Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Water SA
On-line version ISSN 1816-7950
Print version ISSN 0378-4738
Water SA vol.35 n.5 Pretoria Oct. 2009
Biological sulphate reduction with primary sewage sludge in an upflow anaerobic sludge bed (UASB) reactor – Part 3: Performance at 20°C and 35°C
J Poinapen, GA Ekama*; MC Wentzel
Water Research Group, Dept. of Civil Engineering, University of Cape Town, Rondebosch 7701, South Africa
ABSTRACT
The performance of 2 biological sulphate reduction (BSR) upflow anaerobic sludge bed (UASB) reactors fed primary sewage sludge (PSS) and sulphate, one at 20ºC (R2) and one at 35ºC (R1) is described. To maintain the effluent sulphate concentration below 250 mgSO42-/ℓ, the hydraulic retention time (HRT) and bed solids retention time (SRT or sludge age) both needed to be longer and the feed primary sewage sludge (PSS) COD to SO42- ratio higher at 20ºC than at 35ºC, viz. 20.4 to 21.0 h, 24 d and 1.75 gCOD/gSO42- at 20ºC and 16.4 to 17.0 h, 21 d and 1.75 gCOD/gSO42- at 35ºC respectively. The longer HRT, SRT and higher feed PSS COD/ SO42- ratio is a consequence of a slower PSS hydrolysis/acidogenesis rate at 20ºC resulting in a lower biodegradable particulate organics conversion to volatile fatty acids (VFA). Solid liquid separation in both systems was good yielding average particulate and soluble organic COD concentrations of (150 and 100 mgCOD/ℓ for R1; 138 and 96 mgCOD/ℓ for R2). The sulphate reduction was >90% in both systems. The UASB reactor R1 (at 35ºC) was also operated at an increased influent sulphate concentration (1 800 mgSO42-/ℓ) to investigate the inhibition effect by un-dissociated hydrogen sulphide generated from the reduction of this high sulphate concentration. It was found that a high sulphate reduction (~ 92%) was maintained even at the relatively low HRT of 18.5 h. The COD and S mass balances above 95% were achieved over both systems indicating that the performance data obtained from them is reliable for developing and calibrating mathematical models.
Keywords: biological sulphate reduction, hydrolysis, hydraulic retention time, UASB reactor
Nomenclature
| Alk H2S | alkalinity with respect to the H2S reference species excluding the water species |
| AMD | acid mine drainage |
| BPO | biodegradable particulate organics |
| BRT | bed retention time |
| BSR | biological sulphate reduction |
| COD | chemical oxygen demand |
| FBR | fluidised bed reactor |
| fcv | COD to VSS ratio |
| fn | orgN/VSS ratio |
| fPS'up | influent and biodegradable particulate COD fraction of primary sludge |
| FRBCOD | fermentable readily biodegradable COD |
| FSA | free and saline ammonia |
| H2CO3*Alk | alkalinity with respect to the H2CO3 reference species including the water species |
| HAc | acetic acid |
| HRT | hydraulic retention time |
| KI | sulphide inhibition kinetic constant |
| OLR | organic loading rate |
| PBR | packed bed reactor |
| pH | negative log of hydrogen ion activity |
| pK'S1 1st. | dissociation constant for the sulphide weak acid base system corrected for ionic strength effects |
| PSS | primary sewage sludge |
| R1 | UASB Reactor 1 |
| R2 | UASB Reactor 2 |
| Rs | sludge age |
| Sbp | biodegradable particulate COD concentration |
| SBR | sequencing batch reactor |
| SCFA | short chain fatty acids |
| SLR | sludge loading rate |
| SRB | sulphate reducing bacteria |
| SRT | solids retention time |
| SS | steady state |
| SSD | sample standard deviation |
| Sup | un-biodegradable particulate COD concentration |
| TKN | total Kjeldahl nitrogen |
| Total Alk | sum of weak acid/base subsystem alkalinities |
| UASB | upflow anaerobic sludge bed reactor |
| UPO | un-biodegradable particulate organics |
| USCOD | un-biodegradable soluble COD |
| Vb | bed volume |
| VFA | volatile fatty acids |
| Vup | hydraulic upflow velocity in UASB reactor |
Introduction
The feasibility of a novel system for BSR of AMD using PSS as carbon source in a UASB reactor configuration (R1) was described in Part 1 (Poinapen et al. , 2009a). From the successful operation of the UASB reactor R1 at 35ºC (fed 1 500 mgSO42-/ℓ for a period of 280 d), the performance of BSR using PSS was evaluated also in a second identical UASB reactor (R2) operated in parallel to R1 but at ambient (20ºC) temperature. Accordingly, from Day 280 of the investigation, Reactor R2 (T=20ºC) was operated with feed sulphate and PSS concentrations of 1 500 mgSO42-/ℓ and 1995 mgCOD/ℓ respectively, representing a feed PSS COD to sulphate ratio of 1.33 mgCOD/mgSO42-. This ratio for R2 was slightly higher than the R1 ratio of 1.25 because the hydrolysis/acidogenesis rate of PSS at 20ºC is slower than at 35ºC, but how much slower was not known. Later on Day 395, the PSS COD/SO42- R2 ratio was increased to 1.75 (see Fig. 2 in Poinapen et al. , 2009a) to reduce the effluent sulphate concentration to below 250 mgSO42-/ℓ. In this paper, the operation and performance of UASB R2 at 20ºC are assessed, evaluated and compared with that of UASB R1 at 35ºC.
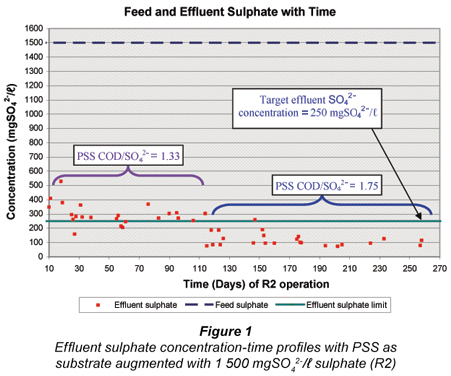
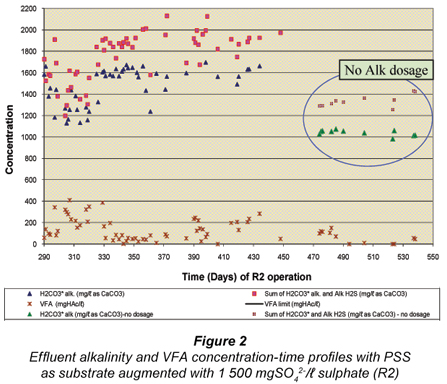
After the feasibility study with R1 from Day 1 to 280 (Poinapen et al. , 2009a), the influent sulphate concentration to R1 was increased from 1 500 mgSO42-/ℓ to 1 800 mgSO42-/ℓ. It was expected that the higher feed sulphate concentration would result in a higher sulphide concentration in the system. Even though compared with other anaerobic micro-organisms (namely methanogenic archaea) the sulphate SRB have the highest tolerance to sulphide, their activity is nevertheless affected by the presence of sulphide, especially un-dissociated hydrogen sulphide (H2S). Un-dissociated H2S is inhibitory to SRB and decreases their growth activity (Reis et al. , 1992; Maillacheruvu et al. , 1993; Konishi et al. , 1996; Kalyuzhnyi et al. , 1997; O'Flaherty et al. , 1998). Investigating the effect of high feed sulphate concentration on the system performance was therefore necessary in order to assess the extent of un-dissociated hydrogen sulphide (H2S) inhibition on BSR using PSS as substrate in a UASB reactor.
Methodology
Reactor R2 (T=20ºC) was inoculated with waste sludge from R1 (Day 280) and operated for 270 d to Day 550. Initially, R2 performance was not as good as R1, despite complete seeding with R1 sludge. The relatively short HRT of ~28 h at which R2 was operated during the start-up period was probably too short. It was thought that R1 waste sludge would result in a quick and effective start-up of R2 biological processes since R1 was at its maximum efficiency. However, it appeared that an adaptation period was required for the selected population group of sulphidogens to develop at the lower temperature (20ºC). After 50 d of operation to Day 330, granulation was observed and the system performance started to stabilise. The effluent sulphate concentration remained stable from periods Day 333-350 and Day 371-392 with a sulphate removal of 83.7% and 79.8% respectively, even though the HRT was incrementally decreased from 28 h to 24 h (see Table 5 later), implying that the reactor was at steady state. However, the effluent sulphate concentration (303 mgSO42-/ℓ) was still above the specified requirement (250 mg SO42-/ℓ in South Africa). In addition, during this period the VFA concentration was low suggesting that PSS hydrolysis was incomplete and was the rate-limiting step under the lower temperature conditions.
During the 270 d of operation of R2 (i.e. from investigation Day 280 to 538, or equivalently from R2 Day 0 to Day 258), the influent feed flow rate was varied to vary the HRT. The UASB R2 operational conditions during the 270 d are set out in Table 1. The different steady state periods with respect to the operating HRT with 2 PSS COD/SO42- ratios, the days at which bed profile tests were conducted, the period when alkalinity dosage was stopped and the days on which mass balances were determined are listed.
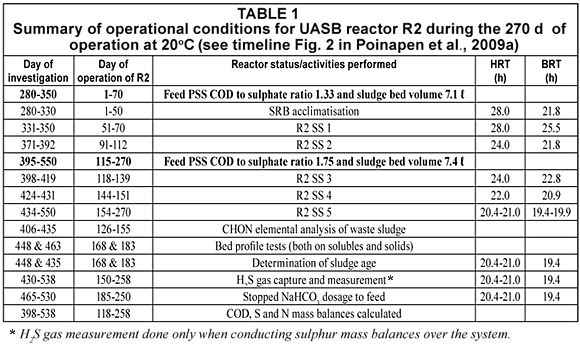
Influent characteristics
Similar to UASB Reactor R1 (Poinapen et al. , 2009a), the prepared feed for R2 (from dilution of the PSS) augmented with 1 500 mgSO42-/ℓ was regularly analysed for its COD, TKN, FSA, VFA and H2CO3* alkalinity concentrations and pH. Two influent PSS COD/SO42- ratios (namely 1.33 and 1.75 mg COD/mgSO42-) were applied. A summary of the results from the measurements of the above parameters over the 2 steady state periods at feed COD/SO42- ratio = 1.33 (SS2, R2 operation Day 371 to Day 392) and 1.75 (SS5, R2 operation Day 520 to Day 538) is listed in Table 2.
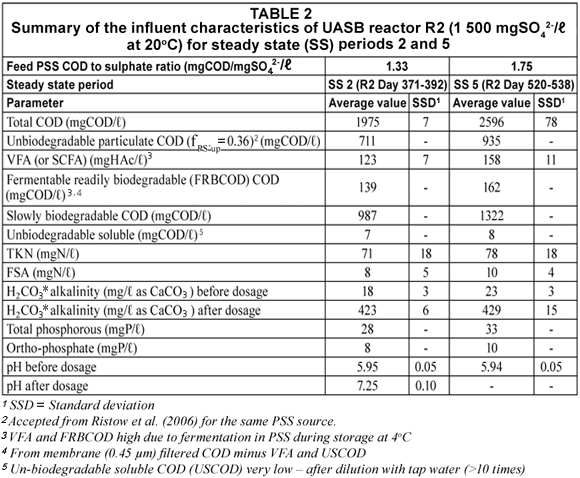
It was expected that at 20ºC, the hydrolysis/acidogenesis of biodegradable particulate organics (BPO) would not be complete but no information was available to indicate what PSS COD/SO42- ratio was appropriate to supply the required VFA from the hydrolysis/acidogenesis of PSS at 20ºC. After a few trial ratios, it was found that a ratio of 1.75 appeared most favourable to supply sufficient VFA for BSR, i.e. effluent sulphate < 250 mg SO42-/ℓ. The results obtained from the 1.33 and 1.75 ratios are discussed below. Also, towards the end of the experimental programme on UASB R2 (R2 Day 465 to 530), influent alkalinity (NaHCO3) dosage (~ 400 mg/ℓ as CaCO3) was stopped in order to ascertain the pH at which the system would stabilise itself.
Results
Effluent sulphate concentration with time
The variation in UASB R2 effluent sulphate concentration with time from R2 Day 290 is shown in Fig. 1 for the whole experimental period of R2 at the 2 feed PSS COD/SO42- ratios. For the period R2 Day 331 to Day 392 (comprising SS Periods 1 and 2), on average the effluent sulphate was not reduced below 250 mgSO42-/ℓ because of the low PSS COD/SO42- ratio (1.33) and consequently low VFA generation by hydrolysis/acidogenesis. From R2 Day 395 onwards the feed COD/SO42- ratio was increased to 1.75 which resulted in a marked decrease in the effluent sulphate concentration to around 100 mgSO42-/ℓ. Clearly VFA generation via hydrolysis/ acidogenesis was the limiting rate for BSR.
Effluent H2CO3* alk, Alk H2S and VFA concentrations with time
As expected, the effluent total Alk (sum of H2CO3* alk, Alk H2S and Alk VFA) increased due to the increase in the PSS COD/SO42- ratio (R2 Day 395 to Day 450 in Fig. 2) as a direct consequence of an increase in sulphate reduced, hence increasing the total sulphide (ST = H2S+HS-), dissociated sulphide (HS-) and Alk H2S (proportional to HS-) concentrations.
The data points circled in Fig. 2 represent the period (R2 Day 475 onwards) over which the feed NaHCO3 dosage was progressively decreased to zero by R2 Day 510. This elimination of feed alkalinity dosage did not affect the system performance in any way as shown in Figs. 1 and 2 by the low sulphate and VFA concentrations. This is an important result for the UASB BSR performance as it shows that BSR:
- Occurs successfully at low temperature (20ºC)
- Regulates the reactor pH near neutrality (~7) by itself even though the sulphate rich AMD is acidic. This is because the BSR bioprocesses reduce sulphate to sulphide and take up 2 protons (H+) per mole SO42-, i.e. SO42- + 2H+ + 8(H+ + e-, supplied by the organics) → H2S + 4H2O.
The stoichiometry of BSR with organics in general and PSS in particular, are considered in Part 5 of this series, currently in preparation by Poinapen and Ekama.
Reactor performance with COD/SO42- ratio of 1.33
Table 3 shows the performance of UASB Reactor R2 when operated at an HRT of 24 h (SS2). It can be seen that R2 performed satisfactorily because it achieved a sulphate removal efficiency of ~80% (± 2%) and an organic removal efficiency of ~77% (± 1%). However, despite the relatively high sulphate removal efficiency, the average effluent sulphate remained above the upper limit of 250 mgSO42-/ℓ. The cause for this fairly high effluent sulphate concentration (~ 303 mgSO42-/ℓ) was attributed to the unavailability of sufficient VFA which remained very low in the effluent, despite the higher feed PSS COD/SO42- ratio of 1.33 compared with the 1.25 of R1. It was concluded that at low temperature, the hydrolysis rate of the PSS biodegradable particulate organics (BPO) was reduced thereby generating insufficient VFA and H2 for sulphate reduction.
In order to increase the production of VFA, the PSS COD/SO42- ratio was increased to 1.75 which increased the feed BPO COD concentration and consequently the concentration of BPO hydrolysed, thereby generating more VFA and H2 for BSR. Increasing the PSS COD/SO42- ratio also resulted in higher un-biodegradable particulate organics (UPO, Sup) and BPO (Sbp) not hydrolysed. Hence more particulate organics needed to be wasted to maintain the bed volume. So to provide a larger bed volume (and concomitantly a longer sludge age), the reactor sludge bed volume (and height) was increased from 7.1 to 7.4 ℓ. The increased sludge age allowed more time for the BPO to be hydrolysed as PSS hydrolysis is the rate-limiting process.
R2 performance with a PSS COD/SO42- ratio of 1.75 at lowest optimal HRT of 20.4 to 21.0 h
At the higher feed PSS COD/SO42- ratio of 1.75, the performance of R2 improved to the extent that the effluent quality concentrations were now met (i.e. sulphate <250 mgSO42-/ℓ and VFA <100 mgHAc/ℓ ). The performance of UASB Reactor R2 at the lowest optimal HRT of 20.4 to 21.0 h with alkalinity dosage (~ 424 mg/ℓ as CaCO3) from R2 Day 434 to Day 464 (SS period 5a) was good (see Table 4) with an average sulphate removal efficiency of 93.3 ± 1.5%.
Influent alkalinity (NaHCO3) dosage was discontinued from R2 Day 465 to the end of the experimental programme on R2 Day 550. Table 4 lists also the effluent parameter values when R2 was operated at an HRT of between 20.4 h and 21.0 h with no alkalinity dosage to the feed for the period Day 520 to Day 538. With the supply of additional carbon (i.e. more PSS), the sulphate removal efficiency increased from 80% (± 2.0%), when fed a PSS CO/SO42- ratio of 1.33, to > 93% (± 1.3%), with PSS COD/SO42- ratio of 1.75. From the high organic COD removal efficiency of 91% (± 1.3%) it was found that the increase in PSS to meet the VFA demand did not impact the effluent quality in terms of suspended solids. In fact, the particulate organic COD concentration remained low with an average value of 138 (± 25) mgCOD/ℓ.
The aqueous sulphide concentration (ST = H2S+HS-) was measured via the COD with the modified method described by Poinapen et al. (2009b). This sulphide concentration matched very closely the theoretical sulphide produced from the sulphate reduced (see below for S mass balance). In addition, discontinuing the alkalinity dosage to the influent (from R2 for the period Day 468 to Day 550) did not affect the system performance in any way. The alkalinity produced by the BSR increased the pH from the influent value of 5.94 to a reactor value of 7.21. This shows that the system is pH self-regulatory, which is a major advantage of BSR to reduce the cost of chemical dosing in AMD treatment.
Summary of UASB R2 performance
A summary of the average experimental data at the different HRTs and BRTs is listed in Table 5. The minimum HRT for which the specified effluent criteria were met was found to be between 20.4 to 21.0 h, where sulphate removal efficiency was ~ 95% at a PSS COD/SO42- ratio of 1.75.
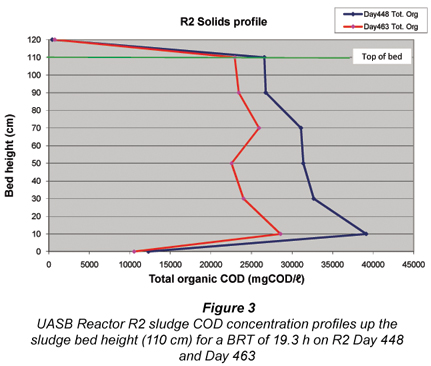
Profile of solids – Calculation of sludge age (Rs)
Figure 3 illustrates the variation of R2 solids concentration along the bed axis. Above the 10 cm height there is little difference in the solids concentration along the bed axis probably largely due to the sludge recycle (at half the influent flow). From these measurements, the mass of sludge in the bed was calculated, which in relation to the mass of sludge wasted (see below) and the mass of sludge in the effluent, yielded the sludge age (Rs) of the UASB Reactor R2 at around 24.1 d.
Analysis of R2 waste sludge
UASB R2 waste sludge was analysed on 6 operational days (Table 6) and was found to have an average particulate organic COD/VSS (fcv) and TKN/VSS (fn) of 1.611 ± 0.041 and 0.068± 0.016 respectively.
Mass balances on R2
Mass balances were conducted on R2 to check experimental data reliability. Similar to UASB R1 when fed 1 500 mgSO42-/ℓ during the first 280 d (Poinapen et al. , 2009a), prior to the modification of the analytical procedures for accurate measurement of aqueous sulphide, the R2 system COD mass balance was relatively low (91.7 ± 5.2%) while the S mass balance was poor (69.4 ± 6.4%). However, these mass balances, particularly the S mass balance, improved considerably with the modified analytical method for H2S measurement. The COD mass balance increased to 98.0% (± 0.2%) while the S mass balance averaged 95.9% (± 0.2%). These good mass balances confirm the reliability of the measured data and the modified methods for aqueous H2S measurement (Poinapen et al. , 2009b). Nitrogen (N) mass balance was also very good and averaged 104.5 (± 4.9%).
Operation and performance of R1 fed 1 800 mgSO42-/ℓ
During the initial phase of this investigation (R1 Day 0 to Day 280), the design and operational parameters for UASB R1 (T=35ºC) fed 1 500 mgSO42-/ℓ were established (R1 Day 1 to Day 280, Poinapen et al. , 2009a). Sulphate, H2CO3* alkalinity and VFA concentration profiles through the sludge bed were conducted to gain insight into the bioprocess behaviour along the reactor bed. Solids profile tests were used to determine the sludge age of the system at the minimum HRT (13.5 to 14.0 h) at which effluent quality criteria were met (i.e. effluent sulphate < 250 mgSO42-/ℓ and effluent VFA < 100 mgHAc/ℓ). After the initial phase of R1, the influent sulphate was increased to 1 800 mgSO42-/ℓ on R1 Day 280 to investigate the effect of higher sulphate concentration on the system. It is well documented that the biological treatment of sulphate-rich wastewater involves the potential effect of sulphide inhibition on bacterial growth and activity. [In the BioSURE® process design for AMD treatment, the treated effluent is recycled to blend with the raw AMD. The high alkalinity (sulphide and carbonate alkalinities) in the recycle stream neutralises the pH, precipitates the heavy metals and almost halves the inflow sulphate concentration of the raw AMD. Therefore, in a situation where an effluent sulphate of 200 mgSO42-/ℓ is recycled on a 1:1 effluent to incoming raw AMD flow rates, the raw AMD can have a feed sulphate concentration of up to 3 400 mgSO42-/ℓ]. Operating R1 with a higher feed sulphate of 1 800 mgSO42-/ℓ gave insight into the extent of sulphide inhibition on the bioprocesses in the system. The feed PSS COD concentration was also increased to conform to a feed PSS COD/sulphate ratio of 1.44 mgCOD/mgSO42-. This higher ratio than 1.25 was applied to ensure that BSR in the system was not VFA limited. The operation and performance of R1 at 1 800 mgSO42-/ℓ are described and analysed below.
R1 experimental programme (fed 1800 mgSO42-/ℓ )
R1 was fed 1 800 mgSO42-/ℓ from Day 281 to Day 530 (the day it was stopped). It took around 20 d for the reactor to reach the first steady state at which the effluent sulphate concentration (< 250 mgSO42-/ℓ) was met at an HRT of 19 h. Thereafter, the HRT was gradually decreased (by increasing the influent flow rate) to establish the lowest optimal HRT. It was found that at an HRT below 18 h, the effluent sulphate concentration could not be maintained below 250 mgSO42-/ℓ either due to sludge bed expansion (which reduces the bed sludge age) and/or insufficient sulphate contact time with the biomass. Accordingly, the lowest HRT was set at 18.0 to 18.5 h. The BRT was 16.4 to 16.8 h based on a sludge bed volume of 7.1ℓ and the upflow velocity, Vup, was 0.093 to 0.096 m/h. The sludge age determined from the bed profile test was 21 d. The various experimental stages and tests conducted on R1 (fed 1 800 mgSO42-/ℓ) including batch tests during the experimental period from Day 281 to Day 530 are listed in Table 7.
R1 performance (fed 1 800 mgSO42-/ℓ) at the lowest optimal HRT of 18.0 to 18.5 h
Table 8 summarises the concentrations of the effluent parameters of interest when R1 (fed 1 800 mgSO42-/ℓ) was operated at its lowest optimal HRT of 18.0 to 18.5 h (Day 420 to Day 473). The influent values are also listed including pH because alkalinity was not dosed to the influent during this period.
The low effluent organic COD, sulphate and VFA concentrations with the concomitant production of high alkalinity underline the very good performance of R1 even at a relatively low HRT and no alkalinity dosage to the feed. The system achieved ~92% sulphate removal efficiency implying that inhibition of un-dissociated hydrogen sulphide on the growth and activity of SRB was negligible. This aspect is important in calibrating the value of hydrogen sulphide inhibition constant (KI) in the calibration and validation of the dynamic kinetic BSR model (UCTADM1-BSR) (to be presented in Part 6 of this series; currently in preparation by Poinapen and Ekama). Interestingly, the reactor/effluent pH was very close to the pK'S1 value of the H2S/HS- system suggesting that in the absence of gaseous CO2 production, the system pH is governed by the sulphide system. This aspect will be explored in greater depth in the steady state model development to be presented in Part 5 (currently in preparation by Poinapen and Ekama).Importantly for model development, the aqueous hydrogen sulphide recovery matches closely the theoretical sulphide from the sulphate reduced and COD, S and N mass balances were all very good averaging 96.5 (± 0.8), 95.8 (± 2.9) and 103.3 (± 8.3) respectively.
With the excellent performance of R1 from Day 280 to Day 400 when fed 1 800 mgSO42-/ℓ (detailed results not given), NaHCO3 dosage to the feed was stopped on R1 Day 400. Figure 4 shows the variations in feed and effluent pH with time. Particularly striking is that the effluent pH was unaffected implying that the system pH is self-regulatory at pH >7.0 even at the high sulphate feed concentration of 1 800 mgSO42-/ℓ due to the high alkalinity generation. Because no CO2 gas was generated (no bubbling was observed in the FeCl3 H2S gas collection bottle, see Part 1, Poinapen et al., 2009a), it seems that all CO2 produced by the breakdown of the PSS biodegradable organics remain in solution as HCO3-. Because the CO2 (or HCO3-) generation may be insufficient to supply the alkalinity required by the sulphate reduction because the organics donate more electrons and protons than supply C for HCO3-, the sulphide system provides the alkalinity shortfall as dissociated sulphide (HS- which is proportional to Alk H2S; to be presented in Part 5 of Poinapen and Ekama, in preparation).

Comparison of R1 (35ºC) and R2 (20ºC)(both fed 1 500 mgSO42-/ℓ)
Since the objective of operating the UASB Reactor R2 was to study the performance of BSR using PSS at ambient temperature (namely 20ºC), a comparison between R1 and R2 both fed 1 500 mgSO42-/ℓ was important because most full-scale systems are not likely to be heated to 35ºC. Table 9 lists the performance of the 2 UASB reactors when both were operated at their lowest optimal HRT. The influent characteristics for both R1 and R2 feeds are also listed because this highlights the carbon requirement when operating BSR using PSS at the 2 reactor temperatures. With regard to the effluent quality, both reactors achieved successful BSR and met the set performance criteria (effluent SO42- <250 mgSO42-/ℓ, VFA <100 mgHAc/ℓ) with excellent solid liquid separation (Table 9). In terms of the design and operating parameters, such as influent flow (Qi), HRT, BRT, Rs and bed volume (Vb), Reactor R1 at 35ºC outperforms R2 at 20ºC because the hydrolysis rate of the PSS is strongly decreased by a temperature reduction of 15ºC (~ 30% reduction in BPO hydrolysed). The feed PSS COD/SO42- ratio of R2 had to be increased to 1.75 to make up for the BPO not hydrolysed. Moreover, R2 had a longer HRT and Vb in order to increase the BRT and sludge age (Rs) thereby improving the utilisation of BPO. Economically these factors favour R1 as it has a more efficient use of BPO (~90%) than R2 (~60%). However, in practical terms, this advantage might be offset as a UASB reactor would need to be heated to 35ºC to achieve the R1 performance. A cost-benefit analysis of these 2 reactors' design and operating parameters would be required in order to assess the economics of the 2 systems. The successful operation of R2 demonstrates that sulphate reduction using PSS at ambient temperature (20ºC) can be achieved provided a higher feed PSS COD/SO42- ratio and/or a longer Rs is applied.
Comparison of operating parameters and performance with previous studies
A summary of the performance of the UASB reactors operated in this study along with the performance of various one-stage bioreactors for the treatment of sulphate-rich waters is listed in Table 10. Overall the use of PSS as carbon source (substrate) in UASB systems matches closely (and sometimes exceeds) the performance of systems fed with soluble substrates (namely ethanol, lactate and molasses). This demonstrates the benefits of PSS as a low cost energy source for BSR.
Design and operating parameters
A summary of the different design and operating parameters applied to the 2 BSR UASB systems of this investigation is listed in Table 11. Two of these parameters are critical and govern the system performance and economics:
- The sludge age (Rs) which controls the sludge aspect ratio (bed height/diameter) and hence the reactor bed volume. This in turn will govern the biomass concentration for BPO maximum hydrolysis rate, which is the limiting rate of the system; Ristow et al. (2006) showed that this rate is closely similar to that in methanogenic AD systems treating PSS.
- The HRT controls the reactor volume comprising the bed and liquid phases. A short HRT is always desirable because it reduces the reactor volume. However, too short an HRT leads to high upflow velocity and disproportionate bed expansion and sludge wash out. Therefore, the optimum HRT should be established so as to allow sufficient contact time of the sulphate with the sludge bed (biomass) and to optimise the upflow velocity (Vup) which governs the bed expansion ratio which in turn dictates the sludge age if a constant bed height is required. Sludge settleability and bed expansion are considered by Poinapen et al. (2009c).
The remaining parameters will then be a consequence of how the sludge age (Rs) and HRT are optimised. As far as temperature is concerned, it was found that the feed PSS COD/SO42- ratio had to be increased to 1.75 to cater for incomplete biodegradable particulate organics (BPO) hydrolysis (and consequently low VFA generation) due to the slower hydrolysis/acidogenesis rate at a temperature of 20ºC compared with 35ºC. If a high % of biodegradable organics utilisation is required at the lower temperature, the sludge age of the UASB reactor needs to be increased by increasing the reactor area so that a higher sludge mass is retained in the reactor in relation to that wasted to maintain the bed height, i.e. in effect reducing the volumetric COD (and SO42-) loading rate.
Conclusions
The successful operation of the UASB Reactor R2 (fed 1 500 mgSO42-/ℓ) at 20ºC is of significant importance because full-scale systems are not likely to be heated due to the absence of methane gas. Besides the 2 critical parameters (Rs and HRT) which are both longer at 20ºC than at 35ºC, the other important parameter in the operation of BSR systems using PSS is the feed PSS COD/SO42- ratio. By decreasing the temperature from 35ºC to 20ºC, the PSS biodegradable particulate organics (BPO) hydrolysis rate is decreased and less than 70% of the BPO is hydrolysed thereby generating less VFA for BSR. Increasing the PSS COD/SO42- from 1.25 (applied to R1) to 1.75 (applied to R2) increased the BPO concentration hydrolysed to meet the VFA demand for sulphate reduction. Alternatively, increasing the sludge age of the system by reducing the volumetric COD and sulphate load would increase the % BPO hydrolysed while maintaining the feed PSS COD/SO42- at 1.25.
UASB R1 was operated at an increased influent sulphate concentration (1 800 mgSO42-/ℓ) to investigate the inhibition effect by un-dissociated hydrogen sulphide generated from the reduction of this sulphate concentration level. It was found that high sulphate reduction (~ 92%) was achieved even at the relatively low HRT of 18.5 h. This observation is important in calibrating the value of the acetotrophic (acetate using) SRB inhibition constant (KI) in the dynamic kinetic simulation model (UCTADM1-BSR) developed in Part 6 of this series, which is currently in preparation by Poinapen and Ekama.
Acknowledgements
This research was supported financially by the Water Research Commission, the National Research Foundation and the University of Cape Town and is published with their permission.
References
BARNES LJ, JANSSEN FJ, SHERREN J, VERSTEEGH JH, KOCH RO and SCHEEREN PJH (1991) A new process for the microbial removal of sulphate and heavy metals from contaminated waters extracted by a geohydrological control system. Trans. Inst. Chem. Eng. 69 184-186. [ Links ]
KAKSONEN HA (2004) The Performance, Kinetics and Microbiology of Sulphidogenic Fluidised-Bed Reactors Treating Acidic Metal- and Sulphate-Containing Wastewater. Ph.D. dissertation. Institute of Environmental Engineering and Biotechnology, Tampere University of Technology, Finland. [ Links ]
KALYUZHNYI SV, DE LEON FRAGOSO C and RODRIGUES MARITNEZ J (1997) Biological sulphate reduction in a UASB reactor fed with ethanol as electron donor. Mikrobiologiya 66 687-693. [ Links ]
KONISHI Y, YOSHIDA N and ASAI S (1996) Desorption of hydrogen sulphide during batch growth of the sulphate-reducing bacterium Desulfovibrio desulphuricans. Biotechnol. Prog. 12 322-330. [ Links ]
MAILLACHERUVU KY, PARKIN GF, PENG CY, KUO W, OONGE ZI and LEBDUSCHKA V (1993) Sulphide toxicity in anaerobic systems fed sulphate and various organics. Water Environ. Res. 65 100-109. [ Links ]
MAREE JP and STRYDOM WW (1987) Biological sulphate removal from industrial effluents in an upflow packed bed reactor. Water Res. 21 141-146. [ Links ]
O'FLAHERTY V, MAHONY T, O'KENNEDY R and COLLERAN M (1998) Effect of pH on growth kinetics and sulphide toxicity thresholds of a range of methanogenic, syntrophic and sulphate-reducing bacteria. Proc. Biochem. 33 1-15. [ Links ]
POINAPEN J, EKAMA GA and WENTZEL MC (2009a) Biological sulphate reduction with primary sewage sludge in an upflow anaerobic sludge bed (UASB) reactor – Part 1: Feasibility study. Water SA 35 (5) 525-534. [ Links ]
POINAPEN J, EKAMA GA and Wentzel MC (2009b) Biological sulphate reduction with primary sewage sludge in an upflow anaerobic sludge bed (UASB) reactor – Part 2: Modification of simple wet chemistry analytical procedures to achieve COD and S mass balances. Water SA 35 (5) 535-542. [ Links ]
POINAPEN J, EKAMA GA and WENTZEL MC (2009c) Biological sulphate reduction with primary sewage sludge in an upflow anaerobic sludge bed (UASB) reactor – Part 4: Bed settling characteristics. Water SA 35 (5) 553-558. [ Links ]
REIS MAM, ALMEIDA JS, LEMOS PC and CARRONDO MJT (1992) Effect of hydrogen sulphide on growth of sulphate reducing bacteria. Biotechnol. Bioeng. 40 593-600. [ Links ]
RISTOW NE, SÖTEMANN SW, WENTZEL MC, LOEWENTHAL RE and EKAMA GA (2006) The effects of hydraulic retention time and feed COD concentration on the hydrolysis rate of primary sewage sludge. Water Sci. Technol. 54 (5) 91-100. [ Links ]
Received 13 January 2009; accepted in revised form 31 July 2009.
* To whom all correspondence should be addressed.
+2721 650 2585; fax: +2721 689 7471;
e-mail: George.Ekama@uct.ac.za














