Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
SA Orthopaedic Journal
On-line version ISSN 2309-8309
Print version ISSN 1681-150X
SA orthop. j. vol.22 n.2 Centurion 2023
http://dx.doi.org/10.17159/2309-8309/2023/v22n2a5
CURRENT CONCEPTS REVIEW
PAEDIATRIC ORTHOPAEDICS
Acute haematogenous osteomyelitis in the paediatric population: a current concepts review
Mari Thiart*; Adisha Nansook
Division of Orthopaedic Surgery, Department of Surgical Sciences, Faculty of Medicine and Health Sciences, Stellenbosch University, Cape Town, South Africa
ABSTRACT
Acute haematogenous osteomyelitis (AHOM) is a bacterial infection localised in bone that usually occurs after an episode of bacteraemia. This infection is commonly encountered by doctors in low- and middle-income countries (LMICs) and, if not recognised early and managed appropriately, can harbour significant early and late complications, including death.
This narrative review aims to summarise the current management of AHOM, highlight the controversies and report on new advances in diagnosis and treatment.
AHOM is typically a monomicrobial disease. Staphylococcus aureus remains the most common pathogen globally, accounting for 70-90% of all cases. Diagnostic work-up includes complete blood cell count, serum C-reactive protein, erythrocyte sedimentation rate, imaging and blood culture.
Management of AHOM includes empiric intravenous (IV) antibiotics based on the most likely causative agents; source control entailing surgery to drain any abscesses and obtain specimens for microscopy, culture and sensitivity (MCS), as well as debridement of any necrotic bone; and subsequent targeted antibiotic therapy effective against the identified pathogen with the narrowest spectrum.
Treatment response is monitored with repeat CRP every 48-72 hours. The decision to switch from IV to oral antibiotics is made if there is clinical improvement and the CRP is < 20 mg/L. The total duration of antibiotics is six weeks. Treatment of paediatric AHOM is multidisciplinary and includes orthopaedic surgeons, paediatricians, infectious diseases specialists, physiotherapists, dieticians, nurses and social workers. AHOM can cause devastating destruction of the bone due to tissue necrosis, leading to late sequelae. These complications are more common in children in LMICs.
Level of evidence: Level 5
Keywords: acute haematogenous osteomyelitis, paediatric
Introduction
Acute haematogenous osteomyelitis (AHOM) is a bacterial infection localised in bone that usually occurs after an episode of bacteraemia.1 Acute haematogenous osteomyelitis is commonly encountered by doctors in low- and middle-income countries (LMICs) and, if not recognised early and managed appropriately, can harbour significant early and late complications, including death.
Premature growth arrest, limb deformity and leg length discrepancy lead to severe disability, especially in this young population.2-6 Predictors of poor outcome include concomitant septic arthritis, as damage to the joints in these young patients leads to severe degenerative arthritis.4,6-9 Although perhaps not very high, a mortality rate of 1.06% is significant, especially for a treatable condition.10
This narrative review aims to summarise the current management of AHOM, highlight the controversies and report on new advances in diagnosis and treatment. PubMed, Cochrane and Google Scholar search engines were searched for articles published since 2018 with the keywords 'Acute Haematogenous Osteomyelitis' AND 'Paediatric'. The titles were then screened for relevance. Those included were then read for content. The bibliographies of included articles were also screened for relevant articles. If novel ideas were quoted, the original articles were sought and cited.
Epidemiology
The annual incidence of AHOM in high-income countries is reported as 8 per 100 000, while the incidence in LMICs is as high as 43 to 200 per 100 000.11-13 Munshi et al. speculated that this increased prevalence is due to the warmer tropical climate, low socioeconomic status, use of traditional medicine and predisposing genetic factors.14
Seasonal variations have been observed, with a peak in late summer and autumn.15 Lindsay et al. reported that most children were admitted in summer and found a correlation between the severity of illness and the average temperature during the summer season and a correlation with the average humidity change during the winter months.15
Children under the age of 5 years are generally affected and present three to four days after the onset of symptoms.2-4'7,12,16-18 Some studies have reported a higher median age of presentation of between 6.7 and 11.1 years.5,8,14,19-37 Males appear to be between 1.2 and 3.6 times more affected than females.
The femur (23-29%), tibia (19-26%) and humerus (5-13%) are most commonly affected, but 10-25% can involve the short or non-tubular bones, and 5% are polyostotic.2-4,7,11,16,18,38-41 Preceding trauma is reported in 30-60% of cases.4,12,15 Concomitant septic arthritis is present in 30% of cases, but this could be as high as 75% in cases involving neonates.2,7
Most infections occur in healthy children with no predisposing conditions.7,18,38,42 Some specific associations have been described, including sickle cell disease and Salmonella infections, upper respiratory tract infections and Kingella kingae (K. kingae) infections,42-44 and trauma or varicella blisters and group A Streptococcus infections.4,7,11,16,18,42,44 Methicillin-resistant Staphylococcus aureus (MRSA) has an increased incidence in children with prolonged prior hospital stays, such as neonates, and in regions like the USA, where the incidence of MRSA is between 30% and 40%.2
Bacteriology
Despite a low positive yield (10-50%), a blood culture should be performed before starting empiric antibiotics.2-4,7,12,19,39-42,45,46 When blood cultures are taken before the administration of systemic antibiotics, the positive yield improves from 69-95%.45,47 If the blood culture is positive, it should be repeated every 48 hours until negative.4,16
AHOM is typically a monomicrobial disease48 (Table I). Staphylococcus aureus (S. aureus) remains the most common pathogen globally, accounting for 70-90% of all cases.1-4,7,12,17-19,29,31,40-42,44,45,48-65 Other causative organisms include Streptococcus spp (S. pyogenes and S. pneumoniae being the most common),16,18,48 S. agalactiae in infants under three months,7,18 K. kingae,11,12,19,40,43,45,48,66,67 Haemophilus influenzae, anaerobes, Gram-negative bacilli including Enterobacter spp and Neisseria gonorrhoeae, and Candida species.2-4,7,12,17,19,29,31,42,45,48-64,68
Staphylococcus aureus
The prevalence of S. aureus infection can be ascribed to 25-30% of the global population and 50-70% of healthcare workers are colonised with the bacteria.48 Multiple factors contribute to S. aureus' virulence, including cytolytic toxins, immune-evasion factors, superantigens, antioxidant systems and adhesions.43,48
Adhesion to host tissues is a critical first step in the pathogenesis of AHOM.43,48 S. aureus releases toxins and degradative exoenzymes that incapacitate the immune cells and expresses surface-bound and secreted immune-evasion factors.48 S. aureus protein A (Spa) promotes immune evasion by binding Fc regions of antibodies, interfering with complement activation and serving as a B-cell superantigen.48 Spa also binds to the osteoblasts via tumour necrosis factor receptor-1 (TNFR-1), leading to apoptosis.4,43,48 The secretion of proinflammatory cytokines leads to bone resorption via osteoclast activation.43,48 These factors could provide potential targets for immunotherapeutic agents and vaccines.36
Panton-Valentine leukocidin (PVL) is a necrotising toxin seen in methicillin-sensitive S. aureus (MSSA) and methicillin-resistant S. aureus (MRSA) bacteria, and is seen with increasing frequency.7,11,17,40,69,70 This toxin targets white blood cells causing destruction and tissue necrosis.7,18,70 PVL-producing S. aureus strains are particularly virulent in the paediatric population, causing profound inflammatory responses and severe complications.4,41,68,69,71-74 It results in more extensive disease and septic shock, leading to extended hospital stays, prolonged antibiotic requirements and more surgical interventions.1,2,12,52-55,57,60,74-78 The incidence of PVL-positive S. aureus in Europe is 18%, while limited data is available in LMICs.7
MRSA infections are associated with higher C-reactive protein (CRP), erythrocyte sedimentation rate (ESR) and white cell count (WCC) levels.7,18,19 MRSA also present clinically with high fever, tachycardia and a painful limp.2 MRSA infection often has an increased severity of local infection (abscess formation, myositis, pyomyositis, interosseous involvement and septic arthritis).8
Kingella kingae
Gram-negative bacteria account for between 60% and 82% of cases in children under the age of 4 years.60,71 K. kingae is a fastidious Gram-negative coccobacillus, extremely difficult to culture, even when inoculating infected bone fluid into blood culture bottles.65 Olijve et al. reported the increased incidence of K. kingae in young children coincides with the eruption of deciduous teeth.44 K. kingae infection presents indolently with low fever, moderately elevated inflammatory markers and is most frequently seen in children between 6 months and 5 years.2,3,7,16,18,42,44,45,48,65,67,68,79,80
Thakolkaran and Shetty reported that less than 15% of children with K. kingae infection had fever on admission, and 39% had normal CRP levels.2
K. kingae represents 15-31% of all cases of AHOM.65 The epiphyses and apophyses, normally spared by other organisms, are frequently involved in K. kingae infections.18,65 Because of the indolent nature, K. kingae infections can often evolve into subacute osteomyelitis, forming a lucent lesion within the bone (Brodie's abscess).65
Haemophilus influenzae
H. influenzae accounts for 10-15% of AHOM cases in unvaccinated children under three months in low-income countries.2 This bacteria typically leads to pneumonia and meningitis in infancy.27 The South African vaccination schedule includes the vaccine against H. influenzae type B and is given at six weeks, ten weeks, 14 weeks and 18 months.81
Streptococcus pneumoniae
Infections caused by H. influenzae and S. pneumoniae are more common in children under 5 years of age.27 Unvaccinated children who develop S. pneumoniae AHOM have a higher risk of developing severe disease with resultant bacteraemia, meningitis, pneumonia and bone and joint infections.2 It is also worth noting that not all S. pneumoniae sub-types are covered by vaccination, and S. pneumoniae AHOM can still be seen in vaccinated children.27 The South African vaccination schedule includes the pneumococcal conjugated vaccine (PCV1) given at six weeks, 14 weeks and nine months.
Streptococcus pyogenes
While the incidence of S. pneumoniae has declined due to vaccination, S. pyogenes is still a bacteria implicated in AHOM.45
Salmonella
Giaccai and Idriss reported that Salmonella (typhi and paratyphoid B) caused AHOM in 0.2-0.85% of all cases.82 The incidence increases to between 1.5% and 12% in children with sickle cell disease.83 It is interesting to note that a prior review article by Burnett et al. in 1998 indicated a higher ratio (1.4:1) of Salmonella AHOM compared to S. aureus in patients with sickle cell disease.84 This ratio was even lower than a report from 1981, which reported that 50 out of 68 positive cultures yielded Salmonella.85 This seems to indicate a decreasing incidence of Salmonella infection over time.
Multifocal osteomyelitis is common, but the disease is mild with minimal bone destruction.82 Some authors have speculated that children with sickle cell disease suffer small gastrointestinal tract infarctions, leading to a Salmonella bacteraemia.86 The increased incidence of bone infarction, combined with the sluggish microcirculation, causes the Salmonella bacteraemia to localise to the metaphysis of bone and present like the typical AHOM.86 However, Salmonella osteomyelitis has been reported as typically affecting the diaphysis of long bones, particularly the femur and humerus, as well as the vertebrae.82,83
Negative cultures
Negative cultures are seen in 33-55% of cases.3,16,48,60,66 This could be due to a low bacteria load, a fastidious organism or an inaccurate microbiology sampling modality preceding antibiotic therapy.48,65,87 Searns et al. reported that culture-negative infections have a distinct clinical phenotype: they are younger, less febrile and less likely to have an abscess, and postulated that a smaller volume of blood collected, decreased inoculum, and a more difficult surgical approach may all contribute to negative cultures.87
Nucleic acid amplification methods (real-time polymerase chain reaction [PCR]) have improved the detection of bacteria not cultured by routine methods.7,42,88 This becomes important in cases of prior antibiotic use and pathogens notoriously difficult to culture such as K. kingae..3,4,42,45 Supplemental techniques to improve culture yield include inoculation of blood or chocolate agar plates intraoperatively.3,12,16 When available, culture-negative specimens can be sent for further molecular testing to better identify the underlying causative agent and guide antibiotic therapy.3,4,87 O'Rouke et al. reported that real-time PCR testing (testing for S. aureus, group A Streptococcus, S. pneumoniae and K. kingae) could play a role in culture-negative cases as a second-line investigation,80 and this was confirmed by a few authors.2,7,38,44,66,67,79 16S rRNA PCR utilisation showed no additional yield when compared to target real-time PCR and should be reserved for cases where the culture and targeted PCR remained negative.80,87
Diagnosis
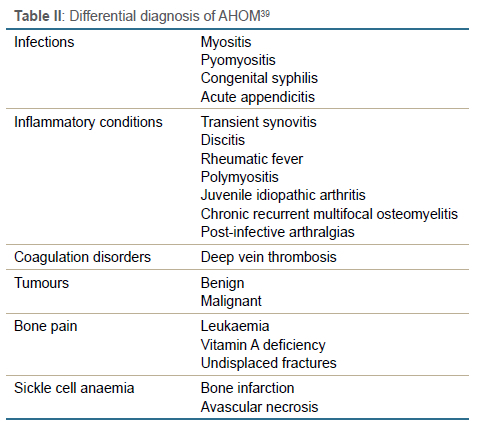
Early diagnosis is the key to successful management and the prevention of long-term sequelae.7,37,40 A high suspicion index is required to diagnose AHOM, especially in neonates.7,12,16,48 The differential diagnosis list is given in Table II.
The diagnosis of AHOM can be made using symptoms (present for 14 days or less) together with at least one of the following four criteria:7,16,19,20,89
• Positive culture or Gram stain of bone
• Positive blood culture
• Abnormal imaging
• An abnormal clinical examination45,49-54,75
Waldvogel added an additional criterion: surgical findings of an intraosseus pus collection.38,90,91
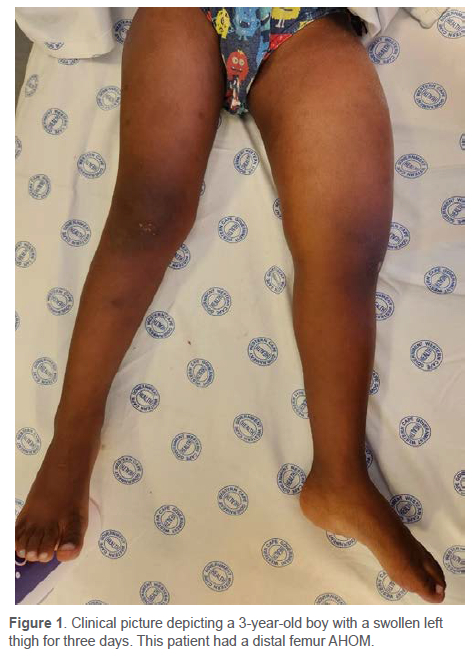
The presentation can vary from a well-localised infection to multifocal infection and septic shock.39 For toddlers, pain can be expressed as failure to bear weight or pseudoparalysis, as seen in neonates.9,39,41 A 2012 systematic review reported the most common clinical features to be: pain (81%), localised signs/ symptoms (70%), fever (62%), decreased range of motion (50%) and inability to bear weight (49%)2,11 (Figure 1). A limp or refusal to walk, along with back pain and fever, may be associated with vertebral osteomyelitis.2,4,39
Children with pelvic or sacroiliac infections can present nonspecific abdominal, back or flank pain.4,16 Morgan and Yates in 1966 reported pelvic osteomyelitis presented with four clinical syndromes: hip joint; abdominal; buttock and sciatic syndrome.92 The hip joint syndrome presents with clinical features like those of a hip septic arthritis.92 The abdominal syndrome features are similar to acute appendicitis with iliac fossa pain.92 Buttock syndrome presents with pain radiating down the thigh to the knee; this differs from sciatic syndrome which presents with pain along the distribution of the sciatic nerve and represents sacroiliac joint arthritis secondary to iliac AHOM.92
Lack of coordinated care may result in missed or delayed diagnosis, and current level III evidence suggests a multidisciplinary team to streamline the diagnostic work-up, improve efficiency and increase the rate of positive cultures.88
Special investigations
Diagnostic work-up includes complete blood cell count, serum C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), radiographs and blood culture.3,4,7,12,16,48,80,88 The blood culture should be drawn on admission, even if the child is apyrexial, and repeated during a temperature spike.7 It is recommended that 2-4 ml blood be drawn in a child weighing 1-2 kg, 6 ml in a 2-12 kg child, 10-20 ml in a 13-40 kg child and 40 ml in a child weighing > 40 kg.16
Because the CRP level rises within six hours, has a half-life of 19 hours, and normalises by 7-10 days, it is used to monitor the response to treatment.2-4,11,12,42,48,92 CRP is elevated in 81-98% of patients and up to 100% by day 3 of the disease.4 The 2021 Pediatric Infectious Diseases Society and the Infectious Diseases Society of America guidelines recommend performing a serum CRP on the initial evaluation because of its sensitivity.19,39 Although raised inflammatory markers are not specific to AHOM, a normal CRP and ESR are rarely seen in these patients.48 Van der Merwe et al. found that the CRP on presentation was the most reliable predictor of culture yield, duration of hospital stay and the number of debridements needed.33
ESR is elevated in 91-95% of children but peaks in three to Ave days and normalises by three weeks.2-4,7 It has a lower specificity for AHOM.4 White blood cell count (WBC) is a poor indicator of disease and is only elevated in 35% of patients; it also correlates poorly with response to treatment.4,7,11 The use of serum procalcitonin (PCT) is not advocated as it has a sensitivity of 85% and specificity of 87% and thus does not outperform CRP.4,39
Imaging
Radiographs of the affected limb should be requested to exclude other diagnoses such as fractures and malignancy.2-4,11,12,39,41,46,48 X-rays are often normal in early AHOM as it takes up to two weeks for radiographic changes to appear, because lytic changes in bone require 50% of the bone mineral density to be depleted before becoming evident on X-rays.3,7,16,37,42,48,94
Ultrasound is a cheap, readily available and non-ionising investigation.20 Ultrasound can guide diagnostic or therapeutic aspiration of fluid collections, but the sensitivity and specificity are low.4,7,12,16 Ultrasound is used to screen for possible deep vein thrombosis (DVT).3 The advantage of ultrasound is that it is non-invasive and does not require sedation but it is operator dependent.3 The ultrasound criteria needed to diagnose AHOM include any one of the following:20
• Deep soft tissue fluid collection around the bone
• Periosteal thickening or elevation with a thin layer of subperiosteal fluid collection
• Increased vascularity within or around the periosteum on Doppler sonography
MRI is particularly useful in detecting pelvic osteomyelitis and discitis.7 MRI can document bone oedema within 24-48 hours from the onset of infection and is considered the gold standard for imaging of AHOM.4,7,18,37,39,42,48,56,68,93,95,96 MRI sensitivity is 80-100%, and the specificity is 50-100% compared to X-rays or bone scintigraphy.2,7,12,19,38,40,46,94
The MRI criteria to diagnose AHOM includes:20,41,96
• Alteration of normal signal intensity (low signal on T1-weighted images and high signal of STIR or T2-weighted images). On fat-suppressed gadolinium-enhanced T1-weighted images, it is seen as increased enhancement relative to the adjacent marrow
• Subperiosteal collection
The MRI scan details bone and soft tissue involvement and helps with surgical planning.2-4,39,42 (Figure 2). Gadolinium may enhance clinical efficacy by detecting intramedullary or muscular abscesses and necrosis.4'7'16,95 Diffusion-weighted imaging (DWI) relates to the composition of tissue and fluid, for example, high cellularity and the viscosity of pus.95,96 Habre et al. reported that DWI can be used to detect AHOM (restricted diffusion in soft tissue or bone) and could potentially negate the use of contrast.95 However, one does need an experienced radiologist.95,96 Contrast-enhanced sequences should be used for young children under 3 years of age, where proximal femoral head involvement is suspected, as the non-contrasted images are less sensitive due to the proximity of the epiphysis.95,96
Repeat MRI does not have a role in routine surveillance but could be useful if the current treatment fails.4 End-of-therapy imaging (MRI or X-rays) is only recommended if the physis was involved.39
MRI does not come without limitations - scan times can potentially be long, and the child needs anaesthesia or deep sedation to prevent movement.2,4,7,16,42 A diagnostic MRI can be successful in non-sedated infants younger than six months when swaddled and asleep.96 The cost of MRI can be justified because its use leads to fewer surgeries and days spent in hospital.22
Where MRI is not possible, contrasted computed tomography (CT) to detect abscess formation, bone scan or scintigraphy and ultrasound have been used.42,48 CT involves high-dose radiation with decreased sensitivity for marrow and soft tissue pathology but is excellent at defining bony pathology.2-4,20
Technetium radionuclide scan can be used to identify multifocal osseous involvement.7,16,42 It has high sensitivity but less specificity and can give false positive results in infancy and with virulent bacteria like MRSA.4,7,11,12,42 Triphasic Technetium scanning includes a flow phase (2-5 seconds after injection), a blood pool phase (5-10 minutes after injection) and a delayed phase (2-4 hours after injection).7 AHOM leads to focal absorption in the third phase, and the brighter the signal, the more osteoblastic activity.7 This modality is a functional scan and cannot give information on whether pus collections are present.4,7 Technetium radionuclide scan involves a significant amount of radiation exposure (200-750 chest X-rays) but could still be faster and more accessible than MRI in some centres.42
Management
The management for AHOM includes:3,43,48,68
• Empiric IV (intravenous) antibiotics based on the most likely causative agents3,42
• Source control: surgery to drain any abscesses and obtain specimens for microscopy, culture and sensitivity (MCS), as well as debride any necrotic bone3,12,29,39,41,42,48,68
• Targeted antibiotic therapy that is effective against the identified pathogen with the narrowest spectrum, lowest adverse effect profile and the fewest side effects39,45,48
• Monitoring response with repeat CRP every 48-72 hours10,16,39,45
• IV antibiotics until clinical improvement and CRP is < 20 mg/L, followed by oral antibiotics for a total period of six weeks
In children who are systemically well, systemic antibiotics should be delayed until after culture samples are taken.46 In children who appear severely ill, starting empiric antibiotics is recommended as the yield of positive cultures obtained within 24-48 hours of initiation of antibiotics is equivalent to those obtained before the administration of antibiotics.39,46 Van der Merwe et al. reported that, in matched groups, antibiotic administration before surgery did not decrease surgical culture yield.33
Some children with AHOM can be severely ill with septicaemia.5 Septicaemia could lead to acute multiorgan failure, including septic pulmonary emboli and DVT.60,72 The early course of infection may be complicated by persistent bacteraemia, prolonged fever, sepsis, thrombophlebitis, longer hospital stays and prolonged course of parenteral antibiotics.5,12 The prolonged bacteraemia may reflect inadequate source control, the establishment of endovascular foci (endocarditis and septic venous thromboembolism) or metastatic infection.97
Gouveia et al. reported a 30% association with concomitant pyomyositis and a 68% association with septic arthritis.98 Manz et al. reported that the incidence of concomitant AHOM and septic arthritis was higher than initially thought in children older than 6 years.37 The incidence of concomitant AHOM and septic arthritis has been reported as 46% below 2 years of age; 28% in 2 to 5 year olds, and for 6 years and older, 40%.26,37,38
Yi et al. reported that children with concomitant infections have a higher intensive care unit (ICU) admission frequency and significantly longer hospitalisation.59 Concomitant infection also produced higher inflammatory markers.59 Primary treatment failure can occur, and if so, the adequacy of the antibiotic treatment should be assessed and the need for surgical intervention evaluated.39 The rate of complications has been reported at 6-9%, and patient non-adherence to treatment accounted for 43% of treatment failures.59
Amaro et al. showed a link between the peak level and duration of CRP elevation and the risk of thrombotic complications in children with musculoskeletal infections.99 The theory is that the prolonged acute phase response due to the infection predisposes to thromboembolism.99 At the peak CRP of 180 mg/L, the predicted probability of venous thromboembolism was 4.9%, while the risk increased to 50.9% at a peak CRP of 420 mg/L.99
The risk of DVT and pulmonary embolism increases from 0.4-6%3,12 to as high as 40% in children older than 8 years old with MRSA infection and a CRP > 60 mg/L.4,7,100 Some studies suggest that each child with MRSA infection should be screened for a DVT.42,54,71,74,76,77 Those at particular risk are infections of the femur and tibia.74
Hester et al. aimed to improve the care of their patients by implementing four quality improvement interventions: 1) infectious disease education, 2) centralisation of admission, 3) communication between orthopaedics and radiology, and 4) application of a management algorithm.101 They aimed to decrease peripherally inserted central catheter (PICC) use by 50%, reduce the number of sedation episodes, and reduce empirical vancomycin by 50%.101 The use of PICC lines decreased, but this was already noticed before the improvement intervention was implemented. Sedation episodes decreased as the use of PICC lines decreased. Empiric use of vancomycin did not decrease.101
Treatment of AHOM is multidisciplinary and includes orthopaedic surgeons, paediatricians, infectious diseases specialists, physiotherapists, dieticians, nurses and social workers.2,48,71 Figure 3 depicts a proposed treatment algorithm for the South African system. Copley and colleagues established guidelines in the context of a multidisciplinary team.48,102 This included daily ward rounds by orthopaedic surgeons, paediatricians, infectious disease specialists, nurses and social workers.48,102 The key to successful treatment included a dedicated MRI slot for children with suspected AHOM, with an immediate transition to the operating room if necessary.3,48,102 A multidisciplinary approach with interdisciplinary MRI protocols has been shown to decrease time to MRI and surgery and thus decrease days in hospital; MRI has great utility as an initial screening test.3 Quick et al. reported that involving the paediatric orthopaedic services immediately upon admission improved communication and created a timely diagnosis and treatment plan.88 The prearranged early morning MRI slot and reserved operating room facilitates the surgeon's availability to perform the relevant surgery following a positive MRI.88
Griswold et al. reported on this protocol: the MRI is scheduled for 7 am the morning after the admission. The consultant orthopaedic surgeon and radiologist review the MRI in real-time.22 A scout view was done to scan for any unexpected foci; T1 with or without contrast, T2 fat-suppressed and STIR images in all planes were done.22,96 An operating room was kept on hold, and the patient was transported to the operating room for surgery without being awakened.22 The authors studied the patients treated before and after the implementation of the MRI protocol and found that preoperative MRI significantly reduced the number of surgeries and unplanned returns to the operating room.22 The duration of hospital stay was reduced, and this was clinically significant.22 This protocol led to a reduction of after-hours MRI scans and after-hour surgical debridements being done.22
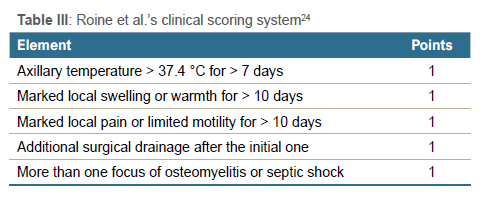
In 1997, Roine et al. reported on the early detection of sequelae.24 They used a clinical scoring system tabulated in Table III and the patient's CRP level. The CRP cut-off limits were as follows: day one 190 mg/L, day two 202 mg/L, day three 178 mg/L, day Ave 86 mg/L. Their research reported that a clinical score of > 1, plus a CRP exceeding the cut-off on the taken day, was statistically significant in predicting sequelae.24
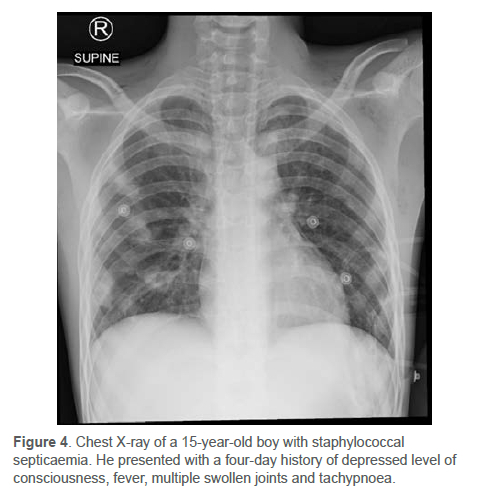
Copley et al. subsequently created a scoring system to assess the severity of AHOM.25,48 This scoring system used clinical and laboratory findings (Table IV).25 Severity was the sum of the parameters, with mild being (0-3), moderate (4-7), and severe (8-10).3,25 Copley et al. reported that their clinical experience suggested that the clinical and laboratory response of the child during the first four to five days of treatment served to determine the severity score.3,25 Disseminated disease included children with meningitis, septic shock, toxic shock, multifocal infection, endocarditis, DVT, septic pulmonary emboli, empyema or pneumonia (Figure 4).3,9,25,40,59
It is not clear but assumed that a patient would only need one of these associated symptoms to deem it a disseminated disease. Mignemi et al. also reported a classification system.25,103 In their study, disseminated infection was defined as AHOM with subperiosteal abscess extension, while local infection was defined as isolated AHOM with no subperiosteal pus formation.103 These patients with disseminated infection were more likely to have longer hospital stays as well as ICU admissions, more surgeries performed, more positive blood cultures, longer duration of antibiotics, more pyrexial days and more imaging studies.103 Inflammatory markers were also higher in disseminated infections.103 No extrapolation was given regarding predictive factors which could be useful on admission to determine prognosis.
Sanchez et al. reported that children with severe severity scores had concomitant endocarditis, thrombophlebitis and septic pulmonary emboli.97 Children with severe illness underwent more surgical debridement and cultured MRSA as the primary pathogen more frequently.10,97
Empiric antibiotics
Empiric antibiotic therapy is determined by the geographic resistance patterns, age of the child and laboratory results, and targets the most likely causative agent.1,2,4,7,39,44,48,57,60,76,77,104
Antibiotic bioavailability and bone penetration should also be considered.7 A summarised version of the empiric antibiotics per age group is reported in Table V.
First-generation cephalosporins and anti-staphylococcal penicillins such as cloxacillin or clindamycin form the mainstay of antibiotic treatment with additional Gram-negative cover (third-generation cephalosporin) for neonates and young children.2,4,9,17,18,45,54,56,57,60,68,72,77,105 In Europe, empiric guidelines include clindamycin plus a first-generation cephalosporin.4,42,48 Cefuroxime 150 mg/kg/day IVI q8h can be used empirically if you suspect H. influenzae in unvaccinated children under 4 years of age.16
First-generation cephalosporins provide dual cover against K. kingae and S. aureus. It is important to note that the anti-staphylococcal penicillins, clindamycin and glycopeptides are ineffective against K. kingae.18,42,68
In cases of MSSA PVL-positive infections, first-generation cephalosporins plus clindamycin is suitable.2,18,42 In vitro evidence has shown that clindamycin, linezolid and rifampicin inhibit the production of PVL, and thus, adding these antibiotics will inhibit toxin production.7,42,56,70
If the MRSA prevalence is > 10%, the Infectious Disease Society of America has recommended the empirical antibiotic choice be vancomycin or clindamycin.2,4,7,12,18,48,53,68,72,74,76,77,93,100 Clindamycin has excellent bone penetration and is safe, inexpensive and effective against MSSA and MRSA.2,18,68,100 Other alternatives for MRSA include daptomycin and linezolid in patients who do not respond to vancomycin.2,18,68,93,100,106 Linezolid has excellent bone penetration.18
In some European studies, amoxicillin-clavulanate is widely used in low MRSA prevalent areas.68 Serrano et al. reported using amoxicillin-clavulanic acid in more than 60% of their cases as empirical oral therapy.17 The epidemiology and pathogen distribution in Serrano et al.'s study was similar to other literature.17
Other options include nafcillin or oxacillin in conjunction with vancomycin for severely ill children with MSSA, although they are not typically used in South Africa due to lack of availability.74
Vancomycin-resistant S. aureus (VRSA) infections are emerging.41 If a VRSA strain is suspected, a paediatric infectious disease specialist should be consulted.41
In cases of a negative culture, the antibiotic recommendation is a first-generation cephalosporin in MRSA-low regions and clindamycin with or without first-generation cephalosporin in high MRSA regions.2,4,40,42 The dosages of the empiric antibiotics as well as the susceptible organisms are tabulated in Table VI. These dosages have been advised using the European literature and verified using the South African Medical Formulary.68,107,108
Biopsy and source control
Nonoperative management with only antibiotics can cure early AHOM in up to 90% of cases.2,4,11,12,35,40,42,68 There is insufficient literature on indications and techniques for the surgical management of AHOM, and consensus regarding the need for surgery is lacking.3,4,21,42,98 Bacterial biofilm, abscess formation and bone necrosis impact antibiotic delivery.48 These factors highlight the importance of surgery to decompress the abscess, decrease disease burden by debriding any necrotic tissue and obtain tissue cultures.21,48,89 Clinical response to antibiotic therapy, the presence of an abscess, persistent fever or elevated CRP and MRSA or PVL-positive bacterial infections should be considered when deciding on surgical management.2-4,16,21,41,42,48 Woods et al. recommended surgical debridement in patients with sepsis or rapidly progressive infection and stable patients with large abscesses (greater than 2 cm).11,39,46
Independent predictors of surgical intervention in Upasani et al.'s study were multifocal infection, ambulatory status, elevated CRP on admission and elevated platelet count on admission.21 Upasani reported that there is marked variability in the surgical management of these patients in centres in the USA, and the overall surgical rate was 62%.21 The decisions were based primarily on institutional practice or dogma. Secondarily, the clinical presentation did affect the surgery rate in centres that did not frequently use surgical management to treat AHOM.21 There was more consensus if patients had multifocal disease, and even in institutions where surgery was not the norm, these patients were taken to theatre for debridement.21
Once AHOM is diagnosed, a biopsy is needed to obtain a culture to guide antibiotic therapy.7,21,42,46,48 This can be done through a percutaneous biopsy or open debridement.48 Deep cultures are not influenced by preceding antibiotics if taken within 72 hours of initiating antibiotic therapy.4,41,45 Interventional radiology-guided cultures avoid surgical morbidity and could potentially be used to obtain deep cultures in mild disease.40 Direct microbiological samples from bone or the abscess collection have improved yield (64-87%) compared to blood culture yield and are higher if obtained through an open procedure versus a percutaneous biopsy.4,7,45
All bone, tissue and fluid samples should be placed in sterile containers.16 The use of pus swabs is discouraged due to low yield.16 Any fluid should be placed in blood culture bottles.16 Some samples should be stored for further molecular testing if no bacteria are cultured.16
The reported rates of surgical management vary widely from 8-80%, primarily due to inconsistencies with the description of the procedure.4,38 The fundamental principle is source control.4 A corticotomy can be performed for intraosseous collections, and drains are routinely placed. Bone drilling is required in patients with subperiosteal or intramedullary abscesses.41 Placing plastic tubing into a drill hole and irrigating the medullary canal has been described89 (Figure 5). Montgomery et al. reported that when not drilling the cortex and irrigating the medulla, patients were six times more likely to need a re-debridement and recommended drilling the cortical bone (even if subperiosteal abscess is present) one to three times and flushing the canal.109
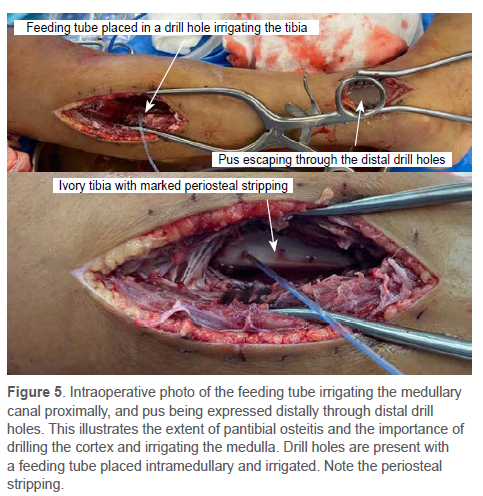
Of those who undergo a surgical debridement, 15-33% need a repeat debridement.4 Infections with MRSA increase the risk for multiple procedures.4,10 Indicators of a secondary procedure include persistent pain, clinical deterioration and increasing inflammatory markers, as well as the presence of MRSA.10
Localised delivery of antibiotics could be advantageous - it leads to a higher drug concentration at the infected site while limiting systemic toxicity.89 Routine surgical-site antimicrobial agents remain controversial, with little evidence recommending their use.39
Wang et al. reported on antibiotic-loaded calcium sulphate beads versus conventional surgery (removal of pus, the entire length of the canal debrided to remove the infected debris and endosteal bone) used in the treatment of AHOM.89 The calcium sulphate beads were impregnated with vancomycin in combination with a negative-pressure dressing.89 Vancomycin beads were placed in the medulla at the initial surgery and changed with each surgery.89 The authors found that the vancomycin group needed fewer surgeries, but the complication rates were the same.89 The vancomycin group had a quicker clinical response with a drop in CRP level seen (4.8 ± 2.5 days versus 13 ± 9.6 days).89
Targeted antibiotic therapy
Cephalosporins and anti-staphylococcal penicillins are bacteriostatic and bactericidal depending on the serum concentration, and continuous IV infusions can increase the serum concentration to make the antibiotic bactericidal.45 High-dose cefazolin (100-150 mg/kg/day divided q8h) is well tolerated and has a clinical cure rate above 95% for susceptible bacteria.16,45 Cefazolin is preferred over nafcillin or oxacillin due to their adverse side effects.45
S. pyogenes has been exquisitely susceptible to ampicillin and amoxicillin.45 Ampicillin-sulbactam and amoxicillin-clavulanic acid should be reserved for situations where narrow-spectrum, first-line drugs cannot be administered.45,68 Serrano et al. reported few cases of recurrence and sequelae (0.9%) when using amoxicillin-clavulanic acid.17 Amoxicillin-clavulanic acid can be used in young children because of its q8h schedule, palatability and seemingly good efficacy in areas with a low prevalence of MRSA.17,105
Intravenous clindamycin should be considered in patients who do not have ongoing bacteraemia and in hospitals with low clindamycin resistance. However, this is controversial as some clinicians have good outcomes with clindamycin, even in the presence of bacteraemia.42,48,93
Rifampicin is bactericidal and may prevent S. aureus resistance to other antibiotics, such as fluoroquinolones.45 Another benefit is the synergism in bacteraemia and its ability to penetrate biofilms, but there is no substantial evidence that this is the case in vitro.18,45 However, the low rate of adverse effects makes this an attractive adjuvant.45,105 It is important to note that rifampicin should never be used as monotherapy.45,105 The rifampicin dose is 10 mg/kg q12h, and the duration should be based on clinical judgement.45 It has excellent oral bioavailability and is also available in an intravenous preparation.18,68
Salmonella infection, common in sickle cell anaemia, should be treated with a third-generation cephalosporin or a fluoroquinolone.2,7,18
Adverse reactions to vancomycin include ototoxicity and nephrotoxicity, so hearing and renal function should be monitored during its use.29,100 As the half-life of vancomycin may be prolonged, it is important to monitor the trough concentration.2,18,29
Fosfomycin is a bactericidal antibiotic against Gram-positive and Gram-negative pathogens.110 It is effective in acidic and low-oxygen environments, like in an abscess.110 Tsegka et al. investigated the efficacy of fosfomycin against AHOM.110 Fosfomycin was successful in 82.2% of patients; it has low toxicity and high bone and abscess penetration, making it a drug to consider.110
Future therapies include dalbavancin, an intravenous concentration-dependent bactericidal drug with a prolonged halflife (202 hours) and thus could potentially be given once daily.100
The duration of antibiotics should be a minimum of four to six weeks, but there is no consensus on duration.2,3,11,16,18,37,45,48,89 Oral therapy should be continued for three to four weeks.94 Shorter durations are recommended in an uncomplicated course where PVL-positive pathogens are not suspected.39,45,94 Some authors have reported that children with negative cultures or K. kingae had a lower mean treatment duration.44,65
Some groups report that neonates should receive four weeks of intravenous antibiotics.18 The European guidelines are shorter, with the total therapy (IV and oral combined) being three to four weeks for AHOM; these children must have CRP levels under 100 mg/L on admission.4,42,64 Longer therapy (up to six weeks) should be given in children with CRP levels above 100 mg/L; resistant or unusual pathogens; children under the age of three months; slow or poor response to treatment; any complications; pelvic or spinal AHOM; septicaemic children or immunocompromised children.4,37,42,64
The decision between oral therapy once ready for discharge versus outpatient parenteral antibiotic therapy (OPAT) via a PICC line sways towards a switch to oral therapy.39,45,88,111 This avoids high costs and harm to the patient.3,39,45,88,111 However, OPAT is preferred over a prolonged inpatient hospital stay if oral therapy is not feasible.39 Prolonged intravenous antibiotics are associated with risks, including catheter-associated complications such as thrombophlebitis.2,4,16,18,45 Oral therapy is contraindicated in cases of poor medication compliance or follow-up, malabsorption or a slow resolution of infection.16
The timing of the switch to oral therapy is still debated.7,18,38,68 Some authors recommend at least five to seven days of intravenous treatment.18 An early oral switch has been used if the child shows clinical improvement.4,41,42,97
Improvements may include:2,4,5,7,12,16,40,42,68,89,105
• Apyrexial for 24-48 hours
• Decreased local inflammation and pain
• Halving of CRP from the maximum value
• No systemic symptoms (endocarditis, pneumonia or DVT)
• Pathogen not Salmonella, MRSA or PVL-positive
• Negative blood cultures after previously being positive
• Mild and moderate illness severity scores39,97
Faust et al. considered an oral switch safe if the CRP value is under 20 mg/L or at least two-thirds of its maximum peak.7,112
Flucloxacillin is a narrow spectrum, semisynthetic penicillin and can be prescribed for sensitive S. aureus but bear in mind the poor taste of the oral suspension.7,11,16,111 Flucloxacillin has bactericidal properties but little evidence that, in vitro, there is good bone penetration.111 Preiss et al. conducted a narrative review on oral flucloxacillin to treat AHOM.111 The experience of most publications reports good outcomes with oral flucloxacillin, despite the poor bone penetration.111 Adverse effects include hypersensitivity, gastrointestinal symptoms, nephrotoxicity and myelotoxicity.111 Drug-drug interactions have been reported with paracetamol and rarely with warfarin and rifampicin.111 Most clinicians recommend 100 mg/kg/day.16
Cephalexin is an oral first-generation cephalosporin and has demonstrated positive outcomes in 82-100% of patients.45 The dosage should be 100-150 mg/kg/day q6h to improve the minimum inhibitory concentration (MIC).45 The half-life is short (one hour); thus, the six-hour dosing is better than eight hours.16
For MRSA, oral therapy with clindamycin, trimethoprim-sulfamethoxazole or linezolid can be considered with careful monitoring.16,68,100 Bradley et al. reported on the efficacy of daptomycin.34 When compared to vancomycin, daptomycin performed as well as vancomycin regarding successful treatment and was better tolerated.34,100 Daptomycin and linezolid should be considered second-line drugs.100
Outcomes
The incidence of complications after AHOM is unclear - high-income countries report the incidence to be 27%113 but it has been reported as high as 48% in South African literature.114 Risk factors for a more prolonged hospital course and long-term complications include high-virulence organisms (such as MRSA), contiguous septic arthritis, positive cultures, younger children, location of the disease and delay in care and source control.4,6,10,11,41,89,94,113-115 Osteonecrosis or chondrolysis, seen in 8% of cases, is correlated with the initial illness severity and leads to premature arthritis.4,6 To protect the hip from dislocation, abduction skin traction can be applied when proximal femur AHOM and hip septic arthritis is diagnosed.118
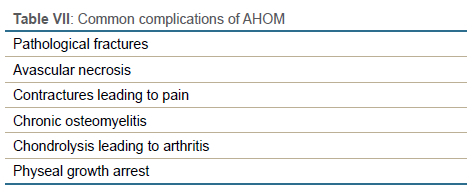
The common complications are tabulated in Table VII.
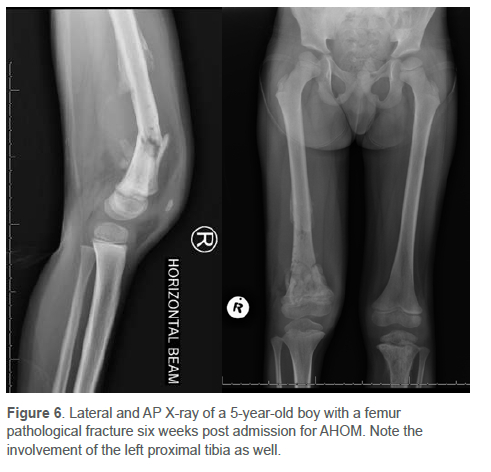
AHOM can cause devastating destruction of the bone due to tissue necrosis, leading to late sequelae like chronic osteomyelitis (1.7%)3 and pathological fractures5,23,31,36 (Figure 6). Complications such as these are more common in children in LMICs.50,54,55 Belthur et al. described the incidence of pathological fracture as 4.6-5%; however, Popsecu et al. reported an incidence of almost 10%.31,119,120 In addition, on admission, large subperiosteal abscesses extending into the muscle were more likely to fracture within 72 days of presentation.3,4,7,71,74 The limb segment should be protected in the first six weeks to prevent a pathological fracture.42 Calvo et al. reported that children with contiguous infections also had the highest incidence of surgery (46%), complications (18%) and sequelae (10%).56
Other complications include avascular necrosis, angular deformities and limb-length discrepancy due to physeal growth arrest or overgrowth.1,5,6,12,49,50,53,54,57,60,72,76,115,121 These children can also develop joint contractures, chronic pain and gait disturbances.9,42,76
Sequelae at one-year follow-up are higher in infants under 3 months.9 Predictors of poor outcome in neonates include hip and shoulder involvement, concomitant septic arthritis with AHOM, bacteraemia and S. aureus infection (especially with MRSA).79 The recurrence rate has been reported as between 3% and 6.8%.3,23,88
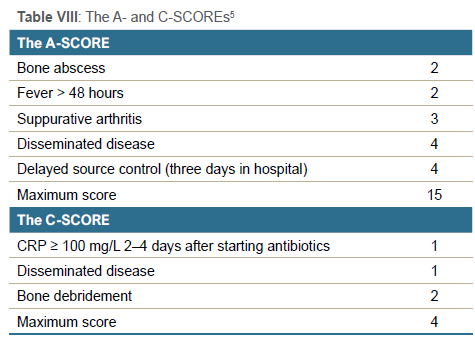
McNeil et al. reported that infection with agr III (accessory gene regulator III) S. aureus organism, fever more than four days after admission and delayed source control were associated with significant increase in orthopaedic complications.31,41 The fracture risk increased significantly with more than three debridements.31 Alhinai et al. developed an A-SCORE and C-SCORE to predict acute and chronic complications (Table VIII).5 The negative predictive value of the A-SCORE < 4 was > 91%, and the C-SCORE < 3 was > 95%.5 Vij et al. found similar results in their study and concluded that the A-SCORE should be used in combination with clinical judgement to determine optimal timing for early care decisions like an oral antibiotic switch.30 The C-SCORE can be used to consider specific decisions regarding the development of chronic osteomyelitis and the need for prolonged (> 12 weeks) antibiotics, as well as to rule out pathological fractures and avascular necrosis.30
Rehabilitation is an integral part of management, with prompt mobilisation crucial to prevent contractures.42 Prolonged orthopaedic follow-up for up to 12 months is recommended in those with infections around the physis and any patient who underwent surgical debridement and even longer if the pelvis, spine or hip were involved.4,39,42
Hunter and Baker reported on the quality of life of children treated for AHOM.23 The most common complaints were pain, stiffness (mostly related to sport) and anxiety.23 There are no validated assessment tools to assess the quality of life after AHOM.23 The recommendation is for a disease-specific assessment tool to analyse the longer-term outcomes.23
Conclusions
A multidisciplinary approach to managing acute haematogenous osteomyelitis should be the standard of care. Proactive MRI protocols may improve patient care and should be implemented in centres where MRI is available. Local antibiogram profiles should govern empiric antibiotics, and clindamycin should be considered for its anti-toxin properties in systemically ill children. If an abscess is present, surgical management to decompress the pus and obtain a biopsy is needed. The bone should be drilled even if there is subperiosteal pus. A total of six weeks of antibiotics is key in complicated cases of AHOM. More research from LMICs should be published to guide local treatment algorithms. A proposed management algorithm is proposed for the South African system (Figure 3).
Acknowledgements
The authors would like to thank Prof. Helena Rabie from the Department of Paediatrics and Child Health, Dr Pieter Nel from the Department of Medical Microbiology and Dr Veshni Pillay-Fuentes Lorente from the Department of Clinical Pharmacology, Faculty of Health Sciences, Stellenbosch University, for their contributions regarding the empiric antibiotic choices, dosages and spectrum.
Ethics statement
The authors declare that this submission is in accordance with the principles laid down by the Responsible Research Publication Position Statements as developed at the 2nd World Conference on Research Integrity in Singapore, 2010. Ethics approval was not obtained (review article).
Declaration
The authors declare authorship of this article and that they have followed sound scientific research practice. This research is original and does not transgress plagiarism policies.
Author contributions
MT: literature search and review, first draft preparation, manuscript revision AN: literature review, manuscript revision
ORCID
Thiart Μ https://orcid.org/0000-0001-9942-8012
Nansook A https://orcid.org/0000-0002-0365-6393
References
1. Castellazzi L, Mantera M, Esposito S. Update on the management of pediatric acute osteomyelitis and septic arthritis. Int J Mol Sci. 2016;17(6):855. [ Links ]
2. Thakolkaran N, Shetty A. Acute hematogenous osteomyelitis in children. Ochsner J. 2019;19(2):116-22. [ Links ]
3. Funk SS, Copley LAB. Acute hematogenous osteomyelitis in children: pathogenesis, diagnosis, and treatment. Orthop Clin North Am. 2017;48(2):199-208. [ Links ]
4. Gornitzky AL, Kim AE, O'Donnell JM, Swarup I. Diagnosis and management of osteomyelitis in children: a critical analysis review. JBJS Rev. 2020 Jun;8(6):e1900202. [ Links ]
5. Alhinai Z, Elahi M, Park S, et al. Prediction of adverse outcomes in pediatric acute hematogenous osteomyelitis. Clin Infect Dis. 2020;71(9):e454-64. [ Links ]
6. Saad L, Hupin M, Buteau C, Nault ML. Late sequelae of osteoarticular infections in pediatric patients: A single-center study. Medicine. 2021;100(8):e23765. [ Links ]
7. Autore G, Bernardi L, Esposito S. Update on acute bone and joint infections in paediatrics: a narrative review on the most recent evidence-based recommendations and appropriate antinfective therapy. Antibiotics. Available from: www.mdpi.com/journal/antibiotics [ Links ]
8. Hartman NR, Gerard JM, Puryear A, et al. Clinical characteristics of acute hematogenous osteomyelitis with and without subperiosteal abscesses in the acute care setting. Pediatr Emerg Care. 2022;38(4):E1224-8. [ Links ]
9. Branco J, Duarte M, Norte S, et al. Osteoarticular infections in infants under 3 months of age. Pediatr Int. 2022;64(1):e15212. [ Links ]
10. Jain MJ, Bradko V, Zhu H, et al. Pediatric osteoarticular infection: Trend in surgically treated patients and association of methicillin-resistant Staphylococcus aureus with requirement of secondary procedures. J Pediatr Orthop B. 2021;30:579-84. [ Links ]
11. Dartnell J, Ramachandran M, Katchburian M. Haematogenous acute and subacute paediatric osteomyelitis. A systematic review of the literature. Bone Joint Surg Br. 2012;94(5):584-95. [ Links ]
12. Alvares PA, Mimica MJ. Osteoarticular infections in pediatrics. J Pediatr (Rio J). 2020;96 Suppl 1(Suppl 1):58-64. [ Links ]
13. Riise 0R, Kirkhus E, Handeland KS, et al. Childhood osteomyelitis-incidence and differentiation from other acute onset musculoskeletal features in a population-based study. BMC Pediatr. 2008 Oct 20;8:45. [ Links ]
14. Munshi B, MacFater W, Hill AG, McCaig EH. Paediatric osteomyelitis in Fiji. World J Surg. 2018 Dec;42(12):4118-22. [ Links ]
15. Lindsay EA, Tareen N, Jo CH, Copley LA. Seasonal variation and weather changes related to the occurrence and severity of acute hematogenous osteomyelitis in children. J Pediatr Infect Dis. e16. JPIDS [Internet]. 2018(7). Available from: https://www.wunderground.com/ [ Links ]
16. Le Saux N. Diagnosis and management of acute osteoarticular infections in children. J Paediatr Child. 2018;23(5):336-43. [ Links ]
17. Serrano E, Ferri I, Galli L, Chiappini E. Amoxicillin-clavulanic acid empirical oral therapy for the management of children with acute haematogenous osteomyelitis. Antibiotics (Basel). 2020;9(8):525. [ Links ]
18. Congedi S, Minotti C, Giaquinto C, et al. Acute infectious osteomyelitis in children: new treatment strategies for an old enemy. World J Pediatr. 2020;16(5):446-55. [ Links ]
19. Stephan AM, Faino A, Caglar D, Klein EJ. Clinical presentation of acute osteomyelitis in the pediatric emergency department. Pediatr Emerg Care. 2022;38(1):E209-13. [ Links ]
20. Paliwal AK, Sahdev R, Deshwal A, Ram B. Role of ultrasound in the diagnosis of paediatric acute osteomyelitis. J Ultrason. 2021;21(84):e34-40. [ Links ]
21. Upasani VV, Burns JD, Bastrom TP, et al. Practice variation in the surgical management of children with acute hematogenous osteomyelitis. J Pediatr Orthop. 2022;42(5):E520-25. [ Links ]
22. Griswold BG, Sheppard E, Pitts C, et al. The introduction of a preoperative MRI protocol significantly reduces unplanned return to the operating room in the treatment of pediatric osteoarticular infections. J Pediatr Orthop. 2020;40(2):97-102. [ Links ]
23. Hunter S, Baker JF. Quality of life in children up to 13 years following acute haematogenous osteomyelitis. J Pediatr Orthop B. 2022;Nov (Published ahead of print). Available from: https://journals.lww.com/jpo-b/Fulltext/9900/Quality_of_life_in_children_up_to_13_years.65.aspx [ Links ]
24. Roine I, Arguedas A, Faingezicht I, Rodriguez F. Early detection of sequela-prone osteomyelitis in children with use of simple clinical and laboratory criteria. Clin Infect Dis. 1997;24(5):849-53. [ Links ]
25. Copley LAB, Barton T, Garcia C, et al. A proposed scoring system for assessment of severity of illness in pediatric acute hematogenous osteomyelitis using objective clinical and laboratory findings. Pediatr Infect Dis J. 2014;33(1):35-41. [ Links ]
26. Monsalve J, Kan JH, Schallert EK, et al. Septic arthritis in children: frequency of coexisting unsuspected osteomyelitis and implications on imaging work-up and management. AJR Am J Roentgenol. 2015;204(6):1289-95. [ Links ]
27. Kawaguchi K, Nakamura T, Wada A, et al. The recent bacterial etiology of childhood osteoarticular infections focusing on the vaccine initiation for Streptococcus pneumoniae and Haemophilus influenzae: A single-center retrospective analysis in Japan. J Orthop. 2022;31:6-12. [ Links ]
28. Zairi M, Amin Mohseni A, Msakni A, et al. Acute hematogenous osteomyelitis in children: Management of pandiaphysitis with extensive bone destruction: A case series of thirteen child. Ann Med Surg. 2022;82:104578. [ Links ]
29. Lv C, Lv J, Liu Y, et al. Pediatric pharmaceutical care with anti-infective medication in a patient with acute hematogenous osteomyelitis caused by methicillin-resistant Staphylococcus aureus. Int J Immunopathol Pharmacol. 2020;34:2058738420925713. [ Links ]
30. Vij N, Singleton I, Kang P, et al. Clinical scores predict acute and chronic complications in pediatric osteomyelitis: an external validation. J Pediatr Orthop. [Internet]. 2022;42(6). Available from: https://pubmed.ncbi.nlm.nih.gov/35405715/ [ Links ]
31. McNeil JC, Vallejo JG, Kok EY, et al. Clinical infectious diseases clinical and microbiologic variables predictive of orthopedic complications following Staphylococcus aureus acute hematogenous osteoarticular infections in children.Clin Infect Dis. 2019;69(11):1955-61. [ Links ]
32. Zhang T, Yu S, Lv X, et al. Paediatric osteomyelitis and septic arthritis pathogen distribution and antimicrobial resistance in a single centre: a 15-year retrospective analysis. J Trop Pediatr. 2022 Apr 5;68(3):fmac038. https://doi.org/10.1093/tropej/fmac038 [ Links ]
33. Van der Merwe M, Rooks K, Crawford H, et al. The effect of antibiotic timing on culture yield in paediatric osteoarticular infection. J Child Orthop. 2019;13(1):114-19. [ Links ]
34. Bradley JS, Arrieta AC, Digtyar VA, et al. Daptomycin for pediatric Gram-positive acute hematogenous osteomyelitis. Pediatr Infect Dis J. 2020;39(9):814-23. [ Links ]
35. Walter N, Bärtl S, Alt V, Rupp M. The epidemiology of osteomyelitis in children. Children. 2021;8(11):1000. https://doi.org/10.3390/children8111000 [ Links ]
36. Dehority Id W, Morley VJ, Domman DB, et al. Genomic characterization of Staphylococcus aureus isolates causing osteoarticular infections in otherwise healthy children. PLoS ONE 2022;17(8):e0272425. https://doi.org/10.1371/journal.pone.0272425 [ Links ]
37. Manz N, Krieg AH, Heininger U, Ritz N. Evaluation of the current use of imaging modalities and pathogen detection in children with acute osteomyelitis and septic arthritis. Eur J Pediatr. 2018;177(7):1071-80. [ Links ]
38. Von Heideken J, Bennet R, Eriksson M, Hertting O. A 10-year retrospective survey of acute childhood osteomyelitis in Stockholm, Sweden. J Paediatr Child Health. 2020;56(12):1912-17. [ Links ]
39. Woods CR, Bradley JS, Chatterjee A, et al. Clinical practice guideline by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America: 2021 Guideline on diagnosis and management of acute hematogenous osteomyelitis in pediatrics. J Pediatric Infect Dis Soc. 2021;10(8):801-44. [ Links ]
40. Thingsaker EE, Urbane UN, Pavare J. A comparison of the epidemiology, clinical features, and treatment of acute osteomyelitis in hospitalized children in Latvia and Norway. Medicina (Kaunas). 2021 Jan 4;57(1):36. [ Links ]
41. El-Sobky T, Mahmoud S. Acute osteoarticular infections in children are frequently forgotten multidiscipline emergencies: beyond the technical skills. EFORT Open Rev. 2021 Jul 8;6(7):584-92. [ Links ]
42. Saavedra-Lozano J, Falup-Pecurariu O, Faust SN, et al. Bone and Joint Infections. Pediatr Infect Dis J. 2017;36(8):788-99. [ Links ]
43. Nasser A, Azimi T, Ostadmohammadi S, Ostadmohammadi S. A comprehensive review of bacterial osteomyelitis with emphasis on Staphylococcus aureus. Microb Pathog. 2020;148:104431. [ Links ]
44. Olijve L, Amarasena L, Best E. The role of Kingella kingae in pre-school aged children with bone and joint infections. J Infect. 2021;83:321-31. [ Links ]
45. DeRonde KJ, Girotto JE, Nicolau DP. Management of pediatric acute hematogenous osteomyelitis, part I: Antimicrobial stewardship approach and review of therapies for methicillin-susceptible Staphylococcus aureus, Streptococcus pyogenes, and Kingella kingae. Pharmacotherapy. 2018;38(9):947-66. [ Links ]
46. Palmer B, Wang ME. Clinical guideline highlights for the hospitalist: Diagnosis and management of acute hematogenous osteomyelitis in children. J Hosp Med. 2022;17(2):114-16. [ Links ]
47. Section J, Gibbons SD, Barton T, et al. Microbiological culture methods for pediatric musculoskeletal infection: a guideline for optimal use. J Bone Joint Surg Am. 2015;97(6):441-19. [ Links ]
48. Urish KL, Cassat JE. Staphylococcus aureus osteomyelitis: bone, bugs, and surgery. Infect Immun. [Internet]. 2020;88(7). https://pubmed.ncbi.nlm.nih.gov/32094258/ [ Links ]
49. Mahmoudi S, Pourakbari B, Borhani K, et al. Acute osteomyelitis and septic arthritis in children: A referral hospital-based study in Iran. Wien Med Wochenschr. 2017;167(11-12):259-63. [ Links ]
50. Branson J, Vallejo JG, Flores AR, et al. The contemporary microbiology and rates of concomitant osteomyelitis in acute septic arthritis. Pediatr Infect Dis J. 2017;36(3):267-73. [ Links ]
51. Kao HC, Huang YC, Chiu CH, et al. Acute hematogenous osteomyelitis and septic arthritis in children. J Microbiol Immunol Infect. 2003;36(4):260-65. [ Links ]
52. Ceroni D, Kampouroglou G, Della Llana RA, Salvo D. Osteoarticular infections in young children: What has changed over the last years? Swiss Med Wkly. 2014;144(June):1-13. [ Links ]
53. Agarwal A, Aggarwal AN. Bone and joint infections in children: acute hematogenous osteomyelitis. Indian J Pediatr. 2016;83(8):817-24. [ Links ]
54. Pääkkönen M, Peltola H. Bone and jointinfections. Pediatr Clin North Am. 2013;60(2):425-36. [ Links ]
55. Osei L, El Houmami N, Minodier P, et al. Paediatric bone and joint infections in French Guiana: A 6 year retrospective review. J Trop Pediatr 2017;63(5):380-88. [ Links ]
56. Calvo C, Núñez E, Camacho M, et al. Epidemiology and management of acute, uncomplicated septic arthritis and osteomyelitis spanish multicenter study. Pediatr Infect Dis J. 2016;35(12):1288-93. [ Links ]
57. Iliadis AD, Ramachandran M. Paediatric bone and joint infection. EFORT Open Rev. 2017;2(1):7-12. [ Links ]
58. Yamagishi Y, Togawa M, Shiomi M. Septic arthritis and acute hematogenous osteomyelitis in childhood at a tertiary hospital in Japan. Pediatr Int. 2009;51(3):371-76. [ Links ]
59. Yi J, Wood JB, Creech CB, et al. Clinical epidemiology and outcomes of pediatric musculoskeletal infections. J Pediatr. 2021;234:236-44.e2. [ Links ]
60. Dodwell ER. Osteomyelitis and septic arthritis in children: Current concepts. Curr Opin Pediatr. 2013;25(1):58-63. [ Links ]
61. Kini AR, Shetty V, Kumar AM, et al. Community-associated, methicillin-susceptible, and methicillin-resistant Staphylococcus aureus bone and joint infections in children: Experience from India. J Pediatr Orthop B. 2013;22(2):158-66. [ Links ]
62. An TJ, Benvenuti MA, Mignemi ME, et al. Similar clinical severity and outcomes for methicillin-resistant and methicillin-susceptible Staphylococcus aureus pediatric musculoskeletal infections. Open Forum Infect Dis. 2017;4(1):1-5. [ Links ]
63. Al-Qwbani M, Jiang N, Yu B. Kingella kingae-associated pediatric osteoarticular infections: an overview of 566 reported cases. Clin Pediatr. 2016;55(14):1328-37. [ Links ]
64. Jagodzinski NA, Kanwar R, Graham K, Bache CE. Prospective evaluation of a shortened regimen of treatment for acute osteomyelitis and septic arthritis in children. J Pediatr Orthop. 2009;29(5):518-25. [ Links ]
65. DeMarco G, Chargui M, Coulin B, et al. Kingella kingae osteoarticular infections approached through the prism of the pediatric orthopedist. Microorganisms. 2021 Dec 24;10(1):25. [ Links ]
66. Ceroni D, Cherkaoui A, Ferey S, et al. Kingella kingae osteoarticular infections in young children: Clinical features and contribution of a new specific real-time PCR assay to the diagnosis. J Pediatr Orthop. 2010;30(3):301-304. [ Links ]
67. Coulin B, Demarco G, Spyropoulou V, et al. Osteoarticular infection in children an update on the epidemiological, clinical, and biological features of Kingella kingae. Bone Joint J. 2021;103 B(3):578-83. [ Links ]
68. Krzysztofiak A, Chiappini E, Venturini E, et al. Italian consensus on the therapeutic management of uncomplicated acute hematogenous osteomyelitis in children. Ital J Pediatr. 2021;47:179. https://doi.org/10.1186/s13052-021-01130-4 [ Links ]
69. Aiblñski MK, Lutz N, Ceronl D, et al. Paediatric musculoskeletal Infections with Panton-Valentine leucocidin. Swiss Med Wkly. 2018;148(3738):w14669. [ Links ]
70. Gijón M, Bellusci M, Petraitiene B, et al. Factors associated with severity in invasive community-acquired Staphylococcus aureus infections in children: a prospective European multicentre study. Clin Microbiol Infect. 2016;22(7):643.e1-643.e6. [ Links ]
71. Montgomery NI, Rosenfeld S. Pediatric osteoarticular infection update. J Pediatr Orthop. 2015;35(1):74-81. [ Links ]
72. Arnold JC, Bradley JS. Osteoarticular infections in children. Infect Dis Clin North Am. 2015;29(3):557-74. [ Links ]
73. Montgomery CO, Siegel E, Blasier RD, Suva LJ. Concurrent septic arthritis and osteomyelitis in children. J Pediatr Orthop. 2013;33(4):464-67. [ Links ]
74. Kaplan SL. Recent lessons for the management of bone and joint infections. J Infect. 2014;68 Suppl 1:S51-56. [ Links ]
75. Arnold SR, Elias D, Buckingham SC, et al. Changing patterns of acute hematogenous osteomyelitis and septic arthritis: Emergence of community-associated methicillin-resistant Staphylococcus aureus. J Pediatr Orthop. 2006;26(6):703-708. [ Links ]
76. Arkader A, Brusalis C, Warner WC, et al. Update in pediatric musculoskeletal infections: when it is, when it isn't, and what to do. J Am Acad Orthop Surg. 2016;24(9):e112-21. [ Links ]
77. Morrison MJ, Herman MJ. Hip septic arthritis and other pediatric musculoskeletal infections in the era of methicillin-resistant Staphylococcus aureus. Instr Course Lect. 2013;62:405-14. [ Links ]
78. Sarkissian EJ, Gans I, Gunderson MA, et al. Community-acquired methicillin-resistant Staphylococcus aureus musculoskeletal infections: emerging trends over the past decade. J Pediatr Orthop. 2016;36(3):323-27. [ Links ]
79. Bréhin C, Claudet I, Dubois D, et al. Assessing the management of pediatric bone and joint infections according to French guidelines. Med Mal Infect. 2020;50(6):515-19. https://doi.org/10.1016/j.medmal.2019.07.016 [ Links ]
80. O'Rourke S, Meehan M, Bennett D, et al. The role of real-time PCR testing in the investigation of paediatric patients with community-onset osteomyelitis and septic arthritis. Ir J Med Sci. 2019 Nov;188(4):1289-95. [ Links ]
81. Immunization - National Department of Health [Internet]. Available from: https://www.health.gov.za/immunization/. Accessed 4 Feb 2023. [ Links ]
82. Giaccai L, Idriss H. Osteomyelitis due to Salmonella infection. J Pediatr. 1952 Jul 1;41(1):73-78. [ Links ]
83. Zacharia DB, Poulosse DS, Xavier DR, et al. Salmonella osteomyelitis and septic arthritis in children. Med Res Arch [Internet]. 2022 Sep 20;10(9). Available from: https://esmed.org/MRA/mra/article/view/3100. Accessed 7 Feb 2023. [ Links ]
84. Burnett MW, Bass JW, Cook BA. Etiology of osteomyelitis complicating sickle cell disease. Pediatrics (Evanston). 1998;101(2):296-97. [ Links ]
85. Givner LB, Luddy RE, Schwartz AD. Etiology of osteomyelitis in patients with major sickle hemoglobinopathies. J Pediatr. 1981 Sep;99(3):411-13. [ Links ]
86. Hernigou P, Daltro G, Flouzat-Lachaniette CH, et al. Septic arthritis in adults with sickle cell disease often is associated with osteomyelitis or osteonecrosis. Clin Orthop Relat Res. 2010 Jun;468(6):1676. [ Links ]
87. Searns JB, DeVine MN, MacBrayne CE, et al. Characteristics of children with culture negative acute hematogenous musculoskeletal infections. J Pediatr Orthop. 2022;42(2):E206-11. [ Links ]
88. Quick RD, Williams J, Fernandez M, et al. Improved diagnosis and treatment of bone and joint infections using an evidence-based treatment guideline. J Pediatr Orthop. 2018;38(6):e354-59. [ Links ]
89. Wang B, Cheng W, Liu F, et al. Efficacy and safety of vancomycin-loaded calcium sulfate versus conventional surgical debridement for pediatric acute osteomyelitis: a retrospective study. BMC Musculoskelet Disord. 2022;23(1):1124. [ Links ]
90. Bonhoeffer J, Haeberle B, Schaad UB, Heininger U. Diagnosis of acute haematogenous osteomyelitis and septic arthritis: 20 years experience at the University Children's Hospital Basel. Swiss Med Wkly. 2001;131(3940):575-81. [ Links ]
91. Waldvogel FA, Medoff G, Swartz MN. Osteomyelitis: a review of clinical features, therapeutic considerations and unusual aspects. N Engl J Med. 1970 Jan 22;282(4):198-206. [ Links ]
92. Morgan A, Yates AK. The diagnosis of acute osteomyelitis of the pelvis. Postgrad Med J. 1966 Feb;42(484):74-78. [ Links ]
93. Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52(3):18-55. [ Links ]
94. Musso P, Parigi S, Bossi G, et al. Epidemiology and management of acute hematogenous osteomyelitis, neonatal osteomyelitis and spondylodiscitis in a third level paediatric center. Children (Basel). 2021 Jul 21;8(8):616. [ Links ]
95. Habre C, Botti P, Laurent M, et al. Benefits of diffusion-weighted imaging in pediatric acute osteoarticular infections. Pediatr Radiol. 2022;52(6):1086-94. [ Links ]
96. Alexander KM, Laor T, Bedoya MA. Magnetic resonance imaging protocols for pediatric acute hematogenous osteomyelitis. Pediatr Radiol. 2022 Jul 7. https://doi.org/10.1007/s00247-022-05435-2 [ Links ]
97. Sanchez MJ, Patel K, Lindsay EA, et al. Early transition to oral antimicrobial therapy among children with Staphylococcus aureus bacteremia and acute hematogenous osteomyelitis. Pediatr Infect Dis J. 2022;41(9):690-95. [ Links ]
98. Gouveia C, Branco J, Norte S, et al. Acute haematogenous osteomyelitis in Lisbon: an unexpectedly high association with myositis and arthritis. Anales de Pediatría (English Edition). 2022;96(2):106-14. [ Links ]
99. Amaro E, Marvi TK, Posey SL, et al. C-reactive protein predicts risk of venous thromboembolism in pediatric musculoskeletal infection. J Pediatr Orthop. 2019;39(1):e62-68. [ Links ]
100. DeRonde KJ, Girotto JE, Nicolau DP. Management of pediatric acute hematogenous osteomyelitis, part II: a focus on methicillin-resistant Staphylococcus aureus, current and emerging therapies. Pharmacotherapy. 2018 Oct;38(10):1021-37. [ Links ]
101. Hester GZ, Nickel AJ, Watson D, et al. Improving care and outcomes for pediatric musculoskeletal infections. Pediatrics. 2021 Feb;147(2):e20200118. https://doi.org/10.1542/peds.2020-0118 [ Links ]
102. Copley LAB, Kinsler MA, Gheen T, et al. The impact of evidence-based clinical practice guidelines applied by a multidisciplinary team for the care of children with osteomyelitis. J Bone Jt Surg. 2013;95(8):686-93. [ Links ]
103. Mignemi ME, Benvenuti MA, An TJ, et al. A novel classification system based on dissemination of musculoskeletal infection is predictive of hospital outcomes. J Pediatr Orthop. 2018;38(5):279-86. [ Links ]
104. Sankaran G, Zacharia B, Roy A, Purayil SP. Current clinical and bacteriological profile of septic arthritis in young infants: a prospective study from a tertiary referral centre. Eur J Orthop Surg Traumatol. 2018;28(4):573-78. [ Links ]
105. Chiappini E, Serrano E, Galli L, et al. Practical issues in early switching from intravenous to oral antibiotic therapy in children with uncomplicated acute hematogenous osteomyelitis: results from an Italian survey. Int J Environ Res Public Health. 2019;16:3557. [ Links ]
106. Kaplan SL. Community-acquired methicillin-resistant Staphylococcus aureus infections in children. Semin Pediatr Infect Dis. 2006;17(3):113-19. [ Links ]
107. SAMF [Internet]. Available from: https://samf-app.com/. Accessed 28 Feb 2023. [ Links ]
108. Sanford Guide - Guidelines & Tools for Antimicrobial Stewardship [Internet]. Sanford Guide - Antimicrobial Stewardship. Available from: https://marketing-staging.sanfordguide.com/. Accessed 28 Feb 2023. [ Links ]
109. Montgomery CO, Porter A, Sachleben B, et al. Treatment of subperiosteal abscesses in children: is drainage of the intramedullary canal required? J Pediatr Orthop B. 2017 Nov;26(6):497-500. [ Links ]
110. Tsegka KG, Voulgaris GL, Kyriakidou M, et al. Intravenous fosfomycin for the treatment of patients with bone and joint infections: a review. Expert Rev Anti Infect Ther. 2022;20(1):33-43. [ Links ]
111. Preiss H, Kriechling P, Montrasio G, et al. Oral flucloxacillin for treating osteomyelitis: a narrative review of clinical practice. JBJI. 2020;5(1):16-24. [ Links ]
112. Faust SN, Clark J, Pallett A, Clarke NMP. Managing bone and joint infection in children. Arch Dis Child. 2012 Jun;97(6):545-53. [ Links ]
113. Johnston JJ, Murray-Krezan C, Dehority W. Suppurative complications of acute hematogenous osteomyelitis in children. J Pediatr Orthop B. 2017 Nov;26(6):491-96. [ Links ]
114. Horn A, Wever S, Hoffman EB. Complications following acute severe haematogenous osteomyelitis of the long bones in children. SA Orthop J. 2019;18(3):23-29. [ Links ]
115. Manz N, Krieg AH, Buettcher M, et al. Long-term outcomes of acute osteoarticular infections in children. Front Pediatr. 2020 Nov 25;8:587740. [ Links ]
116. Nunn T, Rollinson P. Haematogenous pyogenic bone and joint sepsis - Reducing avoidable morbidity. S Afr Med J. 2007;97(6):456-60. [ Links ]
117. Wang E, Zhao Q, Zhang L, et al. The split-heel technique in the management of chronic calcaneal osteomyelitis in children. J Pediatr Orthop B. 2009;18(1):23-27. [ Links ]
118. Prince S, Tulasi R. Case report on pediatric septic arthritis of the hip. EJIFCC. 2020 Sep 29;31(3):248-53. [ Links ]
119. Belthur MV, Birchansky SB, Verdugo AA, et al. Pathologic fractures in children with acute Staphylococcus aureus osteomyelitis. J Bone Jt Surg Am. 2012;94(1):34-42. [ Links ]
120. Popescu B, Tevanov I, Carp M, Ulici A. Acute hematogenous osteomyelitis in pediatric patients: epidemiology and risk factors of a poor outcome. J Int Med Res. 2020;48(4):300060520910889. Available from: https://pubmed.ncbi.nlm.nih.gov/32249643/ [ Links ]
121. Laurent E, Petit L, Maakaroun-Vermesse Z, et al. National epidemiological study reveals longer paediatric bone and joint infection stays for infants and in general hospitals. Acta Paediatr Int J Paediatr. 2018;107(7):1270-75. [ Links ]
Received: February 2023
Accepted: March 2023
Published: May 2023
* Corresponding author: marithiart@sun.ac.za
Editor: Prof. Nando Ferreira, Stellenbosch University, Cape Town, South Africa
Funding: No funding was received for this study.
Conflict of interest: The authors declare they have no conflicts of interest that are directly or indirectly related to the research.














