Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
SA Orthopaedic Journal
On-line version ISSN 2309-8309
Print version ISSN 1681-150X
SA orthop. j. vol.20 n.4 Centurion 2021
http://dx.doi.org/10.17159/2309-8309/2021/v20n4a2
ARTHROPLASTY
Preoperative asymptomatic bacteriuria in patients undergoing total joint arthroplasty in South Africa
Zia Maharaj*; Tristan Pillay; Lipalo Mokete; Jurek RT Pietrzak
Arthroplasty Unit, Division of Orthopaedic Surgery, Charlotte Maxeke Johannesburg Academic Hospital, University of the Witwatersrand, Johannesburg, South Africa
ABSTRACT
BACKGROUND: Periprosthetic joint infections (PJIs) are a leading cause of revision for total hip arthroplasty (THA) and total knee arthroplasty (TKA), worldwide. Asymptomatic bacteriuria (ASB) is an independent risk factor for PJIs; however, a paucity of data relevant to developing countries exists. The aim of this study was to describe the prevalence of preoperative ASB and the subsequent incidence of PJIs in patients undergoing total joint arthroplasty (TJA) in South Africa.
METHODS: We retrospectively reviewed primary THA and TKA patients. All patients were screened for ASB preoperatively. Patients with positive urinalysis for ASB were identified and treated prior to surgery (treated-ASB). The primary outcome was ASB prevalence and the incidence of PJIs and other perioperative complications. Secondary outcomes included risk factors for ASB and subsequent PJIs in treated-ASB patients, respectively, compared to those with no evidence of ASB (non-ASB). Lastly, we aimed to compare the infective microorganisms cultured from preoperative urinalysis and perioperative sampling of PJIs.
RESULTS: We included 179 patients (67 THA; 80% female) with mean follow-up of 2.45 years. ASB prevalence was 22% (n = 39). Patients older than 70 years were 3.5 times more likely to have ASB compared to younger patients (p = 0.005). The prevalence of ASB was 22% (n = 10) for human immunodeficiency virus (HIV) positive and 22% (n = 29) for HIV-negative patients (p = 0.084). PJI incidence was 8% (n = 3) in the treated-ASB and 1% (n = 1) in non-ASB. Treated-ASB patients had an 11.6-fold increased likelihood of PJIs than non-ASB patients (p = 0.046). pJi microorganisms cultured did not correlate to isolates from urine cultures.
CONCLUSION: The prevalence of ASB in a TJA population in South Africa is 22% which is higher than reported findings worldwide. Although the value of preoperative antibiotic therapy for ASB remains controversial, there is a role for routine urinalysis preoperatively to identify patients predisposed to PJI. This is of specific significance in the management of HIV-positive patients and in developing countries to guide healthcare providers in resource-constrained environments.
Level of evidence: Level 2.
Keywords: total hip arthroplasty, total knee arthroplasty, asymptomatic bacteriuria, periprosthetic joint infection, developing country
Introduction
A significant demand for total joint arthroplasty (TJA) exists, with over 1 million procedures performed in the United States of America (USA) annually alone.1 The average rate of total hip arthroplasty (THA) has increased by approximately 30%, while the performance of total knee arthroplasty (TKA) procedures doubled globally between 2000 and 2015.2 Demand for TJA continues to rise and is projected to continue increasing through 2030.3 The increasing demand for TJA translates into a massive economic burden for global healthcare systems further compounded by postoperative readmissions for complications such as periprosthetic joint infections (PJIs).4 PJIs are infective postoperative complications ranging from surgical site infections (SSIs) to deep intra-articular infections. PJIs are the third most common cause of THA revisions and the most common cause of TKA revisions, globally.5 The annual cost for revisions due to PJI is expected to increase to US$1.62 billion by 2020 and has a five-year mortality rate of 21.12% after primary TJA.5 The serious implications of PJI have led to increased efforts to limit infections by the strict adherence to antibiotic prophylaxis, laminar airflow systems in operating theatres, extensive patient perioperative clinical optimisation and stringent sterilisation protocols.6 The incidence of PJI after primary TJA, however, remains at 1.4% rates despite the implementation of preventative measures.7
Asymptomatic bacteriuria (ASB) from the genitourinary tract may be a source of infection for PJI through haematogenous seeding.8,9 A multicentre study including institutions from the United Kingdom (UK), Portugal and Spain identified ASB as an independent risk factor for PJI (p = 0.001), especially those due to Gram-negative microorganisms.8 Similarly, a systematic review and meta-analysis of ten TJA studies showed an increased risk for PJI with ASB (odds ratio [OR]:3.64; 95% confidence interval (CI) 1.40-9.42).9 However, Sousa et al. (2019) reported that the PJI microorganisms were unrelated to those in the urine of the patients with ASB.9 Furthermore, there is evidence demonstrating an association between postoperative urinary tract infections (UTI) and PJI.10-12 Identifying underlying characteristics, especially modifiable risk factors which predispose TJA patients to infection, is essential to mitigate the risk of adverse outcomes.
There is no international consensus guideline for the screening and management of ASB in patients for TJA. The British Orthopaedic Association recommends routine urinalysis for all TJA patients preoperatively; however, they are not specific on management of positive results.13 The Spanish Society of Infectious Diseases and Clinical Microbiology recommends treating ASB while the Antibiotic Therapeutic Guidelines for Australia do not support treatment of ASB preoperatively.14,15 A meta-analysis by Sousa et al. (2019) concluded that preoperative antibiotic treatment for ASB does not influence PJI risk and should not be implemented routinely.9 However, no studies have been reported for an African population, particularly concerning demographic risk factors such as human immunodeficiency virus (HIV) and body mass index (BMI). Furthermore, there is a paucity of data relating to the association between ASB and SSI. There are also economic implications of increased costs for routine screening in less-developed countries that must be considered.
The aim of this study was to assess the prevalence of ASB in patients for primary TJA in South Africa. Secondarily, we sought to determine the incidence of PJIs in TJA patients with no preoperative ASB (non-ASB) compared to those who received TJA after the treatment of ASB (treated-ASB). Lastly, we evaluated risk factors for both ASB and subsequent PJIs in this TJA population. We hypothesise that the high prevalence rates of HIV in South Africa would predispose this sample population to high rates of ASB and subsequent infective complications.
Materials and methods
We conducted a retrospective review of prospectively collected data for patients undergoing primary TJA at an academic referral institution in Johannesburg, South Africa. The study was conducted between January 2015 and December 2016. Patients included in the study were adults aged 18 years or older, undergoing primary, elective THA or TKA and who provided consent for voluntary participation in the study. Exclusion criteria included revision THA, revision TKA and patients for primary TJA who did not provide consent. All patients eligible for study inclusion were screened for evidence of symptomatic UTI. Symptoms of UTI that were assessed included a history of urinary frequency or urgency, foul-smelling urine, abnormal colour of urine, dysuria or burning on micturition and a sensation of incomplete bladder emptying.16 Patients with symptoms of UTI were excluded from the sample population and received appropriate treatment prior to their elective operations.
Demographic data was recorded for all study participants, including age, sex, BMI and tobacco use. Medical comorbidities documented included diabetes mellitus, hypertension and HIV status, and the American Society of Anesthesiologists Classification (ASA class) was noted. All patients provided a mid-stream urine sample that was sent for microscopy, culture and sensitivity (MC&S) by the National Health Laboratory Services (NHLS) of South Africa three days prior to surgery. The urinary specimen was considered positive for bacterial isolation if > 100 000 colony-forming units/ml and antibiotic sensitivity was identified. Patients with evidence of ASB on urinalysis had their operation postponed and were treated for five days with an oral antibiotic according to microorganism sensitivity. Urine MC&S was subsequently performed, and patients received TJA only once their urine sample was sterile.
All patients underwent TJA by the same three fellowship-trained arthroplasty surgeons in a laminar-flow surgical theatre. Both THA and TKA procedures were performed under general anaesthesia (GA). All patients received both tranexamic acid (TXA) and a weight-adjusted prophylactic dose of first-generation cephalosporin intravenously at least 30 minutes before the first surgical incision. Clindamycin was given preferentially in penicillin-allergic patients. Prophylactic antibiotics were continued for 24 hours postoperatively.
All THA procedures were performed using a modified antero-lateral surgical approach, and an uncemented Pinnacle acetabular shell and uncemented Corail femoral stem (DePuy Synthes, Midrand, South Africa) in all cases. All TKA were performed by a medial parapatellar surgical approach after a midline skin incision. A cruciate-sacrificing fixed-bearing cemented TKA, using Genesis II (Smith and Nephew, Durban, South Africa) TKA implants was used in all cases. All components inserted in all TKA cases were cemented using Palacos® R + G antibiotic-loaded cement (Hereaus Group, Hanau, Germany). A drain was used and was removed within 48 hours of surgery. Physiotherapy was initiated the day after surgery and patients were discharged once they were able to mobilise independently and negotiate steps with two crutches. Postoperatively all patients were routinely followed up 14 days after surgery for wound assessment. Subsequent follow-up assessments were at three months, six months and one year after TJA and annually thereafter. All patients' records were assessed for readmission rates and implant failures.
Retrospective review of preoperative and intraoperative patient data was conducted, and all postoperative complications were noted. All infective complications across the spectrum, from wound dehiscence and SSI to deep intra-articular PJI, were investigated to confirm the diagnosis of PJI definitively. The diagnosis of PJI was determined according to the modified criteria proposed by the Musculoskeletal Infection Society (MSIS) in 2014.17 Subsequent advances in the field of PJI diagnosis led to the development of new evidence-based criteria that has demonstrated a higher sensitivity of 97.7% compared to the older MSIS definition (79.3%).17 The retrospective design of our study allowed for the diagnosis of PJI to be confirmed on both the traditional MSIS criteria and the current evidence-based criteria defined by Parvizi et al. (2018).17
All cases suspected of infective complications, including prolonged wound drainage (> 72 hours), deep PJI and infected wound dehiscence, were treated surgically in the same laminar-flow theatre as per treating unit protocol by the surgeon who had performed index primary TJA. At least Ave tissue samples were taken surgically from separate sites. Specimens were hand-delivered immediately postoperatively to the same laboratory for extended MC&S and fungal culture.
For statistical analysis, the patients were divided into two groups: patients who received treatment preoperatively for ASB (treated-ASB) and those without ASB (non-ASB). Bootstrapped statistics with 1 000 samples was performed. Two-sided tests were conducted for sex, age, BMI and comorbidities using chi-squared testing with continuity correction. Odds proportions were used to calculate risk ratio (RR) for an outcome of interest, and all pairwise comparisons were calculated using the Bonferroni correction. Two-sided statistical significance was p < 0.05 and confidence interval of 95% (95% CI) with respective standard error (SE) was determined. All statistical analyses were performed using STATA (version 14) statistical package.
Results
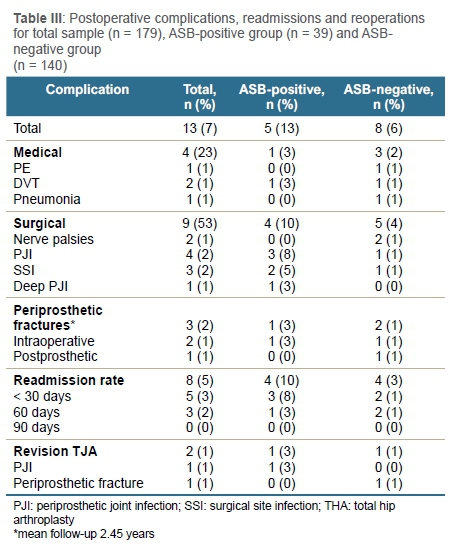
There were 179 patients, including 67 that underwent THA and 112 TKA respectively. All patients (100%) were evaluated at a mean follow-up of 2.45 years. Demographic details of the sample population are shown in Table I. The prevalence of ASB for our TJA sample was 22% (SE 3.1; 95% CI 15.7-27.9).
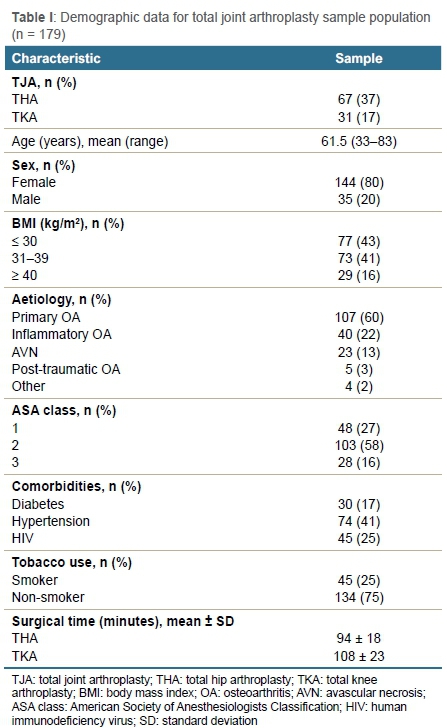
Prevalence of ASB according to demographics and comorbidities is depicted in Table II. Females were 3.6 times more likely to have ASB than males (p = 0.060). The prevalence of ASB according to age was 42% (n = 15) for patients 70 years or older, 18% (n = 20) for those 51-69 years of age and 13% (n = 4) for those 50 years or younger, respectively (p = 0.005). Patients 70 years or older are 3.5 times more likely to have ASB compared to patients younger than 70 (odds ASB positive: > 70 years = 0.71; < 70 years = 0.2). Patients with an ASA class of 3 were 4.6 times more likely to have ASB compared to patients with ASA class 1 (odds ASB positive: ASA 1 = 0.12; ASA 3 = 0.54; p = 0.026). There were seven patients (16%) who used tobacco and 32 patients (24%) who did not use tobacco that presented with ASB, respectively (p = 0.046). The prevalence of ASB was 22% (n = 10) for HIV-positive patients and 22% (n = 29) for HIV-negative patients (p = 0.084).
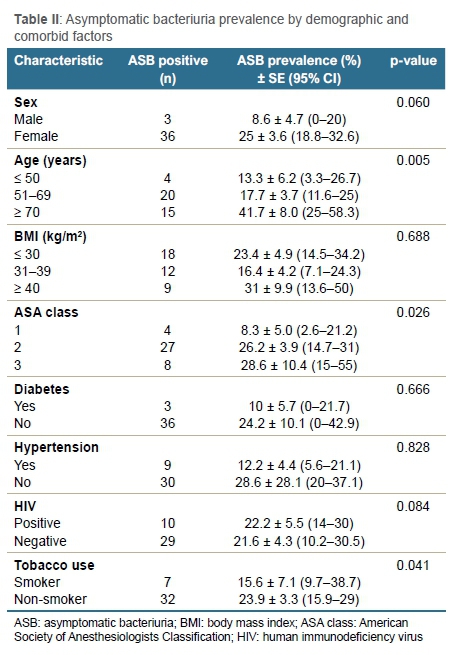
There were 13 (7%) perioperative complications, eight (5%) readmissions and two (1%) revisions at a follow-up of 2.45 years, respectively (Table III). There were two (5%) non-infective complications in the treated-ASB group (95% CI 0-12.8) and seven (5%) in the non-ASB group (95% CI 2.1-9.3), respectively. There were four (2%) infective complications including three (2%) SSIs and one (1%) deep PJI. The microorganisms cultured from urinalysis in patients with ASB (Figure 1) were different from the microorganisms cultured from postoperative infections in treated-ASB patients (Table IV). There were three (8%) PJIs in the treated-ASB group (95% CI 0-17.9) and one (1%) in the non-ASB group (95% CI 0-2.1), respectively. Patients with ASB were 11.6 times more likely to have wound complications than non-ASB patients (p = 0.046).


Discussion
The prevalence of preoperative ASB in patients undergoing TJA in a single referral institution in South Africa was 22% (n = 39). This is higher than reports for similar populations in other countries. Studies indicate that the prevalence of ASB for TJA patients in Spain varies between 5.1 and 18.2%.9,18,19 Similarly the ASB prevalence rates for the UK range from 3.2% to 12.1%,6,8 while Finland and Portugal have reported ASB prevalence of 6.8% and 11.2% respectively for TJA patients.8,20
Patients with preoperative ASB have been reported to be at increased risk for PJI.6,8,9 In our study we found an overall incidence of 2% (n = 4) for postoperative infections, i.e., SSI and PJI after a mean follow-up of 2.45 years. Despite being treated preoperatively, patients with ASB were 11.6 times more likely to have wound complications than non-ASB patients. A similar study of 4 368 patients reported a preoperative ASB prevalence of 3.2%, and all patients received appropriate antibiotic treatment prior to TJA.6 Weale et al. found a significantly higher rate of PJI in the ASB group (4.3%) compared to the non-ASB group (1.4%), respectively (p = 0.001).6 Furthermore, patients with ASB who were not treated preoperatively have been associated with a greater risk of PJI than patients who received antibiotic treatment. In a study of 20 226 TJA patients, Honkanen et al. reported a PJI incidence of 0.3% in patients treated for ASB compared to 0.6% for a control group who were not given treatment.20 In a multicentre study, Sousa et al. reported the respective incidence of PJI as 3.9% and 4.7% for ASB patients who received treatment and did not receive treatment preoperatively.8 No international consensus exists to determine whether ASB should be treated preoperatively despite the association with infective complications.
Although patients for TJA with ASB have an established increased risk for PJI, the causative microorganisms are interestingly not consistently associated.6,8,9 The microorganisms cultured in the urine of ASB patients were all different from the PJI cultures in our study. While some studies report similar isolates between the ASB and PJI microorganisms, a systematic review and meta-analysis including 28 588 TJA patients reported that there was no causal association between microorganisms (OR: 0.98; 95% CI 0.39- 2.44).6,8,9
Therefore, the value of preoperative testing for ASB may be controversial, especially in resource-constrained developing countries. However, an additional consideration in our demographic is that the prevalence of ASB was higher for HIV-positive patients than HIV-negative patients (p = 0.084). Although this was not statistically significant, this finding must be highlighted as South Africa accounts for the most people living with HIV and the highest seroprevalence, worldwide.21 There were 7.7 million people living with HIV in 2020 and 240 000 new adult infections each year. There is an established association between HIV, highly active anti-retroviral treatment (HAART) and osteodegenerative pathology, which predisposes patients to TJA.22 In a study of 1 007 TJA patients in South Africa, the seroprevalence of HIV in patients for THA was higher than the general population.21 Furthermore, the 2018 International Consensus on Orthopaedic Infections determined that HIV is an independent risk for PJI.23 This emphasises the importance of ASB in HIV-positive patients that may determine a further predisposing risk factor for PJI. Screening for ASB may allow healthcare providers to identify patients at increased risk for PJIs to guide management of evolving perioperative complications. It may be used as a surrogate marker to identify those individuals likely to have infective complications. Furthermore, the strong correlation with wound complications may provide motivation for more elaborate wound care postoperatively - this, however, needs to be validated in further studies.
There were several significant findings according to demographic characteristics such as sex, age, ASA class and tobacco use in our study population. There is an associated increased prevalence of ASB associated with increasing age and female sex in the general population.24 In our TJA population, females were 3.6 times more likely to have ASB than males (p = 0.060). Sousa et al. similarly reported an increased ASB prevalence for females (16.3%) compared to males (5%) (p < 0.001).8 Our study also found that patients 70 years or older had the highest prevalence (18%) and were 3.5 times more likely to have ASB compared to patients younger than 70 (p = 0.005). Additionally, patients with an ASA class of 3 were 4.6 times more likely to have ASB compared to patients with ASA class 1 (p = 0.026). However, patients with a higher ASA class were also older in age, which might account for the higher ASB prevalence noted in this group.
To the authors' knowledge, this is the first study to investigate the prevalence of ASB in a TJA population in sub-Saharan Africa. There were several limitations identified in the study. First, the population size was small and there was no stratified sampling to identify significant risk factor associations, and type 2 error should be considered. The ASB prevalence description for a South African population may be incorporated into future systematic reviews and meta-analyses. The study may guide future research for management recommendations in immunocompromised individuals such as a randomised control trial with treated ASB-positive patients to evaluate any perceived benefit. Despite these weaknesses, there were relevant statistically and clinically significant findings to guide further research and add to current knowledge. The results for microorganism cultures may guide future aetiological studies to better ascertain the pathophysiology of PJIs.
Conclusion
The prevalence of ASB in a TJA population in South Africa is 22% and higher than reported findings worldwide. There is an established association between preoperative ASB and increased risk of infective complications, which was reflected in our study. Although the value of antibiotic therapy for ASB remains controversial, there is a role for routine urinalysis preoperatively to identify patients predisposed to infective complications that may warrant more elaborate investigation to identify modifiable risk factors not yet known. The high prevalence of HIV represents a large immune-compromised population in South Africa. Furthermore, these findings may guide improved management of patients in other resource-constrained environments such as better wound care in these individuals at risk - a randomised control trial with treated ASB positive patients would need to be done to evaluate any perceived benefit.
Ethics statement
The authors declare that this submission is in accordance with the principles laid down by the Responsible Research Publication Position Statements as developed at the 2nd World Conference on Research Integrity in Singapore, 2010.
Medical clearance was obtained from the University of the Witwatersrand Human Research Ethics Committee (Medical) registered with the National Health Research
Ethics Council (NHREC) of the National Department of Health (M160716). Informed consent was obtained from all patients prior to being included in the study.
All procedures were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008.
Declaration
The authors declare authorship of this article and that they have followed sound scientific research practice. This research is original and does not transgress plagiarism policies.
Author contributions
ZM: First draft preparation, manuscript revision
TP: Data capture, first draft preparation
LM: Study conceptualisation, manuscript revision
JRTP: Study conceptualisation, study design, manuscript revision
ORCID
Maharaj Ζ https://orcid.org/0000-0001-9172-911X
Pillay Τ https://orcid.org/0000-0001-9202-9449
Mokete L https://orcid.org/0000-0001-9227-0515
Pietrzak JRT https://orcid.org/0000-0001-5694-0016
References
1. American Academy of Orthopaedic Surgeons, American Joint Replacement Registry (AJRR). Fifth AJRR Annual Report on Hip and Knee Arthroplasty Data (2018). Downloaded from: http://connect.ajrr.net/2019-ajrr-annual-report. Accessed 7 Jan 2020. [ Links ]
2. Organisation for Economic Co-operation and Development (OECD) (2017), Health at a Glance 2017: OECD Indicators, OECD Publishing, Paris. https://doi.org/10.1787/health_glance-2017-en. [ Links ]
3. Sloan M, Premkumar A, Sheth NP. Projected volume of primary total joint arthroplasty in the U.S., 2014 to 2030. J Bone Joint Surg Am. 2018;100:1455-60. https://doi.org/10.2106/JBJS.17.01617. [ Links ]
4. Kurtz SM, Lau EC, Ong KL, et al. Which clinical and patient factors influence the national economic burden of hospital readmissions after total joint arthroplasty? Clin Orthop Relat Res. 2017;475:2926-37. https://doi.org/10.1007/s11999-017-5244-6. [ Links ]
5. Beam E, Osmon D. Prosthetic joint infection update. Infect Dis Clin N Am. 2018;32(4):843- 59. https://doi.org/10.1016/j.idc.2018.06.005. [ Links ]
6. Weale R, El-Bakri F, Saeed K. Pre-operative asymptomatic bacteriuria: a risk factor for prosthetic joint infection? J Hosp Infect. 2019;101:210-13. [ Links ]
7. Natshara KM, Shelton TJ, Meehan JP, Lum ZC. Mortality during total hip periprosthetic jointinfection. J Arthroplasty. 2019;34(7 Suppl):S337-42. https://doi.org/10.1016/j.arth.2018.12.024. [ Links ]
8. Sousa R, Munoz-Mahamud E, Quayle J, et al. Is asymptomatic bacteriuria a risk factor for prosthetic joint infection? Clin Infect Dis. 2014;59:41-47. [ Links ]
9. Sousa RJG, MD, Abreu MA, Wouthuyzen-Bakker M, Soriano AV. Is routine urinary screening indicated prior to elective total joint arthroplasty? A systematic review and meta-analysis. J Arthroplasty. 2019;34:1523-30. https://doi.org/10.1016/j.arth.2019.03.034. [ Links ]
10. Cordero-Ampuero J, De Dios M. What are the risk factors for infection in hemiarthroplasties and total hip arthroplasties? Clin Orthop Relat Res. 2010;468(12):3268-77. [ Links ]
11. David TS, Vrahas MS. Perioperative lower urinary tract infections and deep sepsis in patients undergoing total joint arthroplasty. J Am Acad Orthop Surg. 2000;8:66-74. [ Links ]
12. Pulido L, Ghanem E, Joshi A, et al. Periprosthetic joint infection: the incidence, timing, and predisposing factors. Clin Orthop Relat Res. 2008;466:1710-15. [ Links ]
13. British Orthopaedic Association. Primary total hip replacement: a guide to good practice. London: BOA; 2012. Available from: https://www.britishhipsociety.com/uploaded/Blue%20Book%202012%20fsh%20nov%202012.pdf. Accessed Jan 2020. [ Links ]
14. Ariza J, Gomis M, Barberan J, et al. Protocolos Clinicos SEIMC: infecciones osetoarticularles y de partes blandas [SEIMC Clinical Protocols: osteoarticular and soft tissue infections]. Madrid: Sociedad Espanola de Enfermedades Infecciosas y Microbiologia Clinica; 2000. Available from: https://www.seimc.org/contenidos/documentoscientificos/procedimientosclinicos/ seimcprocedimientoclinicovi.pdf. Accessed Jan 2020. [ Links ]
15. Antibiotic Expert Groups. Surgical prophylaxis; therapeutic guidelines: antibiotic. Version 15. Melbourne: Therapeutic Guidelines Limited; 2016. Available from: https://tgldcdp.tg.org.au/index. Accessed Jan 2020. [ Links ]
16. Ipe DS, Sundac L, Benjamin WH Jr, et al. Asymptomatic bacteriuria: prevalence rates of causal microorganisms, etiology of infection in different patient populations, and recent advances in molecular detection. FEMS Microbiology Letters. 2013 Sep;346(1):1-10. https://doi.org/10.1111/1574-6968.12204. [ Links ]
17. Parvizi J, Tan TL, Goswami K, et al. The 2018 definition of periprosthetic hip and knee infection: an evidence-based and validated criteria. J Arthroplasty. 2018;33:1309-14. https://doi.org/10.1016/j.arth.2018.02.078. [ Links ]
18. Martinez-Velez D, Gonzalez-Fernandez E, Esteban J, Cordero-Ampuero J. Prevalence of asymptomatic bacteriuria in knee arthroplasty patients and subsequent risk of prosthesis infection. Eur J Orthop Surg Traumatol. 2016;26:209-14. [ Links ]
19. Garcia-Nuno L, Villamil C, Gonzalez-Cuevas A, et al. Usefulness of urinoculture to patients with dementia and femoral neck fracture at admission to hospital: preliminary results. Geriatr Orthop Surg Rehabil. 2017;8:10-13. [ Links ]
20. Honkanen M, Jamsen E, Karppelin M, et al. The impact of preoperative bacteriuria on the risk of periprosthetic joint infection after primary knee or hip replacement: a retrospective study with a 1-year follow up. Clin Microbiol Infect. 2017;24:376-80. [ Links ]
21. Maharaj Z, Pietrzak J, Sikhauli N, et al. The seroprevalence of HIV in patients undergoing lower limb total joint arthroplasty in South Africa. Sicot J. 2020:6(3). Published online 2020 Sep 19. https://doi.org/10.1051/sicotj/2019042. [ Links ]
22. Pietrzak J, Maharaj Z, Mokete L, et al. Human immunodeficiency virus in total hip arthroplasty: no more (immuno-)compromise. EFORT Open Rev. 2020;5(3):164-71. https://doi.org/10.1302/2058-5241.5.190030. [ Links ]
23. Zainul-Abidin S, Amanatullah DF, Anderson MB, et al. General assembly, prevention, host related general: Proceedings of international consensus on orthopedic infections. J Arthroplasty. 2019;34(2):S13-35. [ Links ]
24. Nicolle LE, Bradley S, Colgan R, et al. Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis. 2005;40:643-54. [ Links ]
Received: October 2020
Accepted: April 2021
Published: November 2021
Editor: Prof. Michael Held, University of Cape Town, Cape Town, South Africa
Funding: No funding was received for this study.
Conflict of interest: The authors declare they have no conflicts of interest that are directly or indirectly related to the research.
* Corresponding author: maharajzia@gmail.com














