecological integrity (RHP, 2005).
Referring to the Crocodile River, the indices that represent general water quality are the SPI, BDI and GDI (Figs. 5A to C). These indices show very similar trends in water quality between the seasons. What is interesting to note is the recovery in water quality integrity (mostly noted in the low flow samples) after the influence of large dams. For example, urban Site C4 (upstream) is separated from urban Site C3 (downstream) by Hartbeespoort Dam; and agricultural Site C2 (upstream) is separated from Site C1 (downstream) by Roodekopjes Dam. This trend may be attributed to the settling out of suspendoids and nutrients in these large dams, adsorption of some nutrients to sediments and phosphate removal by algae and macrophytes (Andersen et al., 2004).
The EPI (Fig. 5D) is designed for the measurement of the effect of nutrients on ionic strength and is influenced most significantly by phosphorus levels (Taylor, 2004; Dell'Uomo, 1996; Dell'Uomo et al., 1999). The EPI showed a similar trend in water quality in relation to the positioning of dams. From the comparison of high-; and low-;flow index scores for the Crocodile River, it is noted that there is an increase in the EPI after Hartbeespoort Dam wall, where after there is a downward trend to the first agricultural site with a very slight recovery after the Roodekopjes Dam wall.
The % PTV is defined as the sum of diatom taxa regarded as being tolerant to organic pollution. Once again, the trend noted in the % PTV Scores (Fig. 5E) indicates that the percentage of pollution-;tolerant valves decreases immediately after the influence of large dams. The only indication of heavy organic pollution (probably due to sewage inputs) was at Site C2 at high flow. This site had a value of 55.9%, indicating serious organic pollution. At C2 there are urban and agricultural impacts at different spatial scales associated with this site. Nutrient impacts from agriculture arise from a combination of smaller sewage inputs, livestock runoff, emissions of NH3-;N to air and leaching and run-;off of nitrogen (Jarvie et al., 1998). Nutrients from urban activities include large inputs of phosphorus to surface waters due to point sources of sewage and industrial effluents entering river systems (Dallas and Day, 2004). These results once again reiterate that the cumulative organic inputs of agricultural and urban impacts have synergistic effects on water quality that have severe impacts on primary producer communities such as diatoms.
There was a general increase in overall classes/scores from high to low flow (Table 4). A study in a catchment with mixed land use by Boyacioglu (2006) showed that under high-;flow conditions, water pollutants mainly originated from urban land use, while water quality was contributed to by agricultural pollutants during the low-;flow period. The reason noted for the results obtained for high flow was due to runoff from an increase in impermeable surfaces which weakens the buffering capacity of stormwater in urban areas. These data go against common belief in that a change in river flow is often inversely related to the concentration of constituents in the water (Stednick, 1991). Taking into consideration data from the current study and the study by Boyacioglu (2006), this would imply that agricultural pollution has a lower impact on overall water quality than urban practices do.
General ecological description and trophic classification
Using diatoms to monitor eutrophication and saprobity in rivers may be undertaken by the collection of a single diatom sample per season as diatoms have varying tolerance for nutrient increases and organic enrichment (Taylor, 2004; Van Dam et al., 1994). The changes in diatom communities that represent changes in nutrient and organic inputs are more holistic than chemical water quality monitoring as they indicate any impacts that may have occurred in the previous 6 weeks (Taylor, 2004). This section refers to Table 5.
Along a longitudinal gradient from the head waters to the middle reaches of rivers, there should be an increase in nutrients and organic material (Vannote et al., 1980). Looking at the Magalies River as a tributary of the Crocodile River, the trophic state was oligotrophic at MR (mountain stream) and M2 (upper/middle reaches) improved from a state of eutrophy to oligo-;mesotrophy between seasons (Table 5). The saprobity was β-;mesoprobous for all sites on the Magalies for all seasons, indicating a slightly to moderately polluted system in terms of organic matter.
The Crocodile River was eutrophic from the headwaters (Site CR) to the site situated most downstream in the middle reaches (Site C1) for both seasons with the exception of agricultural Site C1. This site showed a slight improvement in its trophic status from eutrophic at high flow to mesotrophic at low flow. According to Kelly (1998), in low-;flow conditions, rivers should resemble lentic systems and nutrients are usually retained in rivers in low flow. This contradicts the findings for Site C1. The reason for this is that runoff from rainfall in the summer months is a contributor of nutrients to this point. At low flow, there is less runoff from surrounding land, and thus less inorganic nutrients entering the system from over-;fertilisation. These nutrients would then be flushed off of the agricultural lands after the first heavy spate and return to the river causing the river to return to a eutrophic state in the summer months.
Site CR in the upper reaches of the Crocodile River should be in a state of oligotrophy according to its order (Vannote et al., 1980); however, this site is in a eutrophic state. This eutrophy is indicated by the presence of species that are tolerant to elevated levels of nutrients and that have low DO requirements. The saprobity at CR at low flow was α-;mesoprobous. This was the only site on the Crocodile River that showed definite saprobity problems as the rest were β-;mesoprobous for both seasons. An α-;mesoprobous state suggests that the river has been exposed to organic pollution and is in the initial stages of recovery where fauna is restricted and bacterial counts are high (Kolkwitz and Marsson, 1908 cited in Dallas and Day, 2004). This site does suffer from impacts that are related to high-;density urban activities which would explain the change in saprobity from high to low flow.
Diatom deformities
The presence of cell-;wall deformities in C. pediculus, A. pediculus and N. tripunctata noted at Site C3 at low flow are shown in Figs. 6A to C. Deformities in frustules of diatoms are generally ascribed to silicon limitation, heavy metals and extreme pH (Ruggiu et al., 1998; Dickman, 1998). Referring to water quality data in Table 3, pH may be ruled out as a contributing factor to the deformities as the values were within TWQRs as set out by DWAF (1996). Silicon was not measured for the purpose of this study. Considering that there are numerous mines (one of which is an open cast chrome mine) close to the Hartbeespoort Dam area, heavy metal contamination may very well be a plausible explanation for the deformities noted in diatoms at this site. COD increased to a level of 17 mg/ℓ at Site C3L from 2 mg/ℓ at Site C3H which coincides with the observation of deformities in the diatom species (Table 2).
Researchers have concluded that abnormal cell morphology of diatoms might be a valid indicator of ecosystem health. In case studies in Hong Kong and Hungary more deformed species with teratological frustules were found close to heavy-;metal polluted sources (Dickman, 1998; Szabó et al., 2005).
For deformities to occur in cell walls of diatoms, metal concentrations must be high enough to cause the development of irregular frustules; however, the concentrations need to be low enough to permit at least one division and this range of concentration is often very narrow (Pickett-;Heaps et al.,1990). Converse to the point made in the above paragraph, many authors consider this along with the fact that diatoms may have natural variations in morphology to be a limiting factor in the use of teratological diatom frustules as indicators of heavy metal pollution (Dickman, 1998).
Conclusions
Comparison of community structure for diatoms taken from sites with varying land uses using NMDS and SIMPER analysis reflected differences attributable to land use. Considering the make-;up of the reference site MR, which was comprised of diatom species that had preferences for clean, freshwater, diatom community structures were modified and showed specific variation due to identified agricultural and urban water quality impacts.
Agriculture could be split into high-; and low-;intensity practices, where high-;intensity agriculture was indicated by the presence of motile species of the genus Nitzschia, and low-;intensity agriculture was indicated by motile species of the genus Navicula. Urban sites contained a combination of species that were tolerant of spikes in water quality.
Sites that were impacted by high intensity agriculture were in a 'poor/moderate' class overall, indicating that water quality impacts at these sites were more severe than at urban sites which showed an overall 'moderate' class for diatom community structure. The reference site was classed as 'good' in terms of water quality and diatom assemblage.
There is an increasing acceptance of the view that chemical measurements alone are poor determinants of the biological impacts of pollutants. As a result, there is an increasing movement from the reliance on chemical water quality data to assess the health of ecosystems, to an approach that uses biological communities (biomonitoring) which provide a direct way of observing the impact of contaminants. Because diatoms remain in one place for a number of months, they show cumulative effects and an ecological 'recall' of the water quality over this period. This was evident in the current study as different pollution types were associated with specific diatom species.
Acknowledgements
Appreciation is expressed to the South African Netherlands Research Programme on Alternatives in Development (SANPAD) for the funding of this research. Dr. Jonathan Taylor (North-;West University) is sincerely thanked for his advice and assistance with laboratory techniques, microscopy and identification of diatoms. Simon Milne (University of Johannesburg) is thanked for his assistance with microscopy.
References
ALLAN JD, ERICKSON DL and FAY J (1997) The influence of catchment land use on stream integrity across multiple spatial scales. Freshwater Biol. 37 149-;161. [ Links ]
AMIS MA, ROUGET M, BALMFORD A, THUILLER W, KLEYNHANS CJ, DAY J and NEL J (2007) Predicting freshwater habitat integrity using land-;use surrogates. Water SA 33 (2) 215-;221. [ Links ]
ANDERSEN JM, DUNBAR M and FRIBERG N (EDS) (2004) REBECCA WP4 Rivers Deliverable D6: Report on Existing Methods and Relationships Linking Pressures, Chemistry and Biology in Rivers. Specific Targeted Research or Innovation Project, Denmark. [ Links ]
ANDERSON NJ, RENBERG I and SEGERSTROM U (1995) Diatom production responses to development of early agriculture in a boreal forest lake catchment (Kassjon. Northern Sweden). J. Ecol. 809-;822. [ Links ]
ANSARA-;ROSS TM, WEPENER V, VAN DEN BRINK PJ and ROSS MJ (2008) Probabilistic risk assessment of the environmental impacts of pesticides in the Crocodile (west) Marico catchment, North West Province. Water SA 34 (5) 1-;8. [ Links ]
BAHLS LL (1993) Periphyton Bioassessment Methods for Montana streams. Water Quality Bureau, Department of Health and Environmental Sciences, Montana, USA. [ Links ]
BIGGS BJF (1996) Patterns in benthic algae in streams. In: Stevenson RJ, Bothwell M and Lowe RL (eds.) Algal Ecology: Freshwater Benthic Ecosystems. Academic Press, San Diego, California. 31-;76. [ Links ]
BLINN DW and HERBST DB (2003) Use Of Diatoms and Soft Algae as Indicators of Environmental Determinants in the Lahontan Basin, USA. Annual Report for California State Water Resources Board. Contract Agreement 704558.01.Ct766. California, USA. [ Links ]
BLINN DW and BAILEY PCE (2001) Land-;use influence on stream water quality and diatom communities in Victoria, Australia: a response to secondary salinisation. Hydrobiol. 466 231-;244. [ Links ]
BOYACIOGLU H (2006) Surface water quality assessment using factor analysis. Water SA 32 (3) 389-;394. [ Links ]
BRADBURY JP and VAN METRE PC (1997) A land-;use and water-;quality history of White Rock Lake reservoir, Dallas, Texas, based on paleolimnological analyses. J. Paleolimnol. 17 227-;237. [ Links ]
BROWN CA (1996) Macroinvertebrate community patterns in relation to physico-;chemical parameters measured at two land based trout farms affecting streams in the south-;western Cape, South Africa. Arch. Hydrobiol. 138 (1) 57-;76. [ Links ]
CARPENTER SR, CARACO NF, CORRELL DL, HOWARTH RW, SHARPLEY AN and SMITH VH (1998) Non-;point pollution of surface waters with phosphorus and nitrogen. Ecol. Appl. 8 (3) 559-;567. [ Links ]
CEMAGREF (1982) Etude des méthodes biologiques quantitatives d'appréciation de la qualité des eaux. Rapport Division Qualité des Eaux Lyon. Agence financiè de Bassin Rhone-;Méditerarée. Corse, Pierre-;Bénite. [ Links ]
CHOLNOKY BJ (1960) Beiträge zur Kentniss der Ökologie der Diatomeen in dem Swartkops-;Bache nahe Port Elizabeth (Südost-; Kaapland). Hydrobiol. 16 229-;287. [ Links ]
CLARKE KR and WARWICK RM (2001) Change in Marine Communities: An Approach to Statistical Analysis and Interpretation. PRIMER-;E, Plymouth, United Kingdom. [ Links ]
COMITÉ EUROPÉEN DE NORMALISATION (CEN) (2004) Water Quality – Guidance Standard for the Identification and Enumeration of Benthic Diatom Samples from Rivers, and their Interpretation. European Standard. EN 14407:2004. [ Links ]
COSTE M and AYPHASSORHO H (1991) Étude de la qualité des eaux du Bassin Artois-;Picardie à l'aide des communautés de diatomées benthiques (application des indices diatomiques). Rapport CEMAGREF. Bordeaux – Agence de l'Eau Artois-;Picardie, Douai. [ Links ]
DALLAS HF and DAY JA (2004) The Effect of Water Quality Variables on Aquatic Ecosystems. WRC Report No TT 224/04. Water Research Commission, Pretoria, South Africa. [ Links ]
DAVIES B and DAY J (1998) Vanishing Waters. UCT Press, Cape Town, South Africa. 400 pp. [ Links ]
DE LA REY PA, TAYLOR JC, LAAS A, VAN RENSBURG L and VOSLOO A (2004) Determining the possible application value of diatoms as indicators of general water quality: A comparison with SASS 5. Water SA 30 (3) 325-;331. [ Links ]
DEKSISSA T, ASHTON PJ and VANROLLEGHEM PA (2003) Control options for river water quality improvement: A case study of TDS and Inorganic nitrogen in the Crocodile River (South Africa). Water SA 29 (2) 209-;218. [ Links ]
DELL'UOMO A (1996) Assessment of water quality of an Apennine river as a pilot study. In: Whitton BA and Rott E (eds.) Use of Algae for Monitoring Rivers II. Institut für Botanik, Universität, Innsbruck, Austria. 65-;73 pp. [ Links ]
DELL'UOMO A, PENSIERI A and CORRADETTI D (1999) Epilithic diatoms from the Esino river (central Italy) and their use for the evaluation of the biological quality of the water. Cryptogami. Algol. 20 (3) 253-;269. [ Links ]
DEPARTMENT OF WATER AFFAIRS AND FORESTRY (DWAF) (2008) Resource Quality Services water quality data for Crocodile West and Marico Water Management Area. Available from: www.dwaf.gov.za/iwqs/wms/data/WMA03_reg_WMS_nobor.htm (Accessed 2nd January, 2008). [ Links ]
DEPARTMENT OF WATER AFFAIRS AND FORESTRY (DWAF) (2004) Crocodile River (West) and Marico Water Management Area: Internal Strategic Perspective of the Crocodile River (West) Catchment. Report No. 03/000/00/0303. Directorate: National Water Resource Planning, Pretoria, South Africa. [ Links ]
DEPARTMENT OF WATER AFFAIRS AND FORESTRY (DWAF) (1996) South African Water Quality Guidelines (2nd edn.) Volume 7: Aquatic Ecosystems. DWAF, Pretoria, South Africa. [ Links ]
DICKMAN MK (1998) Epiphytic marine diatom deformities associated with contaminated sediments in Hong Kong. Environ. Int. 24 749-;759. [ Links ]
DODDS WK and WELCH EB (2000) Establishing Nutrient Criteria in Streams. J. N. Am. Benthol. Soc. 19 (1) 186-;196. [ Links ]
EEKHOUT S, BROWN CA and KING JM (1996) National Biomonitoring Programme for Riverine Ecosystems: Technical Considerations and Protocol for the Selection Reference and Monitoring Sites. NAEBP Report Series No 3. Institute for Water Quality Studies, Department of Water Affairs and Forestry, Pretoria, South Africa. [ Links ]
ELORANTA P and SOININEN J (2002) Ecological status of Finnish rivers evaluated using benthic diatom communities. J. Appl. Phycol. 14 1-;7. [ Links ]
GEVREY M, RIMET F, PARK YS, GIRAUDEL J, ECTOR L and LEK, S (2004) Water quality assessment using diatom assemblages and advanced modeling techniques. Freshwater Biol. 49 208-;220. [ Links ]
HARDING WR, ARCHIBALD CGM and TAYLOR JC (2005) The relevance of diatoms for water quality assessment in South Africa: A position paper. Water SA 31 (1) 41-;46. [ Links ]
HART DD and ROBINSON CT (1990) Resource limitation in a stream community: phosphorus enrichment effects on periphyton and grazers. Ecol. 71 1494-;1502. [ Links ]
HASLE GR (1978) Some specific preparations: diatoms. In: Sournia A (ed.) Phytoplankton Manual. United Nations Educational, Scientific and Cultural Organisation (UNESCO), Paris. [ Links ]
HILL BH, STEVENSON RJ, PAN Y, HERLIHY AT, KAUFMANN PR and JOHNSON CB (2001) Comparison of correlations between environmental characteristics and stream diatom assemblages characterised at genus and species levels. J. N. Am. Benthol. Soc. 20 (2) 299-;310. [ Links ]
HUIZENGA JM (2004) Natural and Anthropogenic Influences on Water Quality: An Example from Rivers Draining the Johannesburg Granite Dome. M.Sc. Dissertation. Rand Afrikaans University, Johannesburg, South Africa. [ Links ]
JARVIE HP, WHITTON BA and NEAL C (1998) Nitrogen and phosphorus in east coast British rivers: Speciation, sources and biological significance. Sci. Total Environ. 210/211 79-;109. [ Links ]
KAHLERT M, ALBERT R, ANTTILA E, BENGTSSON R, BIGLER C, ESKOLA T, GÄLMAN V, GOTTSCHALK S, HERLITZ E, JARLMAN A, KASPEROVICIENE J, KOKOCIŃSKI M, LUUP H, MIETTINEN J, PAUNKSNYTE I, PIIRSOO K, QUINTANA I, RAUNIO J, SANDELL B, SIMOLA H, SUNDBERG I, VILBASTE S and WECKSTRÖM J (2009) Harmonization is more important than experience – results of the first Nordic-;Baltic diatom intercalibration exercise 2007 (stream monitoring). J. Appl. Phycol. 21 471-;482. [ Links ]
KELLY MG (2002) Role of benthic diatoms in the implementation of the Urban Wastewater Treatment Directive in the River Wear, North-;East England. J. Appl. Phycol. 14 9-;18. [ Links ]
KELLY MG (1998) Use of community-;based indices to monitor eutrophication in rivers. Environ. Conserv. 25 22-;29. [ Links ]
KELLY MG, CAZAUBON A, CORING E, DELL'UOMO A, ECTOR L, GOLDSMITH B, GUASCH H, HÜRLIMANN J, JARLMAN A, KAWECKA B, KWANDRANS J, LAUGASTE R, LINSTRØM EA, LEITAO M, MARVAN P, PADISÁK J, PIPP E, PRYGIEL J, ROTT E, SABATER S, VAN DAM H and VIZINET J (1998) Recommendations for the routine sampling of diatoms for water quality assessments in Europe. J. Appl. Phycol. 10 215-;224. [ Links ]
KELLY MG and WHITTON BA (1995) The trophic diatom index: a new index for monitoring eutrophication in rivers. J. Appl. Phycol. 7 433-;444. [ Links ]
KOLKWITZ R and MARSSON, M (1908) Ökologie der pflanzlichen Saprobien. Berichte der Deutschen Botanischen Gesellschaft 26 a 505-;519. [ Links ]
KRAMMER K and LANGE-;BERTALOT H (1986) Bacillariophyceae.1. Teil: Naviculaceae. In: Ettl H, Gerloff J, Heynig H and Mollenhauer D (eds.) Süβwasserflora von Mitteleuropa, Band 2/1. Spektrum Akademischer Verlag, Heidelberg, Berlin, Germany. [ Links ]
KRAMMER K and LANGE-;BERTALOT H (1988) Bacillariophyceae. 2. Teil: Bacillariaceae, Epithemiaceae, Surirellaceae. In: Ettl H, Gerloff J, Heynig H and Mollenhauer D (eds.)Süβwasserflora von Mitteleuropa, Band 2/2. Spektrum Akademischer Verlag, Heidelberg, Berlin, Germany. [ Links ]
KRAMMER K and LANGE-;BERTALOT H (1991a) Bacillariophyceae. 3. Teil: Centrales, Fragilariaceae, Eunotiaceae. In: Ettl H, Gerloff J, Heynig H and Mollenhauer D (eds.) Süβwasserflora von Mitteleuropa, Band 2/3. Spektrum Akademischer Verlag, Heidelberg, Berlin, Germany. [ Links ]
KRAMMER K and LANGE-;BERTALOT H (1991b) Bacillariophyceae. 4. Teil: Achnanthaceae, Kritische Ergänzungen zu Navicula (Lineolatae und Gomphonema). In: Ettl H, Gerloff J, Heynig H and Mollenhauer D (eds.) Süβwasserflora von Mitteleuropa, Band 2/2. Spektrum Akademischer Verlag, Heidelberg, Berlin. [ Links ]
KUTKA FJ and RICHARDS C (1996) Relating diatom assemblage structure to stream habitat quality. J. N. Am. Benthol. Soc. 15 469-;480. [ Links ]
LAVOIE I, VINCENT WF, PIENITZ R and PAINCHAUD J (2004) Benthic algae as bioindicators of agricultural pollution in the streams and rivers of southern Qu'ebec (Canada). Aquat. Ecosyst. Health Manage. 7(1) 43-;58. [ Links ]
LECOINTE C, COSTE M and PRYGIEL J (1993) 'OMNIDIA': Software for taxonomy, calculation of diatom indices and inventories management. Hydrobiol. 269/270 509-;513. [ Links ]
LEMLY AD (1994) Irrigated agriculture and freshwater wetlands: A struggle for coexistence in the western United States. Wetl. Ecol. Manage. 3 (1) 3-;15. [ Links ]
LENOIR A and COSTE M (1996) Development of a practical diatom index of overall water quality applicable to the French National Water Board network. In: Whitton BA and Rott E (eds.) Use of Algae for Monitoring Rivers II. Institut für Botanik, Universität, Innsbruck, Austria. 29-;43 pp. [ Links ]
MAGALIES RIVER FORUM MEETING (2007) Personal communication (20 March 2007), Magaliesberg, South Africa. [ Links ]
MOORE SB, WINCKEL J, DETWILER SJ, KLASING SA, GAUL PA, KANIM AR, KESSER BE, DEBEVAC AB, BEARDSLEY A and PUCKETT LA (1990) Fish and Wildlife Resources and Agricultural Drainage in the San Joaquin Valley, California. San Joaquin Valley Drainage Program, Sacramento, California. 974 pp. [ Links ]
MORRISON G, FATOKI OS, PERSSON L and EKBERG A (2001) Assessment of the impact of point source pollution from the Keiskammahoek Sewage Treatment Plant on the Keiskamma River – pH, electrical conductivity, oxygen-;demanding substance (COD) and nutrients. Water SA 27 (4) 476-;480. [ Links ]
MUNN MD, BLACK RW and GRUBER AJ (2002) Response of benthic algae to environmental gradients in an agriculturally dominated landscape. J. N. Am. Benthol. Soc. 21 221-;237. [ Links ]
PAN Y, HERLIHY A, KAUFMANN P, WIGINGTON J, VAN SICKLE J and MOSER T (2004) Linkages among land-;use, water quality, physical habitat conditions and lotic diatom assemblages: A multi-;spatial scale assessment. Hydrobiol. 515 59-;73. [ Links ]
PICKETT-;HEAPS JD, SCHMID AMM and EDGAR LA (1990) The cell biology of diatom valve formation. In: Progress in Phycological Research (Vol. 7). Biopress Ltd. [ Links ]
PRYGIEL J, CARPENTIER P, ALMEIDA S, COSTE M, DRUART JC, ECTOR L, GUILLARD D, HONERÉ MA, ISERENTANT R, LEDEGANCK P, LALANNE-;CASSOU C, LESNIAK C, MERCIER I, MONCAUT P, NAZART M, NOUCHET N, PERES F, PEETERS V, RIMET F, RUMEAU A, SABATER S, STRAUB F, TORRISI M, TUDESQUE L, VAN DER VIJVER B, VIDAL H, VIZINET J and ZYDEK N (2002) Determination of the biological diatom index (IBD NF T 90-;354): Results of an intercomparison exercise. J. Appl. Phycol. 14 27-;39. [ Links ]
QADER M (1998) Diversion of the Ganges water at Farakka and its effects on salinity in Bangladesh. Environ. Manage. 22 711-;722. [ Links ]
RIVER HEALTH PROGRAMME (RHP) (2005) State-;of-;Rivers Report: Monitoring and Managing the Ecological State of Rivers in the Crocodile (West) Marico Water Management Area. Department of Environmental Affairs and Tourism (DEAT), Pretoria, South Africa. [ Links ]
ROTT E, DUTHIE HC and PIPP E (1998) Monitoring organic pollution and eutrophication in the Grand River, Ontario, by means of Diatoms. Can. J. Fish. Aquat. Sci. 55 (6) 1443-;1453. [ Links ]
RUGGIU D, LUGLI'E A, CATTANEO A and PANZANI P (1998) Paleoecological evidence for diatom response to metal pollution in Lake Orta (N. Italy). J. Paleolimnol. 20 333-;345. [ Links ]
SABATER S and ROCA JR (1992) Ecological and biogeographical aspects of diatom distribution in Pyrenean springs. Br. Phycol. J. 27 203-;213. [ Links ]
SCHOFIELD NJ and RUPRECHT JK (1989) Regional analysis of stream salinisation in southwest Western Australia (abstract). J. Hydrol. 112 (2) 19-;39. [ Links ]
SCHONFELDER I (2000) Indikation der Gewässerbeschaffenheit durch Diatomeen. In: Steinberg, CEW, Calmano W, Klapper H and Wilken RD (eds.) Handbuch der angewandten Limnologie 9. 1-;62. [ Links ]
SKINNER J, LEWIS K, BARDON K, TUCKER P, CATT J and CHAMBERS B (1997) An overview of the environmental impact of agriculture in the UK. J. Environ. Manage. 50 111-;128. [ Links ]
STEDNICK JD (1991) Wildland Water Quality Sampling and Analysis. Academic Press, San Diego, USA. [ Links ]
SZABÓ K, KISS KT, TABA G and ÁCS E (2005) Epiphytic diatoms of the Tisza River, Kisköre Reservoir and some oxbows of the Tisza River after the cyanide and heavy metal pollution in 2000. Acta Bot. Croat. 64 (1) 1-;46. [ Links ]
TAYLOR J (2009) Personal Electronic Communication. School of Environmental Sciences, Division of Botany, North-;West University, 14 July 2009. [ Links ]
TAYLOR JC (2007) Personal electronic communication. School of Environmental Sciences, Division of Botany, North-;West University, 22 November 2007. [ Links ]
TAYLOR JC, JANSE VAN VUUREN MC and PIETERSE AJH (2007a) The application and testing of diatom-;based indices in the Vaal and Wilge Rivers, South Africa. Water SA 33 (1) 51-;59. [ Links ]
TAYLOR JC, PRYGIEL J, VOSLOO A, DE LA REY PA and VAN RENSBERG L (2007c) Can diatom-;based pollution indices be used for biomonitoring in South Africa? A case study of the Crocodile West and Marico water management area. Hydrobiol. 592 455-;464. [ Links ]
TAYLOR JC, HARDING WR and ARCHIBALD CGM (2007b) An Illustrated Guide to Some Common Diatom Species from South Africa. WRC Report No TT 282/07. Water Research Commission, Pretoria, South Africa. [ Links ]
TAYLOR JC, HARDING WR, ARCHIBALD GCM and VAN RENSBURG L (2005a) Diatoms as indicators of water quality in the Jukskei-;Crocodile River system in 1956 and 1957, a re-;analysis of diatom count data generated by BJ Cholnoky. Water SA 31 (2) 1-;10. [ Links ]
TAYLOR JC, DE LA REY A and VAN RENSBURG L (2005b) Recommendations for the collection, preparation and enumeration of diatoms from riverine habitats for water quality monitoring in South Africa. Afr.J. Aquat. Sci. 30 (1) 65-;75. [ Links ]
TAYLOR JC (2004) The Application of Diatom Based Pollution Indices in the Vaal Catchment. M.Sc. Dissertation. University of the North West, Potchefstroom Campus: North West Province, South Africa. [ Links ]
TELPY M and BAHLS LL (2006) Diatom Biocriteria for Montana Streams – Middle Rockies Ecoregion. Report by Larix Systems, Montana, USA. [ Links ]
VAN DAM H, MERTENS A and SINKELDAM J (1994) A coded checklist and ecological indicator values of freshwater diatoms from the Netherlands. Neth. J. Aquat. Ecol. 28 (1) 117-;133. [ Links ]
VANNOTE RL, MINSHALL GW, CUMMINS KW, SEDELL JR and CUSHING CE (1980) The river continuum concept. Can. J. Fish. Aquat. Sci. 37 130-;137. [ Links ]
WILLIAMS WD (1987) Salinisation of rivers and streams: an important environmental hazard. Ambio 16 180-;185. [ Links ]
WILLIAMS WD (2001) Anthropogenic salinisation of inland waters. Hydrobiol. 466 329-;337. [ Links ]
WINTER JG and DUTHIE HC (2000) Stream biomonitoring at an agricultural test site using benthic algae. Can. J. Bot. 78 (10) 1319 -;1326. [ Links ]
ZELINKA MA and MARVAN P (1961) Zur Präzisierung der biologischen Klassifikation der Reinheit fliessender Gewässer. Arch. Hydrobiol. 57 389-;407. [ Links ]
*To whom all correspondence should be addressed.
+2782 4222 793; fax: 088 011 673 1192;
e-;mail: gina@ecotone-;sa.co.za
Received 28 October 2008; accepted in revised form 16 July 2009.