Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
SA Orthopaedic Journal
On-line version ISSN 2309-8309
Print version ISSN 1681-150X
SA orthop. j. vol.21 n.4 Centurion 2022
http://dx.doi.org/10.17159/2309-8309/2022/v21n4a7
CURRENT CONCEPTS REVIEW
Truth or DAIR? A review of debridement, antibiotics and implant retention
Neill R Blair*; Johan F van der Merwe; Steven Matshidza
Department of Orthopaedic Surgery, School of Clinical Medicine, Faculty of Health Sciences, University of the Free State, Bloemfontein, South Africa
ABSTRACT
Debridement, antibiotics and implant retention (DAIR) is a viable treatment option in early postoperative and acute haematogenous periprosthetic joint infections (PJIs) with a stable implant. Despite lower success rates compared to one- and two-stage revisions, DAIR maintains satisfactory outcomes in selected patient groups and, if successful, has similar functional outcomes to primary arthroplasty. DAIR remains an attractive treatment option, providing satisfactory outcomes with decreased healthcare costs, reduced surgical burden on the patient and shorter hospital stays. With success rates of 37-90%, various factors need to be considered when deciding on DAIR as the appropriate treatment option for PJI. The risk of DAIR failure needs to be weighed against the potential benefits of DAIR success. Factors that increase success rates include an open DAIR procedure performed for a low-virulence, antibiotic-sensitive organism, within a short duration between symptom onset and/or index surgery and DAIR. The procedure involves intraoperative exchange of mobile components and copious wound irrigation, followed by an appropriate antibiotic regimen for a minimum of six weeks that can be administered either intravenously or orally in a well-optimised host, without significant soft tissue defects or contraindications to surgery. Factors increasing the risk for DAIR failure include chronic/late PJIs with resistant organisms, especially methicillin-resistant Staphylococcus aureus (MRSA) in poor hosts with significant comorbidities, such as chronic obstructive pulmonary disease (COPD), liver cirrhosis, rheumatoid arthritis, advanced age > 80 years, patients with fracture indications for arthroplasty and those who cannot tolerate rifampicin- and fluoroquinolone-based antibiotic regimens. Unfortunately, there is no definitive factor to serve as an indication of whether DAIR will be successful, but with recent data showing that a failed DAIR procedure does not lower success in future staged revisions, then even in the face of a 50% success rate, DAIR can maintain its role as an initial treatment option in the management of PJIs.
Level of evidence: Level 5
Keywords: DAIR, debridement, antibiotics, implant retention, periprosthetic joint infection, PJI, arthroplasty
Introduction
Total joint arthroplasty is a common intervention to relieve pain from advanced joint disease. With improvements in joint replacement surgery and increasing life expectancy, total joint arthroplasties are expected to increase with time.1,2 With escalating joint arthroplasty procedures, increased periprosthetic joint infections (PJIs) are expected.3 The incidence of PJI is estimated at 0.5-2%, with knee arthroplasty at 0.8-1.9% and hip arthroplasty at 0.3-1.7%.4,5 PJI is a devastating complication of total joint arthroplasty and significantly increases the burden on the patient, the surgeon and the healthcare system. Prolonged hospitalisation, multiple surgical procedures, psychological stressors of progressive disease, increased healthcare costs, loss of income and physical disability all add to the burden of PJI and reflect as diminished patient outcomes concerning morbidity, quality of life and mortality rates.2,4,6
PJI is a leading cause of revision arthroplasty, as the management of the condition frequently requires a combination of surgical and medical intervention.7 Eradicating a PJI while retaining a viable and functional prosthesis remains a challenge due to multiple variables, including patient condition, the infective organism, surgical approach and antimicrobial use.3 Debridement, antibiotics and implant retention (DAIR) has been identified as a viable treatment choice in PJI when applied to selected patients, and its use has shown a significantly increasing trend of approximately 0.9-3.4% over a ten-year period.8
This review discusses the use of DAIR for PJI and highlights its potential benefits and pitfalls.
Defining periprosthetic joint infection
Diagnosing PJI with the decision to perform additional surgical procedures and selecting which one of the multiple available interventions to conduct is often difficult. Differentiating superficial wound infection from deep joint infection can be challenging in early presentations, whereas only subtle signs of infection can be present in late PJI, often leading to a delay in diagnosis.
Parvizi et al.9 released revised guidelines for the diagnosis of PJI in 2018. The scoring system uses major and minor criteria to confirm PJI in patients where infection is suspected. The new criteria have demonstrated a sensitivity of 97.7% and specificity of 99.5%.9 The criteria to take into consideration are illustrated in Figure 1.
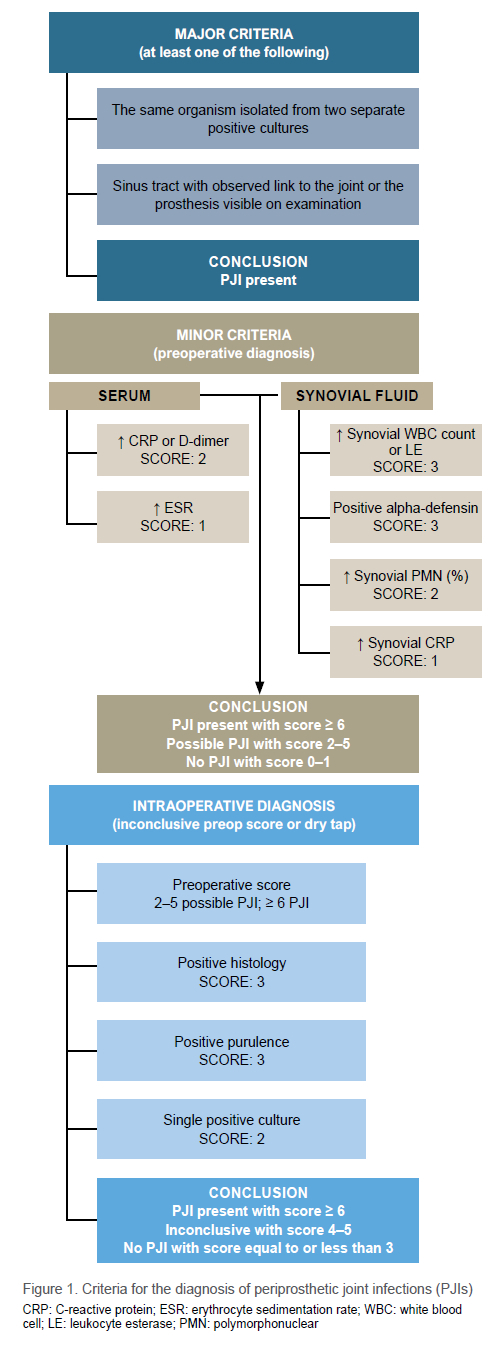
Once PJI is diagnosed, it can be classified as either early/acute postoperative, late postoperative/chronic or acute haematogenous. Various authors have suggested specific time cut-off values paired with these definitions. Tsukayama et al. propose four types of PJI: type I - positive intraoperative cultures; type II - early postoperative infection within four weeks of index surgery; type III - acute haematogenous infection that presents acutely after an asymptomatic period, with a suspected haematogenous origin; and type IV - late chronic infection presenting more than four weeks after the index procedure.10 Zimmerli et al. defined PJIs as early (within three months of surgery), delayed ( 3 to 24 months after surgery) and late (more than 24 months after surgery).11 In 2021, Tarity et al. defined chronicity as acute postoperative (less than six weeks after surgery), chronic (more than six weeks after surgery and more than six weeks of symptoms) and acute haematogenous (less than six weeks of symptoms in a previously well-functioning prosthesis, more than six weeks after surgery).12
Classification of PJI assists with decision making when considering DAIR, as acute postoperative and acute haematogenous infections are more likely to be successful than chronic infections.13
Treatment options
The treatment goals in PJIs are to eradicate infection and maintain pain-free joint function.3,14 Options include prolonged suppressive antibiotics, DAIR, one- and two-stage revisions, resection arthroplasty, arthrodesis and amputation. Chronic suppressive antibiotic therapy is a conservative approach to PJI, usually reserved for patients who are unfit for or refuse further surgical management, and has poor success rates.5,15,16 One- and two-stage revision arthroplasties show the highest success rates for PJI eradication but are paired with more significant patient burden, prolonged hospitalisation, soft tissue and bony defects, and higher costs.4,8,14,15,17 Resection arthroplasty can be considered in low-functioning, non-ambulatory patients with bony or soft tissue defects, those with resistant organism infections, and patients with failed two-stage revisions where antibiotic suppression and further implant intervention are unlikely to be successful.7 Arthrodesis and amputation may be considered as last resort options for patients due to the severe impairment of functionality and quality of life, and are reserved for patients where other surgical options have failed.15 DAIR as a treatment option will be discussed in detail in this article.
Rationale behind DAIR
Two-stage revision arthroplasty is considered the most effective procedure for infection eradication and prevention of infection relapse. Despite its success, it is not without challenges for both patient and surgeon. Two-stage revisions require two extensive surgical procedures, each placing significant strain on the patient, who may already be frail or systemically compromised due to the infection. Increased theatre time, blood loss, surgical difficulty and bone loss associated with implant removal and potential damage to surrounding soft tissues add to a procedure with significant morbidity, prolonged hospitalisation and costs to both patient and healthcare systems.3,14,18
When applied to selective patients, DAIR has shown itself as a cost-effective option in treating PJI while maintaining implants and the surrounding soft tissue envelope. DAIR is associated with an overall decreased surgical demand on both the surgeon and patient, reduced hospital stay and improved functional outcomes when successful. It is regarded as similar to primary arthroplasty in uninfected cohorts with better results compared to two-stage revisions.4,6,15,17,19-21
DAIR approach
Although DAIR is considered less invasive than two-stage revision procedures, it is not to be thought of as a simple washout, and it is suggested that a senior surgeon perform the surgery.4 Pre- and postoperative optimisation of the patient is required to minimise operative risks. DAIR is performed via the index surgery's approach in open procedures. Arthroscopic DAIR has been described and will be discussed later in the article. DAIR is a radical debridement of all potentially infected tissue from the skin to the prosthesis. Previous scar tissue, sinus tracts and inflammatory tissue superficial and deep to the fascia are excised. Debridement of the capsule, synovium and any sequestrum is required, with or without exchange of modular components. Three to Ave wound samples are collected for microbiological culture, and empirical antibiotic therapy is started until the antimicrobial susceptibility profile of the causative organism has been determined. Wound irrigation with normal saline or an antiseptic solution is advised and meticulous closure is undertaken with or without a suction drain. Postoperative antibiotics and infection monitoring are continued until a satisfactory clinical response is achieved.18,20-22
Success rates
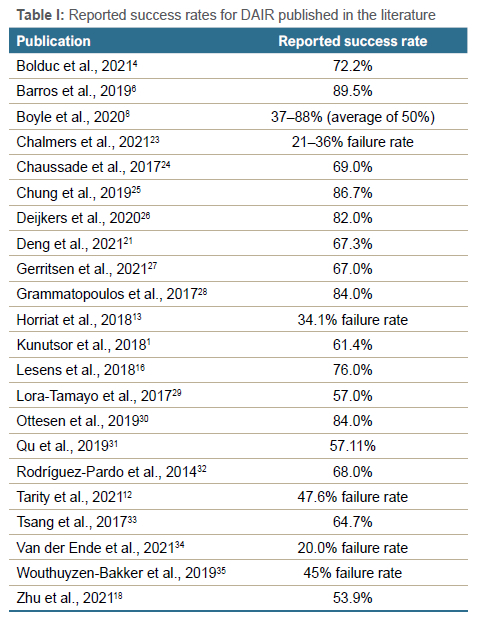
Varying success rates for DAIR have been reported, while several confounding variables are at play. Heterogeneity of cohorts, length of follow-up, inclusion and exclusion criteria, and definitions of success and failure are all factors adding to the overall rates of success reported.4 Table I summarises the success rates for DAIR reported in the literature, but whether a success rate of 50% should discourage DAIR is debatable, as it should be decided on a case-by-case basis whether the potential benefits for a successful DAIR outweigh the consequences of failure.19
Indications for DAIR
Debridement, antibiotics and implant retention is indicated in early postoperative and acute haematogenous infections with a stable, well-fixed prosthesis. Some sources regard early postoperative infections as occurring within one to three months of the index procedure29,36 and acute haematogenous infections with symptoms no longer than three to four weeks,3,19 while others suggest DAIR is a viable option in all cases of PJI with a well-fixed prosthesis, regardless of implant age,28 that is susceptible to anti-biofilm agents.35
Contraindications
The only absolute contraindication to performing DAIR is a loose implant. Despite this, the procedure is discouraged when the odds of failure outweigh the potential benefit.19 Therefore, DAIR is discouraged in chronic PJI, patients with poor soft tissue cover where wound closure problems are expected, and virulent infections where antibiotic susceptibility is uncertain.36
Current concepts in DAIR
Current concepts in DAIR are discussed under surgical and non-surgical factors. These factors play a role in evaluating the risk of success/failure with DAIR, and despite the varying opinions raised, a pattern of successful traits and practices can be identified in the literature.
Surgical factors
Timing
It is widely agreed in the literature that timing plays an essential role in determining the success of DAIR. Many attempts have been made to determine a time cut-off when DAIR is no longer a viable option. However, the decision should not be solely based on the implant or symptom duration but rather on implant stability, organism virulence, the patient's condition and soft tissue viability.37
Three factors need to be considered when assessing the rate of success in DAIR. These factors include the type of PJI, symptom duration and implant age or time from index surgery. Table II summarises the current literature. Acute postoperative PJIs have the best success rates for DAIR, followed by acute haematogenous infections. DAIR is often discouraged in late/chronic PJI due to high rates of failure.1,12-14
A shorter duration of symptoms has also shown more favourable outcomes following DAIR, with some articles advising DAIR in patients with symptoms less than one week,4,33 while others say outcomes remain favourable if DAIR is performed within four weeks of symptom onset.15,37 Despite varying time frames proposed, most authors agree that the sooner DAIR is performed after symptoms have developed, the greater the success rate can be expected to be. Nevertheless, DAIR remains a favourable option in patients with an average duration of symptoms of less than three weeks.7,16,19,31
The time from index surgery or implant age is a heavily debated topic, with some literature suggesting that DAIR should only be performed if the duration from the index surgery is less than one month,7 whereas others have suggested that this time frame could be extended to three months3,8,16,19,34 or even a year.18 It has been suggested that when the implant is well fixed and the PJI is caused by a less virulent organism in an otherwise viable joint, the implant age is not significant.4,33,37 Again, when summarising the literature, the highest success rates in DAIR are seen with an implant age of less than three months. Table II summarises timing recommendations by various authors cited in this review.
Urgency
DAIR is considered an urgent procedure but not an emergency. All efforts should be made to optimise the patient's general condition prior to surgery without significantly delaying the procedure.1,4,8,19,20
Number of surgeries
Consensus seems to have been achieved regarding the appropriateness of follow-up DAIR procedures, from the perspective that if an initial DAIR has failed, a second procedure is unlikely to improve the success rate.19 It has been shown in the literature that follow-up DAIR procedures have similar outcomes to the initial debridement, if not worse. The recommendation is that after one failed DAIR procedure, additional debridement procedures should be avoided and revision or resection arthroplasty considered.19,20,31 Of note is that previous researchers have published a 'double DAIR' protocol, where a planned two-stage DAIR procedure was performed on all patients, with a planned re-examination at Ave days to maximise infection control.25,38 This protocol relied heavily on the short-term use of high-dose antibiotic-loaded beads and modular component exchange, with success rates of 86.7% and an average follow-up of 41.8 months.25,38
Component exchange
The practice of exchanging all mobile components and liners is considered to theoretically improve infection eradication by two means; first, to improve the exposure of the joint to aid debridement of all areas where a potential infectious focus is present, especially posterior aspects of the knee;19 and second, replacing mobile parts to remove a potential site of bacterial adherence that cannot always be achieved by debridement and irrigation, limiting the bacterial load present in the joint.15 Despite some research showing that replacing mobile components does not relate to improved outcomes,27,30 a notable amount of literature reports significant improvements in implant survivability4,19,28 and infection eradication in both early and late PJI.18,28 Exchange of mobile components is an independent factor related to DAIR success.2,14,20,29,33,35 Besides cost implications, the exchange of mobile components has no adverse effects. Therefore, if possible, all mobile components should be exchanged.5
Irrigation
Irrigation with normal saline with or without the addition of an antiseptic solution is strongly recommended by most sources, and the volume recommended ranges between six and nine litres.4,15,19 The addition of antiseptic solutions such as diluted povidone-iodine, chlorhexidine, peroxide and antibiotics to irrigation fluid has been described, but the concentrations used and efficacy over standard irrigation are unclear.19 Pulsed lavage is practically convenient but has comparable efficacy to conventional irrigation.20
Direct antibiotics
Direct intra-articular antibiotic loading using catheters or pumps, antibiotic-loaded beads, sponges and cement spacers has been described, but insufficient evidence supports the routine application of these methods.4 Although antibiotic spacers can provide highdose local availability of a selected drug, it does not supply a consistent amount to remain therapeutic, and often concentrations fall to subtherapeutic levels within 72 hours, therefore reducing its efficacy.19
Sinus tract
A sinus tract is a pathognomonic feature of a PJI,37 and many authors suggest that its presence is an independent risk for DAIR failure,20 or DAIR is contraindicated in patients with a draining sinus.14,15 One source has reported no difference in outcome between patients with or without the presence of a sinus tract, and that meticulous debridement and component exchange improved infection control in these patients.21 There is no consensus to suggest that a sinus tract is an absolute contraindication to DAIR.
Drain
Wound drains left in situ postoperatively are recommended in DAIR to prevent fluid accumulation and decrease potential dead space.4,14 Some authors suggest high negative pressure drains, reporting improved outcomes, but they need to be used selectively due to increased hospital stay, costs and impaired mobility.15
Stability
The radical nature of soft tissue debridement in DAIR can contribute to postoperative instability. A thorough evaluation of joint stability intraoperatively is required, and when modular components are exchanged, more constrained components can be substituted to mitigate such issues. Postoperative precautions, including patient education, splinting and structured physiotherapy, can also be implemented.28
Arthroscopic DAIR
Arthroscopic DAIR without component exchange has been described, but many authors discourage arthroscopic DAIR, identifying it as a risk for failure with lower infection control rates.2,4,20,31 Part of the rationale is that the arthroscopic approach does not allow for sufficient debridement or replacement of mobile components.14,15
DAIR in uni- and mega-prosthesis
The use of DAIR is supported in both uni- and mega-prosthesis patients with PJI. The unique feature of PJI in a uni-knee arthroplasty is that both prosthesis and native cartilage are present, and in the event of failure, conversion to a total knee arthroplasty is required. Mega-prosthesis PJI is often a complex problem, and as revision options are usually limited, DAIR remains a viable initial approach.19
DAIR in revision arthroplasty
Debridement, antibiotics and implant retention should be considered carefully in PJIs with prior revisions. Poorer outcomes have been reported and revision surgery has been identified as an independent risk factor for failure in DAIR, with failure rates of 12-22% higher than in primary joint infections.4,15
DAIR effect on salvage two-stage revision
Despite its high rate of failure, the debate remains on whether DAIR is a viable option for patients with PJI. A salvage two-stage exchange is often warranted when DAIR fails and opinions in the literature are still divided. Some authors suggest no difference in functional outcomes between patients undergoing a direct two-stage procedure versus those having first undergone DAIR, then a subsequent salvage two-stage procedure,3,19 leaving DAIR a promising initial option in managing PJI. Inferior outcomes of salvage two-stage procedures have also been reported, but more data are required to make a definitive decision.14
Non-surgical factors
Organism involved
Identification of the organism involved in PJI is not only valuable to guide antimicrobial therapy but may determine success rates in DAIR. Staphylococcus aureus is the most common organism responsible and represents 27% of all PJIs.16 S. aureus has been associated with early and late PJI and is recognised as an independent risk factor for DAIR failure, independent of infection chronicity.12,14 Infections with methicillin-resistant S. aureus (MRSA) bears the worst prognosis, with success rates reported as low as 30%. Some authors discourage DAIR in cases of MRSA infection.4,14,19,24 Following MRSA, enterococcal and fungal infections also carry a significant risk for DAIR failure due to its high virulence and frequent presentation with early treatment failure.3,20 Mixed cultures or polymicrobial infections pose a moderate risk for failure.20 Coagulase-negative staphylococcal (CoNS) infections are associated with late PJI and due to their non-invasive nature and low virulence, present with a good overall prognosis.14 Streptococci and fluoroquinolone-sensitive Gram-negative bacteria present the best prognosis in PJIs managed with DAIR, but the success rates decrease significantly once antibiotic-resistant Gram-negative bacteria are cultured.1,32 When attempting DAIR in patients with MRSA, resistant Gram-negative and fungal infections, careful consideration of the potential risks should be taken into account when deciding whether to perform the procedure.
Organism identification prior to DAIR
Laboratory-based identification of the causative organism in PJI is a valuable tool to determine if DAIR is an appropriate treatment modality and estimate the probability of success, especially when highly virulent organisms, e.g. MRSA, are responsible for the infection.20 Recommendations are to attempt identification of the organism prior to DAIR, as long as it does not delay definitive treatment.19 When requesting an urgent investigation, commonly used basic laboratory tests and microscopy can assist with a preliminary indication of the causative organism within a reasonably short period of time.
Choice of antimicrobial treatment
Microbial culture and sensitivity results are the basis on which antibiotic therapy in DAIR is based and should be tailored according to the causative organism's antimicrobial susceptibility profile as soon as possible.2 Knowledge of the organism preoperatively limits the transition time between empiric and organism-specific regimens.35 For staphylococcal infections, the addition of rifampicin to the treatment regimen is associated with higher success rates, especially in combination with fluoroquinolones.19,20,28 Studies have shown that a fluoroquinolone and rifampicin combination decreases failure rates by up to 20% in staphylococcal infections.16,35 Fluoroquinolones are also associated with higher success rates in Gram-negative infections.3,19 Beta-lactam antibiotics are the preferred treatment for streptococcal infections.29 In polymicrobial infections, a combination regimen directed against all cultured organisms is used.28
Antimicrobial duration
Three schools of thought prevail in the literature regarding the duration of antibiotic therapy in DAIR, which include chronic suppressive antibiotics, intermediate course antimicrobial therapy and short-course antimicrobial therapy. After a DAIR procedure, chronic suppressive antibiotic therapy remains an appropriate option to prevent infection remission. However, treatment intolerances, potential resistance, and poor prognoses limit chronic suppression as an option for patients who either decline further surgery or are not medically fit to undergo revision surgery.4,14,19
Intermediate course antimicrobial therapy has long been accepted as the ideal duration of therapy. Many authors still recommend this approach which involves an initial course of intravenous therapy for two to six weeks, followed by three to six months of oral antibiotics.4,7,16,20
Newer literature indicates that the duration of treatment does not influence the outcome of DAIR, with long-term treatment failing to show improved outcomes over short treatment schedules. Furthermore, extended duration of antimicrobial therapy masks infective symptoms and postpones the diagnosis of treatment failure.1,15,19 Newer recommendations advise a minimum of six weeks of antimicrobial therapy, with six to eight weeks of therapy being sufficient following the performance of DAIR. An initial course of intravenous therapy and conversion to appropriate oral therapy are proposed if allowed by the causative organism's susceptibility profile.1,19,35,39
Implant factors
Regarding implant indications, DAIR performed for PJI in fracture arthroplasty showed a 20-30% increased failure risk compared to PJI in primary joint arthroplasty.4,19 Along with this trend, DAIR for revision arthroplasties also showed a 12-22% increased risk of failure, with revision arthroplasty being identified as an independent risk factor for DAIR failure.19,20 No consensus has been reached over cemented versus uncemented implants, with some sources citing cemented implants as having a greater failure risk.19,34 Hip arthroplasty shows higher success rates for DAIR over knee arthroplasty,1,20 but shoulder arthroplasty shows a DAIR success rate of 75%, in keeping with hip and knee cohorts.20
Patient factors
Despite optimal surgical and microbial conditions, the general condition of the host also plays a role in the success of DAIR. All efforts should be made to optimise host status prior to treatment. Reversible conditions such as anaemia (haemoglobin < 10 mg/dL), malnutrition, coagulopathy and tobacco use should be addressed before attempting DAIR without causing a significant delay between presentation and treatment.19 Chronic conditions such as rheumatoid arthritis, liver cirrhosis, renal failure, COPD, diabetes mellitus and active malignancy should be considered prior to DAIR, as these conditions significantly increase failure rates, and alternative revision options may be more appropriate.16,19,31 Patient factors such as advanced age (> 80 years), male sex, obesity (body mass index [BMI] > 30 kg/m2) and compromised immune status secondary to disease or steroid use have also been identified as risk factors for DAIR failure.15,19,26,34 Lastly, a high American Society of Anaesthesiology (ASA) score, elevated ESR (> 40 mm/h) and elevated C-reactive protein (CRP); (> 65 mg/L) on presentation are also predictors of failure.2,15,18,26,35 A multidisciplinary team, ideally including specialist nurses, therapists, infectious disease physicians and plastic surgeons in addition to the orthopaedic team, is recommended to treat the PJI, optimise any associated comorbidities, manage reversible conditions, exclude any concurrent extrinsic infections and assist with postoperative rehabilitation.4
Staging tools
Once surgical, microbial, implant and patient factors have been considered, the difficulty remains to decide whether DAIR is an appropriate treatment option and whether it will be successful. No system is available to take all these factors into account and most cases have to be assessed individually. However, staging tools are available to assist in decision making and the use of a scoring system is associated with improved outcomes.20
As summarised in Table III, the KLIC score (kidney, liver, index surgery, cemented prosthesis and CRP value), ranging from 0 to 9.5, was developed to predict DAIR failure in acute postoperative infections.4,36 Taking negative prognostic factors such as chronic renal failure, liver cirrhosis, fracture or revision index surgery, cemented prosthesis and an elevated CRP (> 115 mg/L) into consideration, a score of 4 already suggests a success rate of less than 45%, which can aid in determining an alternative procedure for the patient.4,19,20,36 Researchers have attempted to validate the KLIC scoring system and found that it shows reasonable sensitivity and specificity at higher scores (> 3.5) and facilitates the identification of high-risk patients, but its value at lower scores is uncertain.3,4,23,36
The CRIME80 score (based on the presence of specific predictive factors, namely COPD and an elevated CRP level of > 150 mg/L, rheumatoid arthritis, fracture-related indication for surgery, male sex, exchange of mobile components and more than 80 years of age) was developed as a prognostic tool for acute haematogenous infections.4 Out of 12 points, a score of 3 or more is associated with a less than 40% success rate. The only positive predictive factor was the exchange of mobile components.19,20,35 The CRIME80 score showed an acceptable predictive value for DAIR failure on external validation.23
Conclusion
Debridement, antibiotics and implant retention (DAIR) is a feasible treatment option in acute postoperative and acute haematogenous periprosthetic joint infections in healthy hosts with a well-fixed prosthesis. Despite lower infection eradication rates compared to one- and two-stage revisions, DAIR provides a low-cost option with good functional outcomes and decreased surgical burden, limiting morbidity and mortality. With improvements in surgical techniques, antimicrobial therapy and a multidisciplinary approach, success rates for DAIR have been increasing with time and could continue to improve. Any patient presenting with an acute PJI who has a well-fixed prosthesis, culturing a low-virulence organism with adequate soft-tissue coverage and no significant comorbidities is eligible for DAIR with a mobile component exchange. As it is a far less invasive procedure and does not preclude revision surgery, an ideal treatment algorithm would primarily include DAIR, followed by a staged revision in unsuccessful cases.
Acknowledgement
Dr Daleen Struwig, medical writer/editor, Faculty of Health Sciences, University of the Free State, for technical and editorial preparation of the manuscript.
Ethics statement
The authors declare that this submission is in accordance with the principles laid down by the Responsible Research Publication Position Statements as developed at the 2nd World Conference on Research Integrity in Singapore, 2010.
Declaration
The authors declare authorship of this article and that they have followed sound scientific research practice. This research is original and does not transgress plagiarism policies.
Author contributions
NRB: contributed to the conception and design of the work, literature review and analysis, drafting of the manuscript, and final approval of the version to be published JFvdM: contributed to the conception and design of the work, revising it critically for important intellectual content, and final approval of the version to be published SM: contributed to the conception and design of the work, revising it critically for important intellectual content, and final approval of the version to be published
ORCID
Blair NR https://orcid.org/0000-0002-7468-9969
Matshidza S https://orcid.org/0000-0003-0128-0385
References
1. Kunutsor SK, Beswick AD, Whitehouse MR, et al. Debridement, antibiotics and implant retention for periprosthetic joint infections: a systematic review and meta-analysis of treatment outcomes. J Infect. 2018;77(6):479-88. [ Links ]
2. Kuiper JW, Willink RT, Moojen DJF, et al. Treatment of acute periprosthetic infections with prosthesis retention: Review of current concepts. World J Orthop. 2014;5(5):667-76. [ Links ]
3. Lora-Tamayo J, Mancheño-Losa M, Lumbreras C. To DAIR or not to DAIR: decision-making in the management of acute prosthetic joint infection - a narrative review. Span J Med. 2021;1(2):119-31. [ Links ]
4. Bolduc M, Fischman D, Kendrick B, et al. Contemporary outcomes of debridement, antibiotics and implant retention (DAIR) in hip arthroplasty. Ann Joint. 2021;6:34. https://doi.org/10.21037/aoj-20-87. [ Links ]
5. Bezel P, Fucentese S, Burkhard J, et al. Mini review on the impact of mobile parts' exchange during the DAIR procedure (debridement, antibiotics, irrigation, retention) for infected total joint arthroplasties. J Infectiol. 2019;2(4):13-17. [ Links ]
6. Barros LH, Barbosa TA, Esteves J, et al. Early debridement, antibiotics and implant retention (DAIR) in patients with suspected acute infection after hip or knee arthroplasty - safe, effective and without negative functional impact. J Bone Joint Infect. 2019;4(6):300-305. [ Links ]
7. Osmon DR, Berbari EF, Berendt AR, et al. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2013;56(1):e1-e25. [ Links ]
8. Boyle KK, Kapadia M, Landy DC, et al. Utilization of debridement, antibiotics, and implant retention for infection after total joint arthroplasty over a decade in the United States. J Arthroplasty. 2020;35(8):2210-16. [ Links ]
9. Parvizi J, Tan TL, Goswami K, et al. The 2018 definition of periprosthetic hip and knee infection: an evidence-based and validated criteria. J Arthroplasty. 2018;33(5):1309-14. [ Links ]
10. Tsukayama DT, Estrada R, Gustilo RB. Infection after total hip arthroplasty. A study of the treatment of one hundred and six infections. J Bone Joint Surg Am. 1996;78(4):512-23. [ Links ]
11. Zimmerli W, Trampuz A, Ochsner PE. Prosthetic jointinfections. N Engl J Med. 2004;351(16):1645-54. [ Links ]
12. Tarity TD, Gkiatas I, Nocon AA, et al. Irrigation and debridement with implant retention: does chronicity of symptoms matter? J Arthroplasty. 2021;36(11):3741-49. [ Links ]
13. Horriat S, Ayyad S, Thakrar RR, Haddad FS. Debridement, antibiotics and implant retention in management of infected total knee arthroplasty: a systematic review. Semin Arthroplasty. 2018;29(3):244-49. [ Links ]
14. Qasim SN, Swann A, Ashford R. The DAIR (debridement, antibiotics and implant retention) procedure for infected total knee replacement - a literature review. SICOT J. 2017;3:2. [ Links ]
15. Xu Y, Wang L, Xu W. Risk factors affect success rate of debridement, antibiotics and implant retention (DAIR) in periprosthetic joint infection. Arthroplasty. 2020;2(1):4-9. [ Links ]
16. Lesens O, Ferry T, Forestier E, et al. Should we expand the indications for the DAIR (debridement, antibiotic therapy, and implant retention) procedure for Staphylococcus aureus prosthetic joint infections? A multicenter retrospective study. Eur J Clin Microbiol Infect Dis. 2018;37(10):1949-56. [ Links ]
17. Fisman DN, Reilly DT, Karchmer AW, Goldie SJ. Clinical effectiveness and cost-effectiveness of 2 management strategies for infected total hip arthroplasty in the elderly. Clin Infect Dis. 2001;32(3):419-30. [ Links ]
18. Zhu MF, Kim K, Cavadino A, et al. Success rates of debridement, antibiotics, and implant retention in 230 infected total knee arthroplasties: implications for classification of periprosthetic joint infection. J Arthroplasty. 2021;36(1):305-10. [ Links ]
19. Argenson JN, Arndt M, Babis G, et al. Hip and knee section, treatment, debridement and retention of implant: Proceedings of International Consensus on Orthopedic Infections. J Arthroplasty. 2019;34(2S):S399-S419. [ Links ]
20. Boyer B, Cazorla C. Methods and probability of success after early revision of prosthetic joint infections with debridement, antibiotics and implant retention. Orthop Traumatol Surg Res. 2021;107(1S):102774. [ Links ]
21. Deng W, Li R, Shao H, et al. Comparison of the success rate after debridement, antibiotics and implant retention (DAIR) for periprosthetic joint infection among patients with or without a sinus tract. BMC Musculoskelet Dis. 2021;22(1):895. [ Links ]
22. Zhang C, He L, Fang X, et al. Debridement, antibiotics, and implant retention for acute periprosthetic joint infection. Orthop Surg. 2020;12(2):463-70. [ Links ]
23. Chalmers BP, Kapadia M, Chiu YF, et al. Accuracy of predictive algorithms in total hip and knee arthroplasty acute periprosthetic joint infections treated with debridement, antibiotics, and implant retention (DAIR). J Arthroplasty. 2021;36(7):2558-66. [ Links ]
24. Chaussade H, Uçkay I, Vuagnat A, et al. Antibiotic therapy duration for prosthetic joint infections treated by debridement and implant retention (DAIR): similar long-term remission for 6 weeks as compared to 12 weeks. Int J Infect Dis. 2017;63:37-42. [ Links ]
25. Chung AS, Niesen MC, Graber TJ, et al. Two-stage debridement with prosthesis retention for acute periprosthetic joint infections. J Arthroplasty. 2019;34(6):1207-13. [ Links ]
26. Deijkers RL, Elzakker, Pijls BG. Debridement, antibiotics, and implant retention with the direct anterior approach for acute periprosthetic joint infection following primary THA. J Bone Joint Surg Open Access. 2020;5(2):e0062. [ Links ]
27. Gerritsen M, Khawar A, Scheper H, et al. Modular component exchange and outcome of DAIR for hip and knee periprosthetic joint infection. Bone Joint Open. 2021;2(10):806-12. [ Links ]
28. Grammatopoulos G, Kendrick B, McNally M, et al. Outcome following debridement, antibiotics, and implant retention in hip periprosthetic joint infection - an 18-year experience. J Arthroplasty. 2017;32(7):2248-55. [ Links ]
29. Lora-Tamayo J, Senneville É, Ribera A, et al. The not-so-good prognosis of streptococcal periprosthetic joint infection managed by implant retention: the results of a large multicenter study. Clin Infect Dis. 2017;64(12):1742-52. [ Links ]
30. Ottesen CS, Troelsen A, Sandholdt H, et al. Acceptable success rate in patients with periprosthetic knee joint infection treated with debridement, antibiotics, and implant retention. J Arthroplasty. 2019;34(2):365-68. [ Links ]
31. Qu GX, Zhang CH, Yan SG, Cai XZ. Debridement, antibiotics, and implant retention for periprosthetic knee infections: a pooling analysis of 1266 cases. J Orthop Surg Res.2019;14(1):358. [ Links ]
32. Rodríguez-Pardo D, Pigrau C, Lora-Tamayo J, et al. Gram-negative prosthetic joint infection: outcome of a debridement, antibiotics and implant retention approach. A large multicentre study. Clin Microbiol Infect. 2014;20(11):O911-9. [ Links ]
33. Tsang STJ, Ting J, Simpson AHRW, Gaston P. Outcomes following debridement, antibiotics and implant retention in the management of periprosthetic infections of the hip: a review of cohort studies. Bone Joint J. 2017;99-B(11):1458-66. [ Links ]
34. Van der Ende B, Van Oldenrijk J, Reijman M, et al. Timing of debridement, antibiotics, and implant retention (DAIR) for early post-surgical hip and knee prosthetic joint infection (PJI) does not affect 1-year re-revision rates: data from the Dutch Arthroplasty Register. J Bone Joint Infect. 2021;6(8):329-36. [ Links ]
35. Wouthuyzen-Bakker M, Sebillotte M, Lomas J, et al. Clinical outcome and risk factors for failure in late acute prosthetic joint infections treated with debridement and implant retention. J Infect. 2019;78(1):40-47. [ Links ]
36. Duffy SD, Ahearn N, Darley ES, et al. Analysis of the KLIC-score; an outcome predictor tool for prosthetic joint infections treated with debridement, antibiotics and implant retention. J Bone Joint Infect. 2018;3(3):150-55. [ Links ]
37. Elkins JM, Kates S, Lange J, et al. General assembly, diagnosis, definitions: Proceedings of International Consensus on Orthopedic Infections. J Arthroplasty. 2019;34(2S):S181-5. [ Links ]
38. McQuivey KD, Bingham J, Chung A, et al. The double DAIR: a 2-stage debridement with prosthesis-retention protocol for acute periprosthetic joint infections. JBJS Essent Surg Tech. 2021;11(1):e19.00071. [ Links ]
39. Lora-Tamayo J, Euba G, Cobo J, et al. Short- versus long-duration levofloxacin plus rifampicin for acute staphylococcal prosthetic joint infection managed with implant retention: a randomised clinical trial. Int J Antimicrob Agents. 2016;48(3):310-16. [ Links ]
Received: May 2022
Accepted: October 2022
Published: November 2022
Editor: Dr Jurek Pietzrak, University of the Witwatersrand, Johannesburg, South Africa
Funding: No funding was received for this study.
Conflict of interest: The authors declare they have no conflicts of interest that are directly or indirectly related to the research.
* Corresponding author: neillrblair@gmail.com














