Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
SA Orthopaedic Journal
versión On-line ISSN 2309-8309
versión impresa ISSN 1681-150X
SA orthop. j. vol.22 no.3 Centurion 2023
http://dx.doi.org/10.17159/2309-8309/2023/v22n3a5
TRAUMA AND GENERAL ORTHOPAEDICS
Unexpected high prevalence of Gram-negative pathogens in fracture-related infection: is it time to consider extended Gram-negative cover antibiotic prophylaxis in open fractures?
Nando Ferreira*; Shao-Ting J Tsang; Adrian Jansen van Rensburg; Rudolph Venter; Gadi Z Epstein
Division of Orthopaedic Surgery, Department of Surgical Sciences, Faculty of Medicine and Health Sciences, Stellenbosch University, Cape Town, South Africa
ABSTRACT
BACKGROUND: Gram-negative organisms are increasingly seen as causative pathogens in orthopaedic fracture surgery, which might necessitate a change in antibiotic prophylaxis protocols.
METHODS: A single-centre retrospective review of antibiogram results from all patients treated for fracture-related infection (FRI) was conducted. Subgroup analysis was undertaken to identify any host, injury or treatment variables predisposed to Gram-negative infection.
RESULTS: The bacteriological results of 267 patients who underwent surgical treatment for FRI were analysed. Pathogens were isolated in 216 cases (81%), of which 118 (55%) were Gram-negative infections. Fractures involving the tibia and femur (p = 0.007), the presence of soft tissue defect (p = 0.003) and bone defects (p = 0.001) were associated with an increased risk of developing a Gram-negative FRI.
CONCLUSION: Gram-negative fracture-related infections were associated with injuries experiencing bone loss and those requiring soft tissue reconstruction. It is, therefore, prudent to consider extended Gram-negative directed antimicrobial prophylaxis in these cases to prevent the development of fracture-related infection.
Level of evidence: Level 4
Keywords: open fracture, antibiotics, fracture-related infection, Gram-negative
Introduction
Fracture-related infection (FRI) is a dreaded complication following orthopaedic trauma.1 Extensive pre-, intra- and postoperative care is taken to minimise the risk of infection. One of these strategies is the use of prophylactic systemic antibiotics, primarily aimed at Gram-positive organisms.2-6
Previous studies have reported the spectrum of causative pathogens in FRI to help guide both empirical prophylactic and therapeutic antimicrobial management.7-15 Although Staphylococcus aureus remains the most frequently isolated pathogen in FRI, the prevalence of Gram-negative infections is increasing, with the reported estimates ranging from 21-76%.7-15 It is widely accepted that Gram-negative cover should be added to prophylactic antibiotic protocols in higher-grade open fractures (Gustilo-Anderson 3),16 but this practice has not been generally accepted for closed fractures or elective orthopaedic procedures (hip and knee arthroplasty excluded).2-6,16-20 The inaugural British Aesthetic and Plastic Reconstructive Surgeons (BAPRAS) and British Orthopaedic Association (BOA) guidelines for the management of open fractures recommended co-amoxiclav (1.2 g 8 hourly) or a second-generation cephalosporin (e.g., cefuroxime 1.5 g 8 hourly) as antimicrobial prophylaxis before the first debridement. This is then followed by either co-amoxiclav (1.2 g) or a second-generation cephalosporin plus gentamicin (1.5 mg/kg) given at the time of surgery, and co-amoxiclav/ cephalosporin continued until soft tissue closure or for a maximum of 72 hours.16 However, the most recent iteration of these guidelines refrains from providing specific recommendations for antimicrobial prophylaxis.21
A multicentre audit of major UK trauma centres revealed that 78% of prophylactic antimicrobials used in the management of open fractures provided coverage against only Gram-positive pathogens.6 Similar results were reported in a systematic review of antimicrobial choice for prophylaxis in open fractures, which reported that approximately one-third included papers recommended only agents that covered Gram-positive organisms; just over 50% of studies did, however, recommend regimens that provided cover for both Gram-positive and Gram-negative pathogens.22 In North America, guidelines and consensus statements recommend the use of a first- or second-generation cephalosporin (e.g., cefazolin or cefuroxime) for routine perioperative antimicrobial prophylaxis in hip and knee replacement surgery.23,24 In a survey of North American arthroplasty surgeons, cefazolin was reported to be the antibiotic of choice for prophylaxis in 97% of respondents.25 Similar preferences and recommendations are seen in sarcoma surgery,18,19 foot and ankle surgery26 and spinal surgery.27 The exception to this rule was among UK hip and knee arthroplasty surgeons. In a survey of UK centres performing elective knee and hip arthroplasty surgery, flucloxacillin with gentamicin was the most popular prophylactic regimen, with 57/146 (39%) of surveyed trusts using it. Cefuroxime was the agent of choice in 44/146 (30%), with teicoplanin plus gentamicin being the third most popular 25/146 (17%).28
The study aimed to review the microbiology and antibiogram data of all patients who presented to a tertiary orthopaedic unit with FRI to generate hypotheses for future research in the role of extended spectrum antimicrobial prophylaxis in fracture management surgery. Subgroup analysis was undertaken to identify any host, injury or treatment variables predisposed to Gram-negative infection development.
Methods
A single-centre retrospective review of antibiogram results from all patients treated for FRI at a tertiary level musculoskeletal infection unit was conducted. FRI was diagnosed according to the international consensus definition proposed by Metsemakers et al. and modified by Govaert et al. in 2020.29,30 Patients of any age treated for a fracture-related infection of the appendicular skeleton were included in the study. Patients with chronic osteomyelitis not related to fracture-related infection were excluded. Patient records from January 2016 to October 2022 were reviewed. Data were collected regarding patient demographics, mechanism of injury, site of infection and causative organism, including the antibiogram.
Patient records and laboratory investigations assisted in stratifying the host into A, B or C types according to the modified Cierny and Mader (C&M) classification proposed by Marais et al.31 Laboratory studies were used to identify causative organisms.
In all cases, intraoperatively collected deep samples of infected tissue and/or biofilm were submitted for bacterial culture. Solid media consisted of tryptose blood, boiled blood and MacConkey agar (for aerobic/CO2-enriched conditions) and Brucella and/ or tryptose blood agar (for anaerobic conditions). Liquid media used consisted of cooked meat medium or tryptic soy broth. Tissue samples were crushed, and both crushed tissue and pus samples were inoculated onto the basic solid media listed prior to incubation. Tissue and pus samples were incubated on solid media for at least 48 hours. Pus swabs were incubated on solid media in CO2-enriched conditions for a minimum of 24 hours. Current local laboratory processing guidelines do not include the use of sonication or vortexing of the sample in the absence of submitted prosthetic material.
All pure cultures were identified. Mixed cultures were reviewed by a pathologist and followed up as appropriate. Identification and susceptibility testing was performed using the VITEK 2 automated system (bioMérieux, Marcy-l'Étoile, France) with supplemental rapid biochemical or antigen-based identification and disk or gradient diffusion antibiotic susceptibility testing, as appropriate. Antibiotic susceptibility results were interpreted according to annually published Clinical Laboratory and Standards Institute guidelines. For this paper, organisms falling within the intermediate category were categorised as resistant, as antibiotic activity at the site of infection was likely to be suboptimal.
Statistical analysis was performed using Stata 16.1 (StataCorp, College Station, Texas) and EpiCalc 2000 v1.02 (Brixton Books, UK). Parametric data are reported as mean and standard deviation (SD) with 95% confidence intervals (CI) where appropriate. Non-parametric data are described with median, interquartile range and range. Categorical data are described as frequencies and/ or counts, with 95% CI where appropriate. Associations were investigated using an independent t-test or a Mann-Whitney U test, depending on the distribution. Pearson chi-squared test (or Fisher's exact test, where appropriate) was used to detect significant differences between groups.
Results
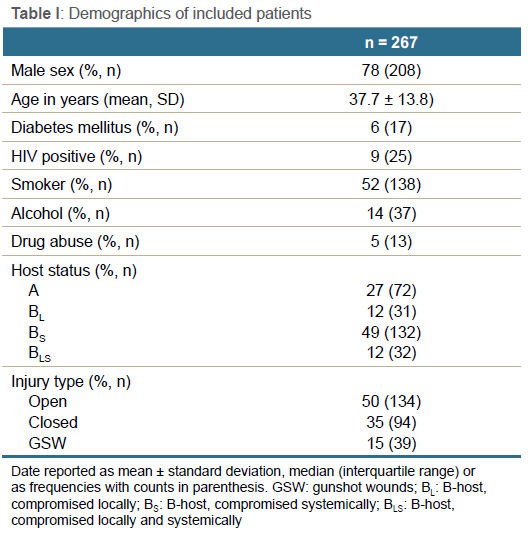
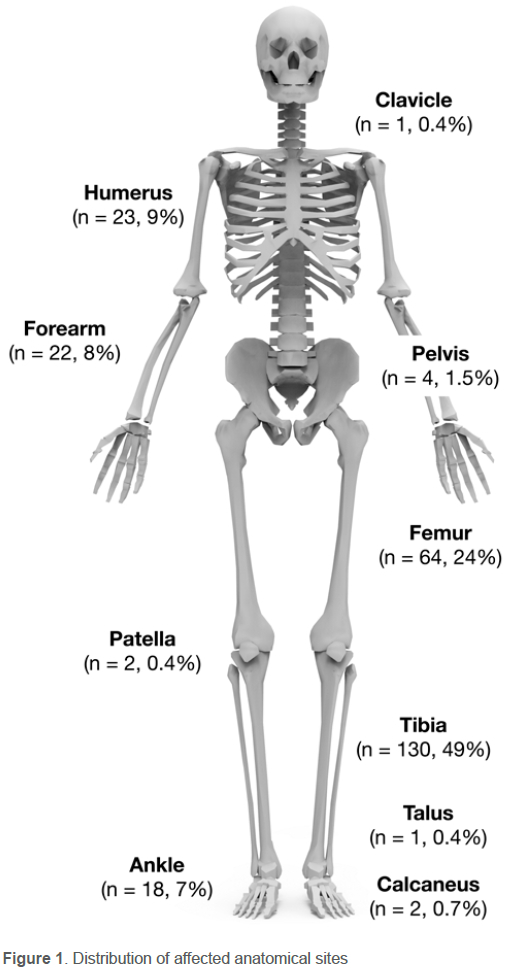
Between January 2016 and October 2022, 267 patients underwent surgical treatment for FRI. No patients were excluded. The final cohort comprised 208 males and 59 females, with a mean age of 37.7 (SD 13.8) years (range 16-84) (Table I). The mechanism of initial injury included 134 (50%) open fractures, 94 (35%) closed fractures, and 39 (15%) gunshot-induced fractures. The anatomical site of infection was predominated by tibias (n = 130, 49%), femurs (n = 64, 24%), humeri (n = 23, 9%) and forearms (n = 22, 8%). The distribution of affected anatomical sites is shown in Figure 1.
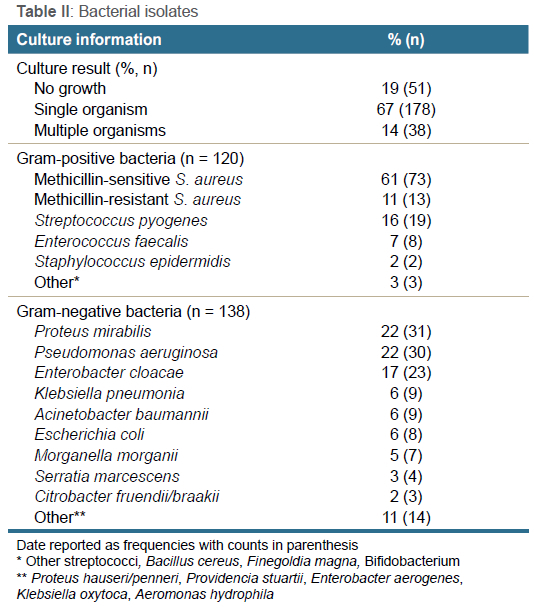
Pathogens were isolated in 216/267 cases (81%) (Table II). A single organism was isolated in 178/267 (67%) patients, while 38/267 (14%) patients showed polymicrobial growth, of which 12/267 (4%) showed both Gram-positive and Gram-negative bacterial growth. A total of 120 Gram-positive species and 138 Gram-negative species were isolated. While 97% of Gram-positive isolates comprised only two genera of pathogens (Staphylococcus spp. and Streptococcus spp.), Gram-negative cultures had a much more diverse range of isolates. Infection with at least one Gram-negative organism was found in 118/216 (55%) patients, while 98/216 (45%) patients showed infection with only Gram-positive pathogens.
No association between Gram-negative FRI and any host factor was observed (Table III). Fractures involving the tibia and femur (p = 0.007), the presence of soft tissue defect (p = 0.003) and bone defects (p = 0.001) were associated with an increased risk of developing a Gram-negative FRI.
Discussion
In this study, the prevalence of Gram-negative associated FRI was 55%. Previous studies have reported lower proportions of Gram-negative infections in FRI. Recent studies examining the microbiology of FRI from northern European centres estimate Gram-negative involvement in 21-28% of cases.7-11 The results of these studies have been used to inform recommendations for the systemic antimicrobial management of FRI.32 Furthermore, they reinforce the widely held belief that Gram-positive organisms should be the primary concern directing the choice of prophylactic antimicrobials.
Sagi et al. examined the causative pathogens of post-traumatic infections following open fracture in 204 patients in seven Level 1 trauma centres across the USA. While overall, S. aureus was the most prevalent pathogen, there were statistically significant regional variations (p < 0.001), with Gram-negative organisms the most commonly encountered pathogens in the mid-Western region (56%).33 A small single-centre cohort study performed in Pittsburgh, USA, published in 2013, reported a similar prevalence with 11/20 cases of FRI following open fracture having Gram-negative bacteria involvement. The authors concluded that consideration should be given to a change in the choice of antimicrobial prophylaxis to allow better coverage of Gram-negative organisms. A multicentre study from northeast China found that 47% of causative microorganisms were Gram-negative pathogens in 328 FRI cases.15 A further single-centre study from China reported a 70% prevalence of Gram-negative FRI in 535 patients.12 Finally, Lu et al. reported that Gram-negative pathogens accounted for 76% of monomicrobial cases of FRI at a major trauma centre in the UK.13 The cohorts reporting 47-76% Gram-negative involvement in FRI were characterised by patients experiencing more high-energy injuries (68% open injuries) of the lower limbs (68%), resulting in more high-grade open injuries (Gustilo-Anderson 3B and 3C) that required soft tissue reconstruction (22%). Similar risk factors were identified in this present study with the development of Gram-negative FRI associated with infections involving the tibia and femur (p = 0.007), the presence of soft tissue (p = 0.003) and bone defects (p = 0.001).
While there was a higher-than-expected prevalence of Gram-negative pathogens compared to previous studies, S. aureus remained the most commonly isolated pathogen (40%). This is comparable to the aggregate estimate of the prevalence of S. aureus FRI from published studies (33%).7-12,14,15 Oxacillin resistance was identified in 18% of S. aureus isolates, which is lower than reports from China (25%), Brazil (35%) and the Middle East (60%).15,34,35 This could be ascribed to the fact that many patients are antibiotic naive at the time of presentation to our limb reconstruction unit.
From the published literature, polymicrobial infections are estimated to affect 22% of FRI cases,7-12,14,15 which was greater than the estimate from this present study (14%). It has been previously postulated that adherence to a structured tissue sampling protocol and stricter diagnostic criteria, such as that implemented in this study, may reduce the prevalence of polymicrobial culture results due to reduced cross-contamination with skin flora and the requirement for isolates to be present in more than one culture.36 As a single-centre cohort, it is more likely that the patients in this current study received a more consistent and homogenous treatment, including intraoperative sampling, which may have resulted in a lower prevalence of polymicrobial infection for the reasons described above.
The call for extended Gram-negative coverage has been raised by various other orthopaedic disciplines. A 2021 review of 989 spinal fusion procedures showed a 54% incidence of Gram-negative infection, and the authors proposed Gram-negative prophylactic antibiotic coverage for a specific subset of patients undergoing spinal fusion.37 Bosco et al. identified that Gram-negative bacilli caused 30% of their periprosthetic joint infections and that the introduction of an extended Gram-negative anti-microbial prophylaxis protocol resulted in a statistically significant reduction in periprosthetic joint infections in their hip arthroplasty patients.38 Previous publications,17,39 as well as guidance from the National Institute of Clinical Excellence in the UK,40 have recommended tailoring both therapeutic and prophylactic antimicrobial choices on local microbiology data.
Several factors limit the generalisability of this study. These include the single-centre cohort and the retrospective nature of the study design. We also acknowledge that contemporary antibiotic prophylaxis is primarily aimed at Gram-positive organisms, which could explain a relative decrease in Gram-positive infections. It is, however, prudent to recognise that Gram-negative FRI is a significant problem in this population and warrants further investigation and discussion.
Conclusion
There is a rising prevalence of Gram-negative fracture-related infection, which is associated with injuries experiencing bone loss and those requiring soft tissue reconstruction. It is, therefore, prudent to investigate whether extended Gram-negative directed antimicrobial prophylaxis in these cases can prevent the development of fracture-related infection.
Ethics statement
The authors declare that this submission is in accordance with the principles laid down by the Responsible Research Publication Position Statements as developed at the 2nd World Conference on Research Integrity in Singapore, 2010.
Ethical approval for this study was obtained before the commencement of data collection from the Health Research Ethics Committee (HREC) of Stellenbosch University: N22/01/007. This retrospective review received a waiver of informed consent from the Stellenbosch University Health Research Ethics Committee.
All procedures were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008.
Declaration
The authors declare authorship of this article and that they have followed sound scientific research practice. This research is original and does not transgress plagiarism policies.
Author contributions
NF: study conceptualisation, data collection, manuscript preparation, approval of final manuscript
SJT: manuscript preparation, approval of final manuscript
AJvR: data collection, approval of final manuscript
RV: data collection, approval of final manuscript
GZE: study conceptualisation, data collection, approval of final manuscript
ORCID
Ferreira N https://orcid.org/0000-0002-0567-3373
Tsang S-TJ https://orcid.org/0000-0002-9862-8503
Jansen van Rensburg A https://orcid.org/0000-0001-6341-0734
Venter R https://orcid.org/0000-0003-0022-6969
Epstein GZ https://orcid.org/0000-0002-4753-5024
References
1. Depypere M, Morgenstern M, Kuehl R, et al. Pathogenesis and management of fracture-related infection. Clin Microbiol Infect. 2020 May;26(5):572-78. [ Links ]
2. Gans I, Jain A, Sirisreetreerux N, et al. Current practice of antibiotic prophylaxis for surgical fixation of closed long bone fractures: A survey of 297 members of the Orthopaedic Trauma Association. Patient Saf Surg [Internet]. 2017 Jan 16 [cited 2022 Dec 5];11(1):1-6. Available from: https://pssjournal.biomedcentral.com/articles/10.1186/s13037-016-0118-5 [ Links ]
3. Gaudias J. Antibiotic prophylaxis in orthopedics-traumatology. Orthop Traumatol Surg Res [Internet]. 2021 Feb 1 [cited 2022 Dec 5];107(1):102751. Available from: https://linkinghub.elsevier.com/retrieve/pii/S187705682030342X [ Links ]
4. Dhammi IK, Haq RU, Kumar S. Prophylactic antibiotics in orthopedic surgery. Indian J Orthop [Internet]. 2015 Aug 1 [cited 2022 Dec 5];49(4):373-76. Available from: www.ijoonline.com [ Links ]
5. Bratzler DW, Dellinger EP, Olsen KM, et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Am J Health Syst Pharm [Internet]. 2013 Feb 1 [cited 2023 Jan 25];70(3):195-283. Available from: https://pubmed.ncbi.nlm.nih.gov/23327981/ [ Links ]
6. Claireaux HA, Beaumont O, Griffin XL, et al. Open lower limb fractures in the UK trauma system: A multicentre prospective audit of current practice. Injury. 2021 Jun;52(6):1374-83. [ Links ]
7. Depypere M, Sliepen J, Onsea J, et al. The microbiological etiology of fracture-related infection. Front Cell Infect Microbiol [Internet]. 2022 Jul 7 [cited 2023 Jan 22];12:934485. Available from: https://pubmed.ncbi.nlm.nih.gov/35873162/ [ Links ]
8. Kuehl R, Tschudin-Sutter S, Morgenstern M, et al. Time-dependent differences in management and microbiology of orthopaedic internal fixation-associated infections: an observational prospective study with 229 patients. Clin Microbiol Infect [Internet]. 2019 Jan 1 [cited 2023 Jan 22];25(1):76-81. Available from: https://pubmed.ncbi.nlm.nih.gov/29649599/ [ Links ]
9. Corrigan RA, Sliepen J, Dudareva M, et al. Causative Pathogens do not differ between early, delayed or late fracture-related infections. Antibiotics. 2022 Jul;11(7). [ Links ]
10. Sliepen J, Corrigan RA, Dudareva M, et al. Does the use of local antibiotics affect clinical outcome of patients with fracture-related infection? Antibiotics. 2022 Oct;11(10). [ Links ]
11. Rupp M, Baertl S, Walter N, et al. Is there a difference in microbiological epidemiology and effective empiric antimicrobial therapy comparing fracture-related infection and periprosthetic joint infection? A retrospective comparative study. Antibiot 2021;10(8):921. [ Links ]
12. Zhang Z, Liu P, Wang W, et al. Epidemiology and drug resistance of fracture-related infection of the long bones of the extremities: a retrospective study at the largest trauma center in southwest China. Front Microbiol. 2022 Jul;13:2345. [ Links ]
13. Lu V, Zhang J, Patel R, et al. Fracture related infections and their risk factors for treatment failure-a major trauma centre perspective. Diagnostics. 2022 May;12(5):1289. [ Links ]
14. Chen AF, Schreiber VM, Washington W, et al. What is the rate of methicillin-resistant Staphylococcus aureus and Gram-negative infections in open fractures? Clin Orthop Relat Res. 2013;471(10):3135. [ Links ]
15. Wang B, Xiao X, Zhang J, et al. Epidemiology and microbiology of fracture-related infection: a multicenter study in northeast China. J Orthop Surg Res [Internet]. 2021 Dec 1 [cited 2023 Jan 31];16(1):1-11. Available from: https://josr-online.biomedcentral.com/articles/10.1186/s13018-021-02629-6 [ Links ]
16. Standards for the management of open fractures of the lower limb [Internet]. [cited 2023 Jan 26]. Available from: https://www.bapras.org.uk/docs/default-source/commissioning-and-policy/standards-for-lower-limb.pdf?sfvrsn=0 [ Links ]
17. Simpson AHRW, Dave J, Ghert M. Prophylactic antibiotics in total joint arthroplasty: evolution or devolution? Bone Joint Res. 2015 Dec;4(12):195-97. [ Links ]
18. Investigators TPAR in TS (PARITY), Ghert M, Schneider P, Giglio V, et al. Comparison of prophylactic intravenous antibiotic regimens after endoprosthetic reconstruction for lower extremity bone tumors: a randomized clinical trial. JAMA Oncol. 2022 Mar;8(3):345-53. [ Links ]
19. The PARITY Investigators. Prophylactic antibiotic regimens in tumour surgery (PARITY): A pilot multicentre randomised controlled trial. Bone Jt Res. 2015 Sep;4(9):154-62. [ Links ]
20. Metsemakers WJ, Onsea J, Neutjens E, et al. Prevention of fracture-related infection: a multidisciplinary care package. Int Orthop 2017 4112. 2017 Aug;41(12):2457-69. [ Links ]
21. BOA. BOAST-Open Fractures. 2017. [ Links ]
22. Chang Y, Bhandari M, Zhu KL, et al. Antibiotic prophylaxis in the management of open fractures. JBJS Rev. 2019 Feb;7(2):e1-e1. [ Links ]
23. AAOS. Information statement recommendations for the use of intravenous antibiotic prophylaxis in primary total joint arthroplasty. 2014. [ Links ]
24. Aboltins CA, Berdal JE, Casas F, et al. Hip and Knee Section, Prevention, Antimicrobials (Systemic): Proceedings of International Consensus on Orthopedic Infections. J Arthroplasty. 2019 Feb;34(2):S279-88. [ Links ]
25. De Beer J, Petruccelli D, Rotstein C, et al. Antibiotic prophylaxis for total joint replacement surgery: results of a survey of Canadian orthopedic surgeons. Can J Surg. 2009;52(6):E229. [ Links ]
26. Modha MRK, Morriss-Roberts C, Smither M, et al. Antibiotic prophylaxis in foot and ankle surgery: a systematic review of the literature. J Foot Ankle Res. 2018 Nov;11(1). [ Links ]
27. Shaffer WO, Baisden JL, Fernand R, Matz PG. An evidence-based clinical guideline for antibiotic prophylaxis in spine surgery. Spine J. 2013 Oct;13(10):1387-92. [ Links ]
28. Hickson CJ, Metcalfe D, Elgohari S, et al. Prophylactic antibiotics in elective hip and knee arthroplasty: An analysis of organisms reported to cause infections and national survey of clinical practice. Bone Jt Res. 2015 Nov;4(11):181-89. [ Links ]
29. Metsemakers W, Morgenstern M, McNally MA, et al. Fracture-related infection: A consensus on definition from an international expert group. Injury. 2018 Mar;49(3):505-10. [ Links ]
30. Govaert GAM, Kuehl R, Atkins BL, et al. Diagnosing fracture-related infection: current concepts and recommendations. J Orthop Trauma [Internet]. 2020 Jan 1 [cited 2022 Nov 30];34(1):8-17. Available from: https://pubmed.ncbi.nlm.nih.gov/31855973/ [ Links ]
31. Marais LC, Ferreira N, Aldous C, et al. A modified staging system for chronic osteomyelitis. J Orthop [Internet]. 2015 Dec;12(4):184-92. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0972978X15000847 [ Links ]
32. depypere m, kuehl r, metsemakers wj, et al. recommendations for systemic antimicrobial therapy in fracture-related infection: a consensus from an international expert group. J Orthop Trauma. 2020 Jan;34(1):30. [ Links ]
33. Sagi HC, Donohue D, Cooper S, et al. Institutional and seasonal variations in the incidence and causative organisms for posttraumatic infection following open fractures. J Orthop Trauma. 2017 Feb;31(2):78-84. [ Links ]
34. Jorge LS, Chueire AG, Fucuta PS, et al. Predisposing factors for recurrence of chronic posttraumatic osteomyelitis: A retrospective observational cohort study from a tertiary referral center in Brazil. Patient Saf Surg [Internet]. 2017 Jun 2 [cited 2023 Jan 31];11(1):1-9. Available from: https://pssjournal.biomedcentral.com/articles/10.1186/s13037-017-0133-1 [ Links ]
35. Fily F, Ronat JB, Malou N, et al. Post-traumatic osteomyelitis in Middle East war-wounded civilians: Resistance to first-line antibiotics in selected bacteria over the decade 2006-2016. BMC Infect Dis [Internet]. 2019 Jan 31 [cited 2023 Jan 31];19(1):1-8. Available from: https://bmcinfectdis.biomedcentral.com/articles/10.1186/s12879-019-3741-9 [ Links ]
36. Hellebrekers P, Rentenaar RJ, McNally MA, et al. Getting it right first time: The importance of a structured tissue sampling protocol for diagnosing fracture-related infections. Injury. 2019 Oct;50(10):1649-55. [ Links ]
37. Al Farii H, Slawaska-Eng D, Pankovitch S, et al. Gram-negative surgical site infections after 989 spinal fusion procedures: associated factors and the role of Gram-negative prophylactic antibiotic coverage. Int J Spine Surg [Internet]. 2021 Apr 1 [cited 2023 Jan 25];15(2):341. Available from: /pmc/articles/PMC8059395/ [ Links ]
38. Bosco JA, Tejada PRR, Catanzano AJ, et al. Expanded Gram-negative antimicrobial prophylaxis reduces surgical site infections in hip arthroplasty. J Arthroplasty [Internet]. 2016 Mar 1 [cited 2023 Jan 25];31(3):616-21. Available from: https://pubmed.ncbi.nlm.nih.gov/26521131/ [ Links ]
39. Tsang S-TJ, Simpson AHR. Antimicrobial rationing in orthopaedic surgery. Bone Joint Res. 2020;9(12):870-72. [ Links ]
40. National Institute of Health and Care Excellence. Surgical site infections: prevention and treatment | Guidance | NICE. NICE; 2019. [ Links ]
Received: February 2023
Accepted: March 2023
Published: August 2023
* Corresponding author: nferreira@sun.ac.za
Editor: Prof. Maritz Laubscher, University of Cape Town, Cape Town
Copyright: © 2023 Ferreira N. This is an open-access article distributed under the terms of the Creative Commons Attribution Licence, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Funding: No funding was received for this study.
Conflict of interest: The authors declare they have no conflicts of interest that are directly or indirectly related to the research.














