Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
SA Orthopaedic Journal
On-line version ISSN 2309-8309
Print version ISSN 1681-150X
SA orthop. j. vol.19 n.3 Centurion Aug./Sep. 2020
http://dx.doi.org/10.17159/2309-8309/2020/v19n3a5
ARTHROPLASTY
Peri-articular infiltration in the resource-restrained environment - still a worthwhile adjunct to multimodal analgesia post total knee replacement
Van Heukelum MI; Blake CAII; Franken TIII; Burger MCIV; Ferreira NV; Gobetz GVI
IMBChB, DA(SA); Registrar, Division of Orthopaedic Surgery, Department of Surgical Sciences, Faculty of Medicine and Health Sciences, Tygerberg Hospital, Stellenbosch University, South Africa
IIBSc, MBChB, MMed; Consultant, Division of Orthopaedics, Faculty of Health Sciences, University of Free State, Bloemfontein, South Africa
IIIMBChB, MMed; Consultant, Division of Orthopaedic Surgery, Department of Surgical Sciences, Faculty of Medicine and Health Sciences, Tygerberg Hospital, Stellenbosch University, South Africa
IVBSc, B(Med)Sc Hons, M(Med)Sc, PhD; Research Coordinator, Division of Orthopaedic Surgery, Department of Surgical Sciences, Faculty of Medicine and Health Sciences, Tygerberg Hospital, Stellenbosch University, South Africa
VBSc, MBChB, FCOrth(SA), MMed, PhD; Associate Professor, Division of Orthopaedic Surgery, Department of Surgical Sciences, Faculty of Medicine and Health Sciences, Tygerberg Hospital, Stellenbosch University, South Africa
VIMBChB, DA(SA); Registrar, Department of Anaesthesia and Perioperative Medicine, Faculty of Medicine and Health Sciences University of Cape Town, Groote Schuur Hospital, South Africa
ABSTRACT
BACKGROUND: Peri-articular infiltrations (PAI) in total knee arthroplasty (TKA) offer effective analgesia, and are cost effective, safe and easy to perform. Currently there is no gold standard technique based on evidence-based medicine; described methods are based on consensus recommendations. The latest literature supports PAI including complex and multiple drug combinations, such as liposomal bupivacaine, ropivacaine and ketorolac, which are not available in all settings. This study aims to prove that a basic PAI technique using widely available and inexpensive agents offers good and effective outcomes in a resource-poor environment
METHODS: A double-blind randomised control trial compared the effectiveness of PAI with a simple, widely available anaesthetic solution (bupivacaine and adrenalin) to a normal saline control group. Infiltration volumes were calculated at 1 ml/kg and the infiltration technique followed a specific protocol. Post-operative outcomes included visual analogue scores (VAS), ambulation scores, morphine use, knee range of motion (ROM) and time to discharge
RESULTS: Two comparable groups of 26 patients each were included (intervention: 81% female, mean age 64.8±8.8 years vs control: 65% female, mean age 67.0±7.6 years). All pain-related measures favoured the intervention group but failed to reach statistical significance at 24 and 72 hours. Mean VAS scores at 48 hours were significantly lower in the intervention group. (VAS score 3.0±1.6 vs 4.1±1.2, p=0.013). The other parameters measured strongly favoured the intervention group but did not prove to be significant
CONCLUSIONS: A volume per kilogram PAI technique making use of widely available, cost-effective agents provides a statistical reduction in VAS scores at 48 hours post TKA. This suggests that in a resource-poor environment PAI is still a valuable addition to the multimodal analgesia pathway in the post-operative management of TKA. Maximum drug doses may show even more promising results, specifically in the first 24 hours post-operatively. Further studies investigating PAI for TKA in resource-restrained environments are indicated
Level of evidence: Level 2
Keywords: standardisation peri-articular infiltration, multimodal analgesia, pre-emptive analgesia
Introduction
Post-operative pain is the most important concern for patients considering a total knee replacement.1 Failure to control pain induces pathophysiological responses which lead to increased morbidity, patient anxiety, impaired rehabilitation, disrupted sleep patterns and decreased patient satisfaction.2 Recently the Joint Commission on the Accreditation of Healthcare Organizations in the United States has declared pain to be the 'fifth vital sign' and acknowledges that patients have the 'right' to adequate pain management.3
Peri-articular infiltration (PAI) as part of a pre-emptive multimodal analgesic protocol has gained widespread popularity among arthroplasty surgeons.2 These infiltrations have proven to provide good analgesia, are cost effective, have minimal side effects, and are easy to perform.4-6 In the era of enhanced recovery after surgery (ERAS), PAI protocols play an important role in knee arthroplasty based on the principle of opioid-free anaesthesia and analgesia. Post-operative in-patient time is not only inconvenient to the patient but adds to the costs involved with arthroplasty surgery. PAI relates to rapid patient rehabilitation and results in shorter length of stay post-surgery.7
Current controversy related to PAI involves the major heterogeneity surrounding infiltration techniques: each institution or surgeon performs the technique in a different manner using different infiltration 'cocktails', varying volumes and non-specific injection techniques and distributions.8,11 The latest literature supports PAI including complex and expensive drug combinations, including agents such as liposomal bupivacaine, ropivacaine and ketorolac, which are not available in all settings.
As part of a multimodal pain management pathway, our bupivacaine and adrenalin-based PAI protocol targets eight areas that have been identified to systematically guide PAI based on knee neuroanatomy and the concentrations of mechanoreceptors.8 The aim of this prospective, double-blinded randomised controlled trial was to assess whether PAI using drug combinations that are widely available offer effective outcomes in a resource-poor environment.
Materials and methods
A double-blind randomised control trial was conducted at Worcester Provincial Hospital between February 2017 and October 2017. All patients undergoing elective total knee arthroplasty (TKA) for osteoarthritis were evaluated for eligibility. Patients were included if they were American Society of Anesthesiologists (ASA) grade 3 or less, had a body mass index under 40 and had no contraindications to spinal anaesthesia. Patients were excluded if they suffered any anaesthetic complications during or following the surgery.
Study participants were stratified into one of two treatment groups using a sealed envelope randomisation technique. A dedicated anaesthetist selected an unmarked envelope pre-operatively and prepared the relevant medication for peri-articular injection. The intervention group received a solution of 1 ml/kg of local anaesthetic solution (concentration 1 mg/ml bupivacaine and 5 ug/ml of adrenalin), while the control group received 1 ml/kg of normal saline. Infiltration volume was calculated on a volume per kilogram (1 ml/kg) basis for each individual patient to standardise volumes and adjust for the large variance in patient size and weight. The preparation of the injectate solution was done away from the attention of the surgeon in order to ensure blinding. The patient, surgical team and physiotherapists as well as everyone involved in data capturing were blinded to the intervention.
All study participants received pre-emptive analgesia in the form of paracetamol 1 g six hourly and tramadol 50 mg six hourly in the period leading up the surgery. They received spinal anaesthesia with 2.8 ml of 0.5% hyperbaric bupivacaine and fentanyl 0.2 ml/10 micrograms. Cefazolin (2 g) was used for prophylaxis against surgical infection 30 minutes prior to skin incision. Clindamycin (600 mg) was used in cases with a penicillin allergy.
TKA surgery was performed via medial parapatellar approach by one of three consultant orthopaedic surgeons. The Sigma posterior stabilised, fixed bearing, cemented system from DePuy Synthes (West Chester, Pennsylvania, United States) was used in all cases.
The PAI was performed according to a specific protocol that targeted eight areas (zones) identified to systematically guide PAI (Figure 1). A 22-gauge 1½ inch needle was used for infiltration, allowing 2-3 ml to disperse per pass; aspiration was performed prior to any injection; the infiltrate was not allowed to elute from the tissue. Once infiltration volumes were calculated (1 ml/kg), 20 ml of the total volume was set aside for surgical wound infiltration. The remaining volume was roughly divided between the eight zones. Zone 6 is difficult to infiltrate as the area is mainly metaphyseal bone. This area and the surrounding soft tissues were infiltrated as well as possible, and any remaining infiltrate volume was included in zone 5.
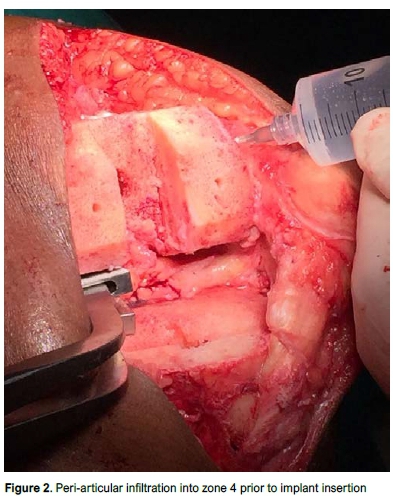
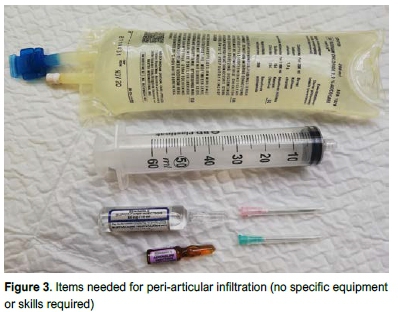
Posterior, posterolateral, posteromedial and intercondylar areas were infiltrated with the knee in flexion with the laminar spreader in place prior to insertion of the tibial component (Figure 2). Specific care was taken to avoid the popliteal artery. Anterior structures including the quadriceps tendon, suprapatellar pouch and infrapatellar fat pad were injected with the knee in extension while the cement was curing. The infiltration technique also included specific infiltration of the surgical wound post-operatively. Although the TKA was performed by three different surgeons, all PAIs in this study were performed by a single surgeon as per specific protocol to ensure continuity in technique. Figure 3 shows the minimal equipment required to perform the infiltration.
Post-operatively both groups received morphine-containing patient-controlled analgesia (PCA) pumps together with a standard analgesia protocol, including paracetamol and tramadol, with breakthrough morphine prescribed. Bilateral compression stockings, early mobilisation and Clexane 40 units daily was standard for deep venous thrombosis prophylaxis.
Participants were assessed daily for pain, opioid use, range of motion (ROM) and mobility during their inpatient physiotherapy session from day 1 post-surgery until discharged. Discharge criteria required patients to walk unaided with crutches, climb and descend stairs and achieve ROM from 0 to 90 degrees. Data was collected by a single dedicated researcher together with a member of the physiotherapy team.
Audit data was used to calculate sample size. A sample size of 52 participants (26 in each group) was adequate to detect differences between groups at a power of 90% and alpha level of 0.05. IBM SPSS version 24 was used to analyse the data. The completeness of the randomisation process was tested by comparing the two groups in terms of demographics and baseline parameters and found to be equivalent. Therefore, any differences were attributed to the intervention. All primary outcomes were measured quantitatively as continuous variables. Their distributions were checked for normality using Kologorov-Smirnov tests. If found to be normally distributed, parametric tests were used to compare the two groups, i.e. two sample t-tests while a non-parametric test, i.e. Mann-Whitney U test, was used to compare the two groups when data was not normally distributed. Normally distributed continuous data was described as mean±standard deviation (SD) while data that was not normally distributed was described as median and interquartile range (IQR). When continuous data was normally distributed within one group but not the other, median (IQR) was presented for both groups.
Results
Fifty-eight patients were consecutively recruited between February 2017 and October 2017. Three patients failed to meet the inclusion criteria and three patients were excluded. The final cohort consisted of 52 patients, 26 in each group (Figure 4). The mean age of the study population was 65.8 years, the mean BMI 32.2, and 73% of the participants were female.
Both intervention and control groups were equally distributed for baseline characteristics and were matched for age, height, weight and BMI (Table I).
The mean surgical time in the intervention group was 80.1±15.5 (95% CI 73.9 to 86.5) minutes and 79.0±16.4 (95% CI 72.4 to 85.6) in the control group. There was no significant difference in terms of surgical time between the groups (p=0.789).
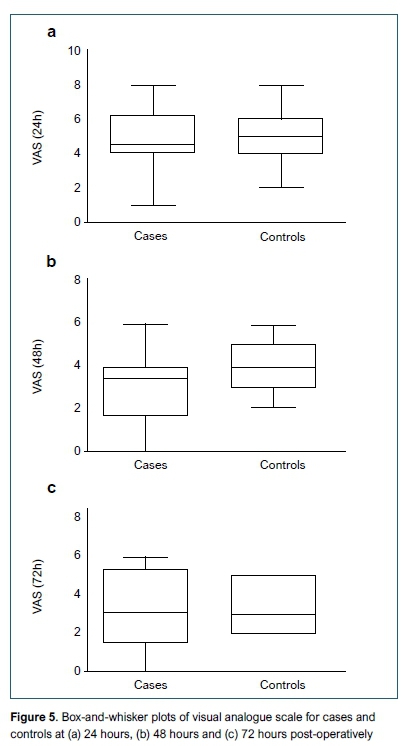
At 48 hours post-operatively the visual analogue score (VAS) for pain showed a significant reduction in the intervention group (p=0 .023) (median 3.5, 2 to 4) when compared to the control group (median 4, 3 to 5). There was no significant difference in VAS score at 24 (p=0.462) and 72 (p=0.808) hours post-operatively (Figure 5).
The mean time to discharge in the intervention group was 2.1±0.6 (95% CI 1.8-2.3) versus 2.4±1.1 (95% CI 2.0-2.9) in the control group. The mean time to discharge between the two groups was 2.3 days. ROM was measured as time (in days) to reach zero degrees extension (p=1.000), 90 degrees flexion (p=0.560) and time to both extension (0°) and flexion (90°) (p=0.743). Cumulative ambulation scores (CAS) at 24 (p=0.726), 48 (p=0.137) and 72 (p=0.808) hours, total volumes (ml) of patient-controlled morphine consumption (PCA) (p=0.146), ROM and mean time to discharge showed no statistical significance between the groups (Table II).
Post-operative drain volumes (ml) showed decreased values in the intervention group (mean 616.2 ml±245.8 [95% CI 516.9715.4] ml) compared to the control group (mean 670.0 ml±262.0 [95% CI 72.4-85.6] ml). The difference was not significant (p=0.448) (Table II).
Complications associated with morphine administration such as pruritus, nausea and vomiting, and urinary retention showed no difference between the groups (p=1.000). There were no complications, which we can attribute to the injected medication.
Discussion
Peri-articular infiltration (PAI) as part of a pre-emptive multimodal analgesic protocol has gained widespread popularity among arthroplasty surgeons.2 These infiltrations offer good efficacy in controlling pain, are cost effective, have minimal side effects, and are easy to perform.46 The technique can be used by all surgeons without the need for further training or specialised equipment. For the above reasons, PAI using an effective, widely available and cost-effective infiltrate offers a very attractive option in resource-poor settings.
Andersen et al. noted that the technique has gained widespread popularity; however, the optimal PAI technique (i.e. infiltration drug mixture, technique, use of catheters) has not been adequately evaluated.9 Turnbull et al. noted that variation in PAI technique such as type of medication used, dose and volume can alter the efficacy of the PAI7 while Kelley et al. concurred that although several peri-articular protocols exist, a gold standard has not been established and that additional research is needed to optimise the technique.10 Currently the leading recommendations are based on consensus recommendations.11
In a recent meta-analysis of 12 randomised control trials that included 770 patients, Gibbins et al. suggest that PAI results in statistically significant reduced pain scores 24 hours after TKA. However, there was significant heterogeneity among the studies urging caution in their interpretation. All 12 studies showed significant variation in the technique of performing PAI. Variations included the location of single intra-operative injections, the content and volume of the injectate and the use of post-operative infusions and boluses via catheter.12 They concluded that further research should focus on the optimum technique for PAI.
Literature supports PAI as an effective adjunct to multimodal pain management post TKA. Issues relating to the heterogeneity and the optimum technique are constantly evolving and may be resolved with time. Most sources agree that optimum outcomes rely on the use of infiltrates including liposomal bupivacaine, ropivacaine and ketorolac in varying combinations.
Liposomal bupivacaine (Exparel) is a long-acting, local anaesthetic. Efficacy of immediate-release bupivacaine HCl for acute postsurgical pain when administered via wound infiltration is well established; however, Exparel offers the advantage of a longer-acting local formulation that can be administered as a single dose.13 Dasta et al. published a pooled analysis of nine studies evaluating the effect of liposomal bupivacaine on pain intensity scores and opioid consumption. The analysis represented a total of five surgical procedures including TKA and compared liposomal bupivacaine with bupivacaine HCl. In all nine studies, patients who received liposomal bupivacaine reported significantly less pain over 72 hours and consumed less opioids compared with patients who received bupivacaine HCl.13 Liposomal bupivacaine costs around US$300 for a 20 ml vial14 and is not available in resource-scarce environments such as state healthcare in South Africa as well as the rest of the developing world.
Most articles do not specify the injection sites used during infiltration. This leads to uncertainty regarding its reproducibility and makes comparing studies difficult. By employing knowledge of intra-articular knee innervation and ensuring a systematic, site-specific approach to peri-articular injection, we aimed to maximise benefits from peri-articular injection and ensure a more reproducible result. Our infiltration technique focused on eight zones with increased number of nerve endings as described by Guild et al., following a systematic review of available literature focusing on knee neuroanatomy, pain generators, and the concentrations of mechanoreceptors.8 We targeted these specific zones (Figure 1) to systematically guide PAI. Infiltration technique included infiltration of the surgical wound post-operatively.
One of the major areas of heterogeneity within PAI is the volume of infiltrate used - a wide range of volumes are reported in the literature ranging from 20 ml to 150 ml.12 In most papers, infiltration volumes are pre-set, and patients receive the same volume irrespective of size or weight. We used 1 ml/kg of local anaesthetic solution (concentration 1 mg/ml bupivacaine and 5 ug/ml of adrenalin) calculated for each specific patient. In our experience, we found that these volumes were practical and provided adequate volume to infiltrate each zone thoroughly without having large excess. To our knowledge this is the first trial where infiltration volumes were calculated using a volume per kilogram basis. Our bupivacaine concentration of 1 mg/kg is well below the maximum dose of 2.5 mg/kg with adrenalin added (to a maximum dose of 225 mg). Higher bupivacaine concentrations may have led to improved outcomes.
Our study aimed to assess whether PAI using drug combinations that are widely available, inexpensive and injected following a well-described, systematic 'eight zone' infiltration technique, offers effective outcomes in a resource-poor environment.
Yeunyongviwat et al.15compared bupivacaine infiltration (20 ml of 0.25% bupivacaine) to saline and showed significantly reduced morphine consumption at six hours post-op but no difference in VAS scores. Busch et al. showed significantly less patient-controlled analgesia at six hours, at 12 hours, and over the first 24 hours after the surgery. In addition, they found lower visual analogue scores during the first four hours after the operation.16
Vendittoli et al. reported that morphine consumption was lower in the PAI group compared to the control group for up to 40 hours postoperatively.5 Chaumeron et al. suggest that when comparing PAI to femoral nerve block, PAI provided equivalent pain control for up to 120 hours without the 37% incidence of motor blockade found in the femoral nerve block group.17
Our findings showed VAS scores, as well as all other pain-related measures recorded at 24 and 48 hours were consistently lower in the intervention group and showed significant improvement in VAS score at 48 hours. This may be as a result of the down-regulation of pain receptors in the surrounding tissue but may also suggest a prolonged advantage offered by PAI compared to what was previously thought, and future research should explore this finding further. Other authors support the idea of prolonged advantage following PAI.5,15,18 Other parameters assessed in our study, including ambulation scores (CAS), narcotic usage (PCA), ROM and time to discharge favoured the intervention group but failed to prove statistical significance.
In modern medicine where the cost related to treatment, specifically surgery, is increasingly important not only to the patient but to the hospital and surgeon as well, post-operative days in hospital amount to increased expenses associated with any procedure. In the present study, we report no significant difference in the time to discharge between the intervention and control groups (2.1 days versus 2.4 days). These patients were discharged directly home and did not visit any form of rehabilitation or step-down facility. The mean time to discharge of 2.3 days between the two groups, however, represents a marked improvement from the mean time to discharge over the past five years in the same centre (mean 3.3 days over the period 2011 to 2016, 295 knees, Foxcroft D, unpublished data) This is in keeping with global standards for fast-track discharge protocols, with an average median time to discharge of 2.5 days.19 This improvement is most likely the result of a well-structured, specific protocol with multidisciplinary involvement and motivated patients.
Limitations of our study include that we did not account for patients' pre-operative medication and therefore cannot adjust for tolerances to narcotics in the peri-operative period; the bupivacaine concentration of 1 mg/kg is well below the maximum dose, and we may have seen improved results had we used higher concentrations. Our study has several strengths: it is a well-constructed, randomised, double blind study using an infiltration technique that is widely available, inexpensive and easily reproducible in any centre. Additionally, all PAIs as well as data capturing were performed by one person, providing continuity across the board.
Conclusion
A widely available, inexpensive PAI, calculated on a volume per kilogram basis and infiltrated according to a specific, eight-zone infiltration technique, leads to statistically significant improvement of pain scores at 48 hours post-operatively. This suggests that in a resource-poor environment, PAI is still a valuable addition to the multimodal analgesia pathway in the post-operative management of TKA. Maximum drug doses may show even more promising results, specifically in the first 24 hours post-operatively. Further studies investigating PAI for TKA in resource-restrained environments is indicated.
Ethics statement
Ethical approval was obtained from the Health Research Ethics Committee of Stellenbosch University, South Africa (protocol number N16/07/087) and the trial was registered with the national health research data base (WC2017RP5679). The author/s declare that this submission is in accordance with the principles laid down by the Responsible Research Publication Position Statements as developed at the 2nd World Conference on Research Integrity in Singapore, 2010. The study was conducted in accordance with the declaration of Helsinki of 1975, as revised in 2008, South African Guidelines for Good Clinical Practice and the Medical Research Council (MRC) Ethical Guidelines for Research.
Declaration
The authors declare authorship of this article and that they have followed sound scientific research practice. This research is original and does not transgress plagiarism policies.
Author contributions
MVH: Conception and design, data collection, data analysis and interpretation, drafting article, critical revision, final approval of article
CAB: Conception and design, data collection, critical revision, final approval of article
TF: Conception and design, critical revision, final approval of article
MCB: Data analysis and interpretation, drafting article, critical revision, final approval of article
NF: Data analysis and interpretation, drafting article, critical revision, final approval of article
GG: Conception and design, data collection, critical revision, final approval of article
ORCID
Van Heukelum M https://orcid.org/0000-0001-9160-7796
Blake CA https://orcid.org/0000-0002-3592^823
Burger MC https://orcid.org/0000-0003-2831-4960
Ferreira N https://orcid.org/0000-0002-0567-3373
Gobetz G https://orcid.org/0000-0002-2116-6750
References
1. Lavernia CJ, Alcerro JC, Rossi MD. Fear in arthroplasty surgery: the role of race. Clin Orthop Relat Res. 2010;468(2):547-54. [ Links ]
2. Dalury DF, Lieberman JR, MacDonald SJ. Current and innovative pain management techniques in total knee arthroplasty. J Bone Joint Surg. 2011;93(20):1938-43. [ Links ]
3. American Pain Society. Principles of analgesic use in the treatment of acute pain and cancer pain. American Pain Society; 1999. [ Links ]
4. Toftdahl K, Nikolajsen L, Haraldsted V, et al. Comparison of peri-and intraarticular analgesia with femoral nerve block after total knee arthroplasty: a randomized clinical trial. Acta Orthop. 2007;78(2):172-79. [ Links ]
5. Vendittoli PA, Makinen P, Drolet P, et al. A multimodal analgesia protocol for total knee arthroplasty. J Bone Joint Surg Am. 2006;88(2):282-89. [ Links ]
6. Parvataneni HK, Shah VP, Howard H, et al. Controlling pain after total hip and knee arthroplasty using a multimodal protocol with local periarticular injections: a prospective randomized study. J Arthroplasty. 2007;22(6):33-38. [ Links ]
7. Turnbull ZA, Sastow D, Giambrone GP, Tedore T. Anesthesia for the patient undergoing total knee replacement: current status and future prospects. Local Reg Anaesth. 2017;10:1-7. [ Links ]
8. Guild GN, Galindo RP, Marino J, Cushner FD, Scuderi GR. Periarticular regional analgesia in total knee arthroplasty: a review of the neuroanatomy and injection technique. Orthop Clin North Am. 2015;46(1):1-8. [ Links ]
9. Andersen L0, Kehlet H. Analgesic efficacy of local infiltration analgesia in hip and knee arthroplasty: a systematic review. Br J Anaesth. 2014;113(3):360-74. [ Links ]
10. Kelley TC, Adams MJ, Mulliken BD, Dalury DF. Efficacy of multimodal perioperative analgesia protocol with periarticular medication injection in total knee arthroplasty: a randomized, double-blinded study. J Arthroplasty. 2013;28(8):1274-77. [ Links ]
11. Joshi GP, Cushner FD, Barrington JW, et al. Techniques for periarticular infiltration with liposomal bupivacaine for the management of pain after hip and knee arthroplasty: a consensus recommendation. J Surg Orthop Adv. 2015;24(1):27-35. [ Links ]
12. Gibbins ML, Kane C, Smit RW, Rodseth RN. Periarticular local anaesthetic in knee arthroplasty: A systematic review and meta-analysis of randomised trials. SA Orthop J. 2016;15(3):49-56. [ Links ]
13. Dasta J, Ramamoorthy S, Patou G, Sinatra R. Bupivacaine liposome injectable suspension compared with bupivacaine HCl for the reduction of opioid burden in the postsurgical setting. Curr Med Res Opin. 2012 Oct 1;28(10):1609-15. [ Links ]
14. Liposomal Bupivacaine (Exparel) 2013-01-14 05:00:00 Ashley N. Lewis, PharmD, BCPS. [ Links ]
15. Yuenyongviwat V, Pornrattanamaneewong C, Chinachoti T, Chareancholvanich K. Periarticular injection with bupivacaine for postoperative pain control in total knee replacement: a prospective randomized double-blind controlled trial. Adv Orthop. 2012;2012:107309. [ Links ]
16. Busch CA, Shore BJ, Bhandari R, et al. Efficacy of periarticular multimodal drug injection in total knee arthroplasty. J Bone Joint Surg Am. 2006;88(5):959-63. [ Links ]
17. Chaumeron A, Audy D, Drolet P, Lavigne M, Vendittoli PA. Periarticular injection in knee arthroplasty improves quadriceps function. Clin Orthop Relat Res. 2013;471(7):2284-95. [ Links ]
18. Nair VS, Radhamony NG, Rajendra R, Mishra R. Effectiveness of intraoperative periarticular cocktail injection for pain control and knee motion recovery after total knee replacement. Arthroplasty Today. 2019 Sep 1;5(3):320-24. [ Links ]
19. Van Egmond JC, Verburg H, Mathijssen NM. The first 6 weeks of recovery after total knee arthroplasty with fast track: A diary study of 30 patients. Acta Orthop. 2015;86(6):708-13. [ Links ]
 Correspondence:
Correspondence:
Dr M van Heukelum
Division of Orthopaedic Surgery, Department of Surgical Sciences, Faculty of Medicine and Health Sciences
Tygerberg Hospital, Stellenbosch University
Cape Town, 7505, South Africa
tel: +27 21 938 4911; email: marcusvanh@gmx.com
Received: May 2019
Accepted: March 2020
Published: August 2020
Editor: Dr Michael Held, University of Cape Town, South Africa
Funding: None
Conflict of interest: The authors declare they have no conflicts of interest that are directly or indirectly related to the research.














