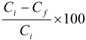Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
Water SA
versión On-line ISSN 1816-7950
versión impresa ISSN 0378-4738
Water SA vol.35 no.3 Pretoria abr. 2009
Acidithiobacillus caldus, Leptospirillum spp., Ferroplasma spp. and Sulphobacillus spp. mixed strains for use in cobalt and copper removal from water
BB MambaI, *; NP DlaminiI; AF Mulaba-BafubiandiII
IDepartment of Chemical Technology, University of Johannesburg, PO Box 17011, Johannesburg 2028, South Africa
IIMinerals Processing and Technology Research Group, Department of Extraction Metallurgy, University of Johannesburg, PO Box 526, Wits 2050, South Africa
ABSTRACT
Bacteria from the genus Acidithiobacillus, Leptospirillum and Ferroplasma, Sulphobacillus are often associated with water remediation. In this study a consortium of Acidithiobacillus caldus, Leptospirillum spp., Ferroplasma spp. and Sulphobacillus spp. was cultured and used to remove Cu2+ and Co2+ from synthetic aqueous sulphate solutions. The influence of experimental conditions such as pH, temperature, time, volume and metal concentration on the efficiency of the biosorption process was investigated. Biosorption of 54 to 67% Cu (pH 2, 37°C, 24 h) and 23-70% Co (pH 2, 37°C, 24 h) was observed from solutions containing 3.86 g·ℓ-1 Cu2+ ions and 3.36 g·ℓ-1 Co2+ ions. Such findings suggest that if optimal conditions for biosorption of the metals by micro-organisms are achieved, this should afford a cost-effective method of removing metal species from water and aqueous solutions.
Keywords: copper, cobalt, adsorption, biosorption, synthetic solution
Introduction
Recent progress in the use of micro-organisms for industrial applications promotes not only bacterial leaching of mineral-bearing ores but also the microbial treatment of metal contaminated water (Brierley, 1982). Water contamination is a serious challenge which is ever increasing in magnitude. With respect to groundwater contamination, about 10% of the population in South Africa uses groundwater for drinking while a large number of industries and agricultural systems depend on clean and safe groundwater supplies (Botha, 2006). Contamination of groundwater can be due to leaching of effluents emanating from garbage dumps, industrial waste dumps, mine dumps and septic tanks (Vullo et al.,2008). Considerable effort is being expended to remediate this water-pollution problem. Contamination of groundwater resources by heavy metals in South Africa is also on the rise, due to large- and small-scale mining activities taking place.
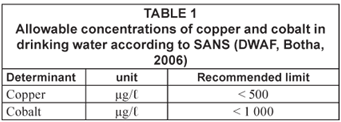
Heavy metals which contaminate water bodies include, among others, copper, cobalt, iron, manganese, chromium, nickel, gold and lead. Although most of these metals have vital human body functions, their ingestion in high quantities can result in severe health implications which in some cases may be fatal. It is, therefore, crucial that concentration levels of these metals in drinking water for example, are closely monitored. Table 1 shows the allowable concentrations of copper and cobalt in drinking water according to the South African National Standards (SANS), 2006.
Most commonly reported mechanisms for metal removal from solution are biosorption, chemisorption or specific adsorption and precipitation in the form of hydroxides (Fe3+, Cr3+, and Al3+), carbonates (Fe2+, Mn2+) or sulphides (Pb2+, Co2+, Cd2+, Cu2+, Ni2+, Fe2+, Zn2+) (Bojinova et al., 2006). While these methods have been successfully used in removing metal species from water, the challenge for research is the use of novel techniques that are less costly, more efficient and environmentally friendly. For example, these conventional methods can be effectively used for solutions of high metal concentrations while for low metal concentrations the same techniques may be costly. With respect to the environment, the removal of metals from solutions emanating from metallurgical and mining operations would serve to protect ground and surface water, especially when taking into consideration repercussions from acid mine drainage. The use of micro-organisms for the removal of metals from metallurgical aqueous solutions has not been reported largely. Recently, reports have been published where bacterial species such as Pseudonomonas spp. have demonstrated capacity to remove significant amounts of heavy metals by biosorption from aqueous solutions (Donalkova et al., 2005; Vullo et al., 2008).
Copper is a major component of haemoglobin, the protein responsible for oxygen transport in blood cells. Copper, along with vitamin C, forms elastin, a protein required to keep skin, blood vessels, and lungs supple and elastic (Araya et al, 2007). It is also an antioxidant and is required by the central nervous system as a component in the production of noradrenalin. However, consumption of copper in large quantities can cause serious ailments and people with ulcerative colitis may tend to accumulate copper, aggravating the disorder. A daily intake of more than 20 mg of copper can cause vomiting and stomach ulcers (Araya et al., 2007). Cobalt, on the other hand, forms part of vitamin B12 which is essential for human health. It is also used to treat anaemia in pregnant woman because it stimulates the production of red blood cells. However, consumption of high amounts of cobalt can cause serious lung ailments like asthma and pneumonia. Drinking water with a high cobalt concentration induces vomiting and nausea, and can cause vision problems, heart-related ailments and thyroid damage (http://www.atsdr.cdc.gov ).
In this study, copper and cobalt were chosen for biosorption experiments due to their wide use in industry and their potential water-pollution impact. What is of significance is that the micro-organisms will be utilised in removing metal species from single-element synthetic solutions as well as from multi-metal synthetic solutions where there would be potential interference by other metal ions present in solution. Hence, the combined effects of two or more metals present in solution and their effect on their removal from aqueous media will be explored. While research on single species of heavy metal-ion uptake in solution by various micro-organisms is well reported, the synergetic effect of two or more metals in the biosorption process has, to date, received less attention (Chang and Chen, 1998).
Experimental
Culturing
A mixed-strain culture of bacteria containing 4 types of bacterial strains namely Acidithiobcillus caldus, Leptospirillum spp., Ferroplasma spp. and Sulphobacillus spp. was obtained from Mintek's Hydrometallurgy Laboratory Division in Randburg, South Africa. It was then grown in a culture medium Postgate's Medium B (Postgate, 1984). The mixed-strain culture sample (20 mℓ) was mixed with 100 mℓ of nutrient medium and thoroughly shaken in a 1 000 mℓ volumetric flask. This was kept at constant stirring using a magnetic stirrer and the temperature was maintained at 36°C and the pH was constantly monitored within a range of 1.5 to 2.0.
Test solutions (synthetic solutions)
Standard synthetic solutions were prepared in our laboratories using copper (II) and cobalt (II) sulphate salts. The metal solutions were divided into 3 categories, i.e. single metal system (Co or Cu), binary metal (Co and Cu mixed system and multi-element system (Co and/ Cu in varying ratios). Concentrations of 3.86 g·ℓ-1, 19.32 g·ℓ-1 and 38.6 g·ℓ-1 Cu2+ ions in solution and 3.3 g·ℓ-1, 16.7 g·ℓ-1 and 33.4 g·ℓ-1 Co2+ ions in solution (which is 0.07 M, 0.33M and 0.66 M for both Cu2+ and Co2+). Their ratios were varied depending on the category of testing.
Extraction experiments
The factors that affect the biosorption rate were examined in a batch system. Five parameters were examined for their effect in the biosorption of metal ions from solutions and these are biomass concentration (bacteria), metal-ion concentration, contact time, presence of co-cations and solution pH. All experiments were carried out with micro-organism suspension in Erlenmeyer flasks in an incubator at 37±0.5°C with constant shaking to create optimum conditions (contact time, pH, initial metal concentration and bacterial dose). The contents of the flasks were thereafter filtered and the filtrates were diluted (10 mℓ was pipetted into 100 mℓ volumetric flask and filled to the mark with distilled water). This was done in order to obtain lower concentrations which could be analysed by flame absorption spectroscopy to obtain residual metal concentrations. The metal adsorbed by biomass was calculated as follows:
% metal adsorbed by biomass =
where:
Ci is the initial metal concentration
Cf is the final metal concentration
Control experiments
Control experiments were conducted to monitor other factors which may affect metal concentrations in solution like precipitation. This was done by having a similar experimental set-up without any bacteria added to the solutions. Solutions from all 3 different concentrations were put in Erlenmeyer flasks in an incubator at 37±0.5°C with constant shaking to emulate optimum conditions for biosorption (contact time, pH, initial metal concentration).
Results
Effect of biomass (bacteria) and metal-ion concentration
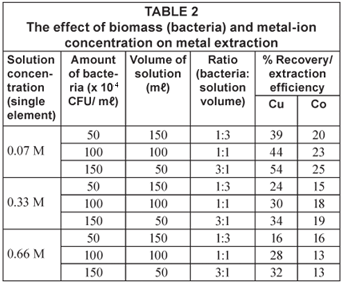
In the first stage of batch biosorption experiments, the combined effects of the amount of biomass (bacteria) and metal ion concentration were examined. Three different amounts and concentrations of bacteria were used (50 × 104 CFU/mℓ, 100 × 104 CFU/ mℓ and 150 × 104 CFU/mℓ ), i.e. 1:1, 1: 3 and 3:1 ratios and 0.07 M, 0.33 M and 0.66 M solutions of both Cu2+ and Co2+ ions, respectively, were used.
These results are presented here as a bar chart in Fig. 1.
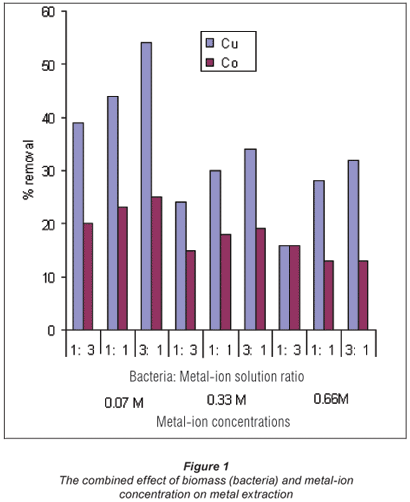
Metal removal with respect to the concentration of Co2+ and Cu2+ ions in solution is shown in Fig. 1. The results demonstrate a decrease in metal uptake with an increase in the equilibrium concentration. This observation is linked to the metal-concentration gradient between the active and available sites of the bacterium, which suggests that metal uptake decreases with an increase in initial ion concentration. This could be due to the fact that biosorption in dilute solutions is mainly through ion exchange, whereas in concentrated solutions biosorption is mainly through precipitation which is much slower compared to the latter (Zachara et al., 1991).
Table 2 and Fig. 1 also shed some light on the effect of biomass concentration on the recovery of the metals. It is observed that an increase in the amount of biosorbent results in an increase in metal adsorption efficiency (removal efficiency). This observation is due to the fact that there is an increase in binding sites (surface area) as the bacterial species increase (Brady and Tobin, 1994).
Effect of contact time
The effect of contact time was also examined for the bacterial species. This was done to ascertain the threshold in terms of the time the microbial biomass is capable of spending in aqueous solution while still active, i.e. recovering (adsorbing metal ions). Figures 2 and 3 illustrate the effect of contact time observed over a period of 12 d.
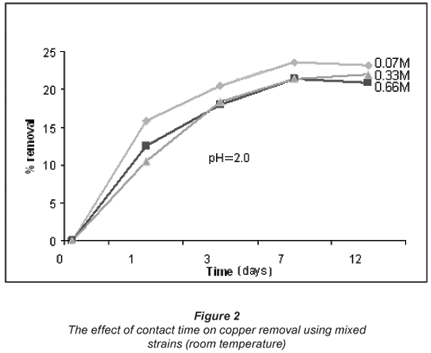
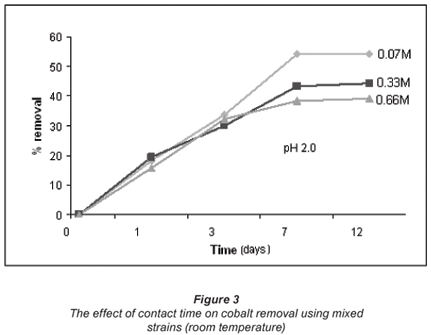
The increase in metal uptake with an increase in the initial ion concentration is linked to the metallic concentration gradient with respect to the active and available adsorption sites of the bacteria, which suggests that metal uptake increased with an increase in initial ion concentration. However, at very high metal concentrations the biosorption rate is reduced. The proposed explanation is that biosorption in dilute solutions takes place mainly through ion exchange whereas in concentrated solutions biosorption is mainly through precipitation which is much slower compared to the latter (Zachara et al., 1991).
Figures 2 and 3 show a trend that is not in line with the typical bacterial growth curve, as the lag phase was very short and almost insignificant. This could have been due to the fact that these strains are indigenous and are well adapted to harsh environments. Copper removal in all concentrations exponentially increases with time and reaches equilibrium after 7 d of exposure. Cobalt removal, on the other hand, shows an increase in all concentrations at a lower gradient when compared to copper removal. The 0.07 M concentrated solution shows high removal efficiencies for both copper (54%) and cobalt (25%). Copper removal decreased with an increase in concentration, whereas cobalt was taken up more effectively from the 0.66 M solution after 12 d than its removal from the 0.07 M concentrated solution.
Viability tests performed using fluorescence microscopy confirmed that in solutions of low concentration, the rate at which bacteria die is less than in the case of highly concentrated solutions. Different biosorbents used for the adsorption of various heavy metals yielded similar observations. For example, in a study conducted by Abu Al-Rub and fellow researchers using C. vulgaris algal cells, a substantial viable biomass remained after biosorption of less concentrated copper solutions (0.1 g/ℓ than in the case of highly concentrated copper solutions (20 g/ℓ) (Kumar et al., 2006; Fiol et al. 2006; Abu Al-Rub et al., 2006, Pan et al., 2006).
In solutions of low concentration, the rate at which bacteria die is less than in the case of highly concentrated solutions. Different biosorbents used for the sorption of various heavy metals yielded similar observations. For example in a study conducted by Abu Al-Rub and fellow researchers using C. vulgaris algal cells, a substantial viable biomass remained after biosorption of low concentrated copper solutions (0.1 g·ℓ-1) than in the case of highly concentrated copper solutions (20 g·ℓ-1) (Kumar et al., 2006; Fiol et al., 2006; Abu Al-Rub et al., 2006, Pan et al., 2006).
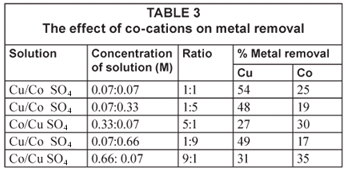
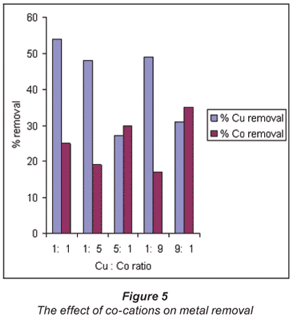
The effect of co-cations on metal extraction
Extensive research has been done on the effect of co-cations on metal extraction (Tsezos, 1983). However, the majority of published work on biosorption only involves one metal species. Very little information is available for binary and multi-metal biosorption systems (Gonzalez Davila, 1990). If biosorption is to be implemented in the fields of water treatment and hydrometallurgy where complex multi-component metal systems are common, further research on such systems is essential. The CuSO4 and CoSO4 solutions were further mixed in order to represent a real sample where the effect of one metal ion on another may be examined. This was done by varying the concentrations of the two metal cation solutions as shown in Table 3.
The occupation of active sites of an adsorbent during ion exchange is also a competitive process whereby one cation is favoured over the other. This could be attributed to cation-cation interactions as well as to water molecules that surround the cations in solution. Inglezakis et al. (2005) documented that selectivity for one ion over the other in a matrix is a result of physico-chemical and stereochemical factors which are hydrated radii, hydration enthalpy of the cation and the space requirements in the micropores of the adsorbent in connection with the incoming ions. It has been reported as shown in Fig. 4 that every metal cation that is dissolved in water possesses its own specific hydrated layer which has a characteristic thickness and degree of stability which determines its capability of being taken up by an ion exchanger (Tansel et al., 2006).
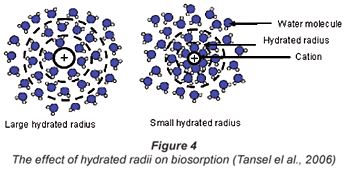
Therefore, for the larger anhydrite ions, with the charge more dispersed, the water of hydration is held less strongly to the cation which enables biosorption to occur.
Mixing of the 2 cations seemed to affect the rate at which biosorption occurred when compared to the single element matrices in Table 2. There was better biosorption in more dilute solution combinations than in concentrated solutions.
The 5:1 and 9:1 copper: cobalt ratio (0.33 M, 0.07 M and 0.66 M:0.07 M respectively) in Table 3 and Fig. 5 shows an almost comparable recovery of copper and cobalt, with cobalt being slightly favoured. This could be due to the fact that the cobalt in solution is more dilute than the copper solution and hence binds easily to the surfaces of the bacteria.
The effect of solution pH
pH is one of the parameters that affects the biosorption mechanism. In order to determine the optimum pH for biosorption, experiments were carried out for 24 h at pH values of 1.5, 3 and 4.5 using 0.07 M as initial concentrations of CuSO4 and CoSO4 CuSO4 and CoSO4 solutions. Usually the pH has a significant effect on the solubility, speciation and biosorption of heavy metals (Abu Al-Rub et al., 2006). The increase in metal removal with increase in pH can be explained using the analogy between the reaction of metal hydrolysis Eq. (1) and the reaction between binding sites of the sorbent and the metal Eq. (2).
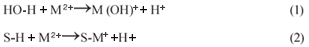
In both reactions the O-H and S-H bonds are broken and hydrogen ions are released followed by substitution by the metal, M (HO–M and S–M) (Pagnanelli et al., 2003). Figure 6 shows metal uptake as a function of pH.
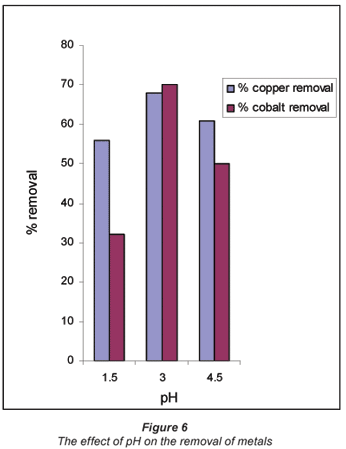
From Fig. 6 it can be observed that the best biosorption results occurred at pH 3.0 for both Cu (II) and Co (II) where 67% and 70% were respectively adsorbed by the biomass.
Such a trend was also observed by other authors that investigated the effect of pH on biosorption using different biomaterials (Kiran et al., 2005; Fiol et al., 2006; Uslu and Tanyol, 2006). The different pH binding values of these components could be due to the nature of the chemical interactions of each component with the microbial cells. At highly acidic pH values, metal ions compete for binding sites on the cell wall, which results in a lower metal uptake (Bueno et al., 2008).
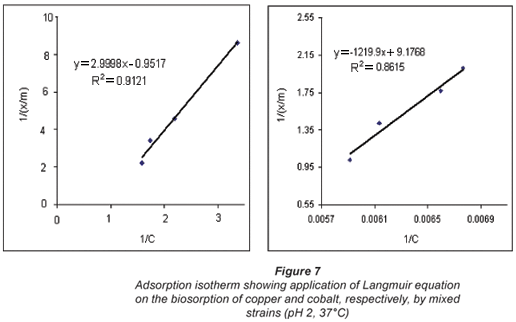
Langmuir biosorption isotherms
It is important to note that sorption studies determine the capacity of the sorbent, which can be described by a sorption isotherm, characterised by certain constants whose values express the surface properties and the affinity of the sorbent (Ho and Mckay, 2000).
The Langmuir model represents a theoretical treatment of non-linear sorption and suggests that uptake occurs on a homogenous surface by monolayer sorption without any interaction between adsorbed molecules. Langmuir isotherms were plotted for the single element category. The Langmuir equation is given by:
x / m = (ab / Cr) / (1 + bCr)
where:
Cr is the equilibrium concentration of the metal in solution left after adsorption (mg/ℓ)
x/m is the number of metal ions adsorbed onto the bacteria
a and b are Langmuir constants related to adsorption capacity and adsorption energy, respectively
constant a is the maximum sorption capacity and represents single layer coverage of the adsorbent (bacteria) in contact with the adsorbate (metal solution)
variable b represents enthalpy of adsorption and changes with temperature
To draw the Langmuir plots, the equation is first linearised as follows:
Cr/[X / m] = (1 / ab) + (1/a)Cr
Cr/(x/m) is then plotted against Cr. to obtain a straight line.
The adsorption data were then fitted in the Langmuir equation as illustrated in Fig. 7.
Interaction of bacteria with metal ions
Interactions of the micro-organisms with the metal species were examined using Scanning Electron Microscopy (SEM), model SEM Joel JSM 5600 and Transmission Electron Microscopy, Joel 100S model as illustrated in Figs. 8 and 9. The SEM micrograph shows the accumulation of metal ions on the cell wall of the bacterial species and the darkish accumulations seen on the cell wall of the bacterium in the TEM image support the SEM observations.
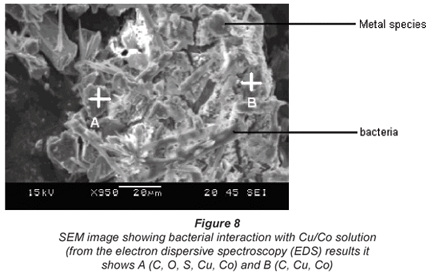
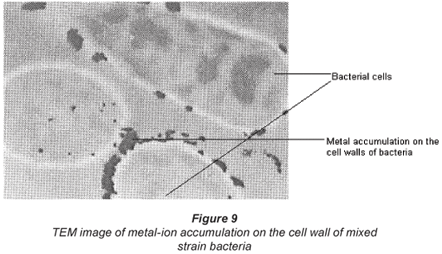
It is also observed that under all conditions there was better biosorption of Cu2+ than Co2+. This may be accounted for by the nature of the 2 metal ions. Although the charges on both Cu2+ and Co2+ ions are the same, the hydrated radii differ whereby Cu2+ has a lower hydrated radius than Co2+. Biosorption of a metal-ion using an ion exchanger largely depends on its hydrated radius. Similar observations on an adsorbent called clinoptilolite were observed by Erdem, who concluded that the larger the diameter of the metal ion the slower its mobility and the less likely its ionic exchange capability will be (Erdem et al., 2004). It would appear that for ease of biosorption there should be fewer water molecules surrounding the cation.
Conclusions
This study has revealed that micro-organisms tend to remove or adsorb more metal ions at low concentrations and for shorter contact periods. Also, the data accumulated thus far suggest that the mixed strain bacterial consortia containing Acidithiobcillus caldus, Leptospirillum spp., Ferroplasma spp. and Sulphobacillus spp. can adsorb both copper and cobalt from their synthetic sulphate solutions. These results demonstrate the potential for using these micro-organisms for the removal of copper and cobalt from sulphate solutions of leached mineral ores. We are currently investigating the use of these bacterial consortia in the removal of metals including copper and cobalt from mine effluents resulting from metallurgical operations.
Acknowledgements
The authors wish to thank Mintek for providing the bacterial strains. Funding from the National Research Foundation (NRF), the University of Johannesburg (UJ) and assistance from Mr E Fosso-Kankeu and Mr N Tshabalala (UJ Clinic) with bacterial isolation is gratefully acknowledged.
References
ABU AL-RUB FA, EL-NAAS MH, ASHOUR I and AL-MARZOUQI M (2006) Biosorption of copper on Chlorella vulgaris from single binary and ternary metal aqueous solutions. Proc. Biochem. 41 457-464. [ Links ]
ARAYA M, OLIVARES M and PIZARRO F (2007) Copper in human health. Int. J. of Environ. Health 1 (4) 608-620. [ Links ]
BOJINOVA DY and VELKOVAR RG (2006) Bioleaching of metals from mineral waste product. Acta Biotechnol. 21 275-282. [ Links ]
BOTHA FS (2006) Groundwater in South Africa, where to from here? Department of Water Affairs and Forestry, Pretoria, South Africa. [ Links ]
BRADY JM and TOBIN JM (1994) Adsorption of metal ions by Rhizopus arrhizus biomass: Characterization studies. Enzym. Microb. Technol. 16 (8) 671-675. [ Links ]
BRIERLEY CORALE L (1982) Microbial mining. Sci. Am. J. 247 42-50. [ Links ]
BUENO BYM, TOREM ML, MOLINA F and DE MESQUITA LMS (2008) Biosorption of lead(II), chromium(III) and copper(II) by R. opacus: Equilibrium and kinetic studies, Miner. Eng. 21 65-75. [ Links ]
CHANG JS and CHEN CC (1998) Biosorption of lead, copper and cadmium with continuous hollow-fiber microfiltration processes. Sep. Sci. Technol. 34 (8) 1607-1627. [ Links ]
DONALKOVA A, MARSHAL MJ, KENNEDY DW, GORBY YA, SHI L, BELIAEV A, APKARIAN R and FREDRICKSON JK (2005) Micros. Soc. Am. 129-132. [ Links ]
ERDEM E, KARAPINAR N and DONAT R (2004) The removal of heavy metal cations by natural zeolites. J. Colloid Interface Sci. 280 (2) 309-314. [ Links ]
FIOL N, VILLAESCUSA I and MARTINEZ M (2006) Sorption of Pb(II), Ni(II), Cu(II) and Cd(II) from aqueous solution by olive stone waste. Sep. Purif. Technol. 50 132-140. [ Links ]
GONZALEZ DAVILA M, SANTANA-CASIANO JM and MILLERO FJ (1990) The adsorption of Cd (II) and Pb (II) to chitin in seawater. J. Coll. Interf. Sci. 137 102-104. [ Links ]
HO YS and MCKAY G (1998) Kinetic model for lead (II) sorption on to peat. Adsorp. Sci. Technol. 16 243-255. [ Links ]
INGLEZAKIS VJ, ZORPAS AA, LOIZIDOU MD and GRIGOLOPOULOU HP (2005) The effect of competitive cations and anions on ion exchange of heavy metals. J. Sep. Purif. Technol. 46 202-207. [ Links ]
KIRAN ISMAIL, SIBEL TUNALI and TAMER AKAR (2005) Chromium (VI) biosorption characteristics of Neurospora crassa fungal biomass. Miner. Eng. 18 (7) 681-689. [ Links ]
KUMAR YP, KING P and PRASAD VS (2006) Equilibrium and kinetic studies for the biosorption system of copper(II) ion from aqueous solution using Tectona grandis L.f. leaves powder. J. Hazardous Mater. B137 1211-1217. [ Links ]
PAN J, GE X, LIU R and TANG GH (2006) Characteristic features of Bacillus cereus cell surfaces with biosorption of Pb (II) ions by AFM and FT-IR. Colloids Surfaces B: Biointerfaces 52 89-90. [ Links ]
POSTGATE JR (1984) The Sulphate-Reducing Bacteria (2nd edn.) Cambridge University Press, Cambridge, UK. 105-107. [ Links ]
TANSEL B, SAGER J, RECTOR T, GARLAND J, STRAYER RF, LEVINE L, ROBERTS M, HUMMERICK M and BAUER J (2006) Significance of hydrated radius and hydration shells on ionic permeability during nanofiltration in dead end and Cross flow modes. Sep. Purif. Technol. 51 40–47. [ Links ]
TOXICOLOGICAL PROFILE FOR COBALT- http://www.atsdr.cdc.gov/toxprofiles/tp33.html Accessed (2 April 2008). [ Links ]
TSEZOS M (1983) The role of chitin in uranium adsorption by Rhizopus arrhizus. Biotechnol. Bioeng. 25 2025-2040. [ Links ]
USLU G, and TANYOL M (2006) Equilibrium and thermodynamic parameters of single and binary mixture biosorption of lead (II) and copper (II) ions onto Pseudomonas putida: Effect of temperature. J. Hazardous Mater. 135 (1-3) 87-93. [ Links ]
VULLO DL, HELLENA C, MARIA AD, SILVANA AM and RAMIREZ AZ (2008) Cadmium, zinc and copper biosoption by Pseudomonas veonii 2E. Bioresour. Technol. 99 5574-5581. [ Links ]
ZACHARA JM and COWAN CT (1991) Sorption of divalent metals on calcite. Geochim. Cosmochim. Acta 55 (6) 1549-1562. [ Links ]
Received 12 November 2008; accepted in revised form 16 March 2009.
* To whom all correspondence should be addressed.
+2711 559 6516; fax: +2711 559 6425;
e-mail: bmamba@uj.ac.za